Abstract
Background
The diagnostic value of thoracic ultrasonography (US) has recently increased. Skilled sonographers with experience in pulmonary medicine have demonstrated the existence of US signs of chest pathology.
Purpose
To detect US findings associated with infectious interstitial pneumonia that can be used to supplement other diagnostic tools.
Materials and methods
Over a period of 5 years (2001–2006), 55 patients were referred to our ultrasonography units for evaluation of probable viral or viral-like infections of the respiratory tract. Each patient was subjected to a work-up that included clinical examination, blood tests, pulmonary function tests, bronchoscopy, chest radiographs, high-resolution computed tomography (HRCT), and thoracic US, which was performed under blinded conditions.
Results
Based on the findings that emerged from the work-up described above, all 55 patients were diagnosed with interstitial pneumonia. Evaluation of the US scans for the signs of interstitial lung disease described by Lichtenstein revealed “comet-tail” artifacts in the anterolateral lung fields in 31 (56.36%) patients and mixed patterns consisting in increased density associated with ring-down artifacts in 24 (46.64%). Pleural involvement was also observed in 34 cases (61.82%).
Conclusions
Thoracic US appears to be a useful adjunct to clinical, laboratory and radiological studies in patients suspected of having infectious interstitial pneumonia.
Keywords: Thoracic ultrasonography, Interstitial pneumonia, Pulmonary function tests, Radiological tests
Sommario
Premessa
L'ecografia del torace soltanto di recente ha assunto una sua completa dignità di metodica diagnostica: esperti ecografisti, con esperienza pneumologica, hanno, infatti, dimostrato la possibilità di una applicazione degli ultrasuoni in ambito toraco-polmonare, soprattutto in situazioni critiche, quali quelle emergenti in urgenza, in terapia intensiva o in ambito pediatrico o in corso di gravidanze.
Scopo
Da queste premesse si origina il lavoro, che si propone di individuare, se esistenti, gli aspetti ultrasonografici delle polmoniti interstiziali a genesi infettiva, e il ruolo di supporto (alla radiologia) degli ultrasuoni anche in questo ambito.
Materiali e metodi
Sono stati studiati 55 soggetti afferiti, nel quinqennio 2001–2006, alla UOS di Ecografia toracica (UOC Pneumologia I) dell'Ospedale M. Santo e dell'UOS di Ecografia Internistica (UOC di Medicina Generale) dell'Ospedale di Rogliano dell'AO di Cosenza, perché affetti da sospetta patologia infettiva respiratoria virale o simil-virale. Tali pazienti sono stati valutati con indagini clinico-funzionali e strumentali (anamnesi + es. obiettivo + esami ematochimici + prove di funzionalità respiratoria + broncoscopia + Rx e HRTC del torace) e con esame ecografico, in cieco, del torace.
Risultati
Dalla valutazione comparativa tra dati clinico-laboratoristico-strumentali e dati ecografici è emerso che i 55 soggetti studiati sono risultati affetti da polmonite interstiziale. In tali soggetti la diagnosi è stata formulata con l'ausilio delle comuni tecniche di studio, ma anche l'esame US ha permesso la individuazione di segni considerati diagnostici (Lichtenstein) di patologia interstiziale. Dei soggetti esaminati, infatti, 31 (56,36%) hanno mostrato all'ecografia la presenza di artefatti a coda di cometa (>5 per lato) nelle regioni anteriore e laterale del polmone (dato patognomico di patologia interstiziale) e 24 (46,64%) di “quadri misti” (aree di addensamento ecografico associate a ring down). In 34 (61,82%) casi sono stati descritti associati aspetti di patologia pleurica.
Conclusioni
Attraverso l'osservazione di segni ultrasonografici e la correlazione di questi con quelli clinico-laboratoristico-strumentali di routine, gli autori valutano la possibilità di attribuire rilievo anche alla indagine US nella diagnosi di polmonite interstiziale ad eziologia infettiva e, senza voler sostituire gli US alle tradizionali e opportune tecniche di approccio e diagnosi, la propongono quale complementare tecnica metodologica di indagine.
Introduction
For years, ultrasonography (US) played a marginal role in the examination of thoracic structures, with the obvious exception of the heart [1]. However, sonographers trained in pulmonary medicine have continued to use this imaging modality to study the thorax and lungs, and thanks to their efforts, interest in this approach and its potential applications has increased in recent years. Sonographic evaluation of the lungs undoubtedly offers interesting possibilities, but its applicability is limited by the absence of objective US findings in the normal lung. Paradoxically, these organs are “revealed” by the disease that affects them [2].
The use of US in the evaluation of the respiratory tract dates back to the 1970s, when it was employed for the study of pleural opacities [3] and for the detection of lung masses in children [4] and adults [5]. Since then, it has also emerged as a fundamental tool for use during thoracentesis (US-guided) [6,7], biopsy of subparietal pulmonary nodules [8], assessment of mediastinal pathology [9], pleural sclerotherapy and chest-tube placement [10], and today contrast-enhanced US (CEUS) is even being used for the characterization of pulmonary nodules [11]. The 1990s witnessed the opening of the fascinating new field of endoscopic US, in which sonography was combined with esophagoscopy or bronchoscopy [12–14]. More recently, US has earned a place in the investigation of a number of lung diseases that were formerly studied exclusively with computed tomography, such as pulmonary fibrosis and interstitial lung diseases [15–19].
The aim of the present study was to define the possible contributions of US in the diagnostic work-up of patients suspected of having infectious interstitial pneumonia and the possible role of thoracic US in infectious disease medicine.
Methods and disease classification
Sonographic examinations of the thorax are generally performed with real-time gray-scale scanners equipped with high-resolution 3.5-, 5.0- or 7.5-MHz transducers. Small sectorial or convex-array models are preferable. Linear transducers can be used to examine the chest wall, the pleurae, and other superficial structures; sectorial transducers are used to evaluate pleural effusions. Sonographic exploration of the thorax is hindered by the presence of the rib cage and air in the lungs. Organs that transmit sound waves well, e.g., liver, spleen, kidneys, have been evaluated as “acoustic windows” for US examination of the thoracic cavity. Access is usually gained through intercostal, suprasternal, parasternal or subcostal routes [2,20,21]. In a normal subject, longitudinal US scans of the thorax reveal the following structures (in order of increasing depth) (Fig. 1) [22–24]:
-
-
a soft-tissue layer composed of skin, subcutaneous fat, and muscle;
-
-
the ribs (with posterior shadowing) and intercostal muscles;
-
-
the surfaces of the pleurae and the lung itself, which has a powdery appearance due to the sound-wave reverberating effects of air;
-
-
mediastinal structures, in particular the heart and great vessels.
The use of US has been validated in three sets of guidelines [25–30].
-
-
Use as a first-line diagnostic tool in patients suffering from acute trauma, bedridden patients or those that cannot be moved or uncooperative patients, when the services of a radiologist are not available.
-
-
Use as an adjunct to traditional modalities for the detection of disease and lesions, for determining the nature (solid vs. fluid-filled) of superficial neoformations in contact with or originating from the chest wall: pleural effusions and tumors; lung disease including peripheral cysts and tumors, abscesses, atelectasis, hepatized lobar pneumonia; mediastinal pathology (cysts, tumors, thymic disease); disease of the chest wall (cysts and tumors involving bones, muscles, and/or cartilage) or diaphragm.
-
-
Interventional uses. Percutaneous punctures for diagnosis or drainage of pleural effusions, needle aspiration and biopsy of the pleurae and peripheral regions of the lungs; pleurodesis; aspiration of the contents of peripheral lung abscesses or pleural empyema for microbiological diagnosis; US-guided transthoracic needle biopsy in patients with severe forms of pneumonia (especially those who are immunocompromised) whose etiology cannot be otherwise determined; and transbronchial biopsies during bronchoscopy (Fig. 2).
Fig. 1.
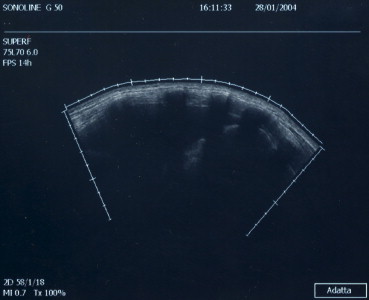
The thorax: anatomical planes in a right paravertebral scan.
Fig. 2.
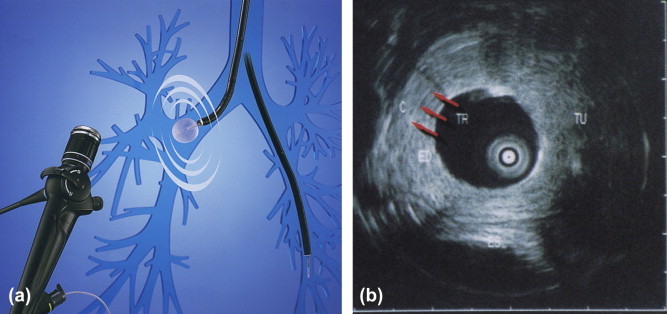
(a and b) Endobronchial US image.
More recently, the use of ultrasound for the study of the pulmonary interstitium has received attention. Although this approach has been assessed in several studies, there is currently no widely accepted classification system of interstitial lung disease based on both clinical and US findings. The one proposed by Fraser et al. based on anatomical and clinical features was incorporated into the European Respiratory Society (ERS)/American Thoracic Society (ATS) International Multidisciplinary Consensus Classification (2002). Of the numerous classification systems that have been proposed, it also seems to be the one best suited for correlation with US findings [31–36] and it was therefore used as the reference point for the present study.
Using this classification, we attempted to define US correlates for acute and chronic inflammatory processes involving Type I and Type II pneumocytes (and therefore the alveolo-capillary membrane), which are characterized by activation of inflammatory cells leading in some cases to the deposition of fibrous tissue in the interstitium. Histologically, three phases can be distinguished: the acute exudative endoalveolar phase, the intermediate stage characterized by interstitial inflammation, and the phase of chronic septal fibrosis. In certain US images, infectious forms of interstitial pneumonia could be visualized, including those caused by bacteria, viruses (cytomegalovirus, coronarovirus, HIV), viral-like agents (mycoplasma, chlamydiae, coxiellae), and protozoa (Pneumocystis carinii).
The US classification is based on the indirect criteria (artifacts) for the diagnosis of interstitial lung disease proposed by Soldati [37] and by Lichtenstein [38–40]: comet-tail artifacts in the anterior and lateral regions of the thorax (B lines), subpleural thickening, and irregularity of the parietal pleural line. The alveolar-interstitial syndrome causes comet-tail artifacts (which are actually expanded in deep tissues to form a “ring down” pattern) that arise from the surface of the pleura and project all the way to the opposite side of the screen, slashing dramatically through the lung fields. These artifacts are regarded as significant only when they appear in the anterior and lateral regions of the chest. Sporadic posterolateral comet tails (fewer than five per side) located in the basal 2–3 cm of the lung are not significant. Other US signs include pleural thickening, which often appears irregular. The anterior and posterior surfaces are affected, particularly those overlying the bases of the lungs. Unilateral or bilateral slowing of the physiological “gliding sign” displays direct correlation with the severity of the disease. Finally, subcostal scans performed with the patient in the supine position will often reveal reduced diaphragmatic excursion.
Materials and methods
In this study, we analyzed thoracic–abdominal sonograms performed in our units during a 5-year period (2001–2006) in patients with diagnoses of probable interstitial pneumonia or bronchopneumonia (based on medical reports and images). All images had been obtained on an ATL 1500 US/color Doppler scanner.
For each case, we attempted to determine the nature of the infectious focus (i.e., viral vs. viral-like) based on an analysis of subjective (cough, dyspnea, fever, asthenia, joint pain) and objective (rales, crepitus, wheezing, rubs) clinical features; findings on plain films and high-resolution computed tomographic (HRCT) scans of the chest; the results of pulmonary function studies (plethysmography, DLCO, blood-gas analysis); laboratory data (hematocrit, antibodies against viruses and virus-like agents, and when available, bacteriological analyses of sputum and bronchial aspirates). The following findings on chest X-rays and CT were considered indicative of interstitial involvement: irregular bands of peribronchial thickening radiating from the hilus; irregular bands of attenuation with distortion of the pulmonary architecture; ground-glass appearance; hilar lymphadenopathy (alone or associated with isolated foci of infection); pleural effusion [41–46].
Results
A total of 70 subjects had thoracic US examinations: 15 controls and 55 (38 males, 17 females) with suspected disease that proved to be viral pneumonia or pneumonia with a viral-like etiology.
In analyzing subjective clinical findings, we regarded the presence of three or more of the following symptoms as indicative of acute infectious interstitial lung disease: dry, hacking or moderately productive cough; asthenia; joint pain; moderate to high fever (37.5–39 °C); chest pain; dyspnea. All 55 of the patients with disease had histories that were positive for three or more of these findings in various combinations.
In 43 of these subjects, the physical examination revealed abnormal auscultatory findings (subtle in some cases) that included subcrepitant rales, crepitation, wheezing or friction rubs.
In 20 of the 55 patients, pulmonary function studies were normal; in the remaining cases, findings were indicative of a restrictive syndrome (n = 10), small-airway obstruction (n = 10) (MEF25) or altered alveolocapillary diffusion (n = 15). Blood-gas analysis yielded normal findings in 44/55 cases and revealed hypoxemia without hypercapnia in the other 11. None of the patients presented hypercapnia, but three were hypocapnic.
Complete blood counts with differential revealed lymphomonocytosis in 38 subjects, neutrophilic leukocytosis in four, and normal leukocyte profiles in the other 13.
In 10 of the patients, assays for antibodies against respiratory viruses and virus-like agents revealed significant titers (>1/80) against influenza virus-like agents (n = 3), adenovirus (n = 5), coxsackievirus (n = 1) or echovirus (n = 1). Eight were also positive for antimycoplasma antibodies and six had positive titers of antibodies against Chlamydiae.
Cultures of sputum or bronchial aspirates grew Pseudomonas aeruginosa in one case and Aspergillus in another.
Radiological examinations (plain films and high-resolution computed tomography [HRCT] of the chest) were carried out in all 55 cases with suspected disease, and the results were compared with those of thoracic ultrasonography. In five cases, the plain film or HRCT disclosed the presence of isolated foci and in 50 various combinations of the following: irregular lines of peribronchial thickening radiating from the hilum, irregular linear attenuation with distortion of the architecture, conglomeration of the bronchi, areas with a ground-glass appearance, hilar lymphadenopathy, accentuated fissures, and moderate pleural effusions [47–50].
No pathological findings emerged from the clinical or instrumental examinations in the group of 15 control subjects.
Thoracic ultrasound examinations in the two groups were performed under blinded conditions by a sonographer who was unaware of the nature of the subjects' acute respiratory disease to minimize bias. The 15 normal control subjects were also examined under blinded conditions to obtain data for comparison purposes.
As shown by the ultrasound examinations performed in all 15 of the normal subjects, the ultrasound beam undergoes attenuation with loss of definition 5–6 cm from the surface. As a result, horizontally and vertically oriented artifacts can be identified within a homogeneous background with variable levels of echogenicity (ground-glass appearance).
In 31 of the 55 patients with interstitial disease, pure pictures emerged with comet-tail artifacts (>5 per side), especially in the anterior and lateral regions (which is typical of interstitial pathology) of the middle-upper lung fields; in the other 24 cases, mixed pictures emerged with areas of increased sonographic density associated with ring-down artifact.
In 34 of the cases, there was evidence of pleural changes, consisting in one or more of the following: effusion (12 cases), accentuated fissures (eight cases), thickening of the leaflets (14 cases), and irregularity (16 cases) (indentation) of the pleural line. In 31 cases, the pneumonia was correlated with pure sonographic findings typical of joint interstitial/pleural involvement. In the other 24, there were mixed forms, with or without pleural involvement.
In all 31 subjects with pure forms, the CT examination confirmed the interstitial nature of the pneumonia (Figs. 3–7).
Fig. 3.
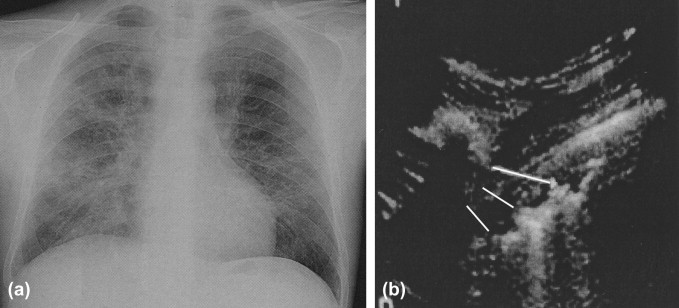
Interstitial pneumonia. (a) Plain film: interstitial pattern; and (b) US: ring-down artifact and pleural irregularity.
Fig. 4.
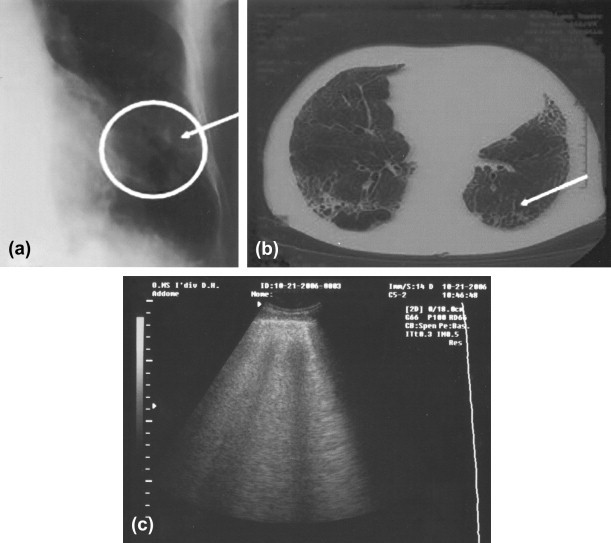
(a–c) Plain film, computed tomography, and ultrasound: interstitial pattern in interstitial pneumonia.
Fig. 5.
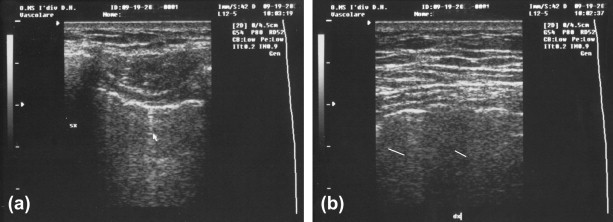
(a and b) Image of the pleura on US in interstitial pneumonia.
Fig. 6.
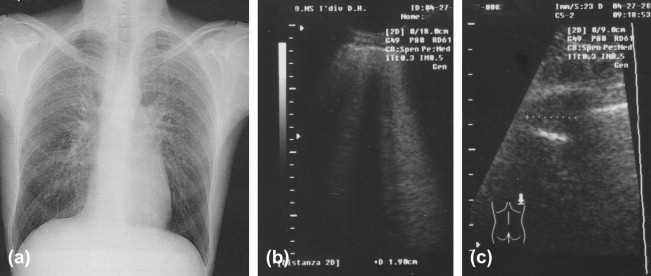
Interstitial pneumonia caused by Chlamydiae: radiological (a) and US (b) findings; and comparison (c).
Fig. 7.
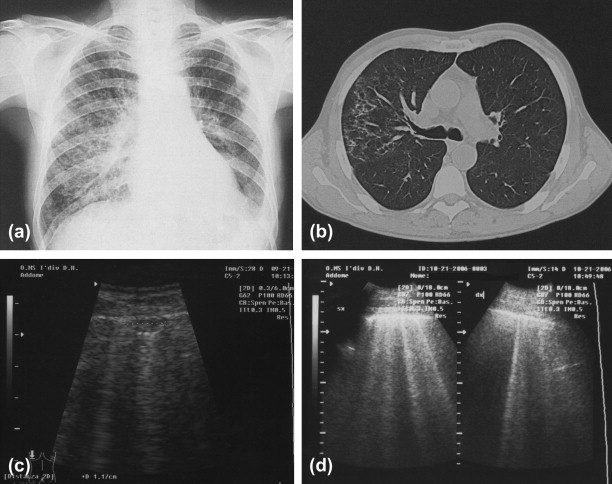
Pneumocystis carinii infection in an HIV+ subject. (a) Radiological findings; (b) CT; (c) subpleural hypoechoic zone; and (d) ring-down artifact.
Conclusions
Ultrasound evidence suggestive of interstitial involvement in patients with pneumonia seems to be related to acoustic interference on small aerated structures. The air remains isolated trapped, in tiny (measured in millimeters or smaller units) units (alveoli, lobules, bronchioles), which prevent its extensive representation (below the wavelength of 3.5 MHz transducer: 0.5 mm). The result is diffusion or diffraction (scattering) with deviation of the incident energy in multiple directions. (This event can be best visualized with higher-frequency transducers, i.e., ≥7.5 MHz.) The vertical comet-tail artifact that results appears as a strong linear echo that extends from the reflector into deeper planes. In the normal lung, these artifacts appear as transverse images (ring-down or comet-tail) that reproduce the pleural line as continuous echogenic bands, which are attenuated in deeper planes by alveolar gas collections. They are formed by small parallel bands of echoes (A lines) arranged transversally, with respect to the beam that generates them; their width decreases with depth [38,51–60]. The presence of comet-tail artifacts extending from the visceral pleura and of a few ring-down artifacts in the basal regions is a normal finding [61]. When there are more than five artifacts per side, especially in the anterolateral and middle-upper regions, this is pathognomonic for interstitial disease [15,38]. The topographic extension is considered an index of the progressive involvement of the interstitium in edematous or inflammatory conditions of the lung (pulmonary edema or pneumonia) [17,62]. Vertical artifacts are also observed in the active phases of interstitial pulmonary fibrosis, where they reflect structural disruption of the connective tissue and expansion of the interstitial space [63,64], and the pleura displays surface irregularities and increased thickness [16].
In conclusion, based on a comparative analysis of clinical, instrumental, laboratory and sonographic findings (Tables 1 and 2), it seems reasonable to affirm that in expert hands, thoracic sonography can also be used to assess interstitial pneumonia. Lichtenstein's view that a good sonographer does not need radiological studies seems overly enthusiastic. However, ultrasonography can be regarded as a useful supplement in the study of infectious forms of interstitial pneumonia, but it should always be used after a thorough clinical and imaging work-up based on plain-film radiography and HRCT to determine the characteristics, distribution and evolution of the interstitial lung lesions.
Table 1.
Diagnosis of interstitial pneumonia
|
|
|
|
Table 2.
Summary
|
Creation of a flow-chart can also be useful in formulating a diagnosis of interstitial pneumonia. As shown in the Flow-chart, the sonographic exam (even when used exclusively to provide C evidence) can be of use in the diagnosis of this disease (Flow-chart), as it has become in the work-up of pleural effusions and lobar pneumonia and in the work-up of patients in whom radiologic techniques cannot be used (bedridden patients, pregnant women, trauma victims, children, etc.) [65–68].
Flow-chart.
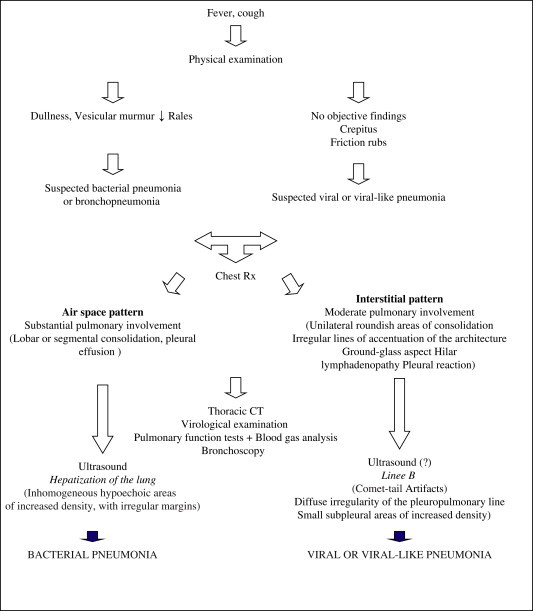
Diagnosis of infectious interstitial pneumonia.
References
- 1.Fraser R.G. WB Saunders; Philadelphia: 1970. Diagnosis of the diseases of the chest. p. 118. [Google Scholar]
- 2.Soldati G., Copetti R. Ecografia toracica. Medico Scientifiche; Torino: 2006. Il significato dell'ecografia del torace; pp. 9–36. [Google Scholar]
- 3.Kangerloo H., Sukov V., Sample F. Ultrasonographic evaluation of juxtadiaphragmatic masses in children. Radiology. 1977;125:785–787. doi: 10.1148/125.3.785. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch J., Carter S., Chikos P. Ultrasonic evaluation of radiographic opacities of the chest. AJR Am J Roentgenol. 1978;130:1153–1156. doi: 10.2214/ajr.130.6.1153. [DOI] [PubMed] [Google Scholar]
- 5.Lillington G.A. Pulmonary nodules: solitary and multiple. Clin Chest Med. 1979;3:361–367. [PubMed] [Google Scholar]
- 6.Harnsberger H., Lee T., Mukuno D. Rapid, inexpensive, real-time directed thoracentesis. Radiology. 1983;146:545–546. doi: 10.1148/radiology.146.2.6849106. [DOI] [PubMed] [Google Scholar]
- 7.Mc Gahan J., Anderson M., Walter P. Portable real-time sonographic and needle guidance systems for aspiration and drainage. AJR Am J Roentgenol. 1986;147:1241–1246. doi: 10.2214/ajr.147.6.1241. [DOI] [PubMed] [Google Scholar]
- 8.Cinti D., Hewkins H. Aspiration biopsy of peripheral pulmonary masses using real time sonography guidance. AJR Am J Roentgenol. 1984;142:1115–1116. doi: 10.2214/ajr.142.6.1115. [DOI] [PubMed] [Google Scholar]
- 9.Wernecke K., Vassallo P., Potter R. Mediastinal tumors. Biopsy under US guidance. Radiology. 1990;172:473–476. doi: 10.1148/radiology.172.2.2664870. [DOI] [PubMed] [Google Scholar]
- 10.Klein J.S., Schultz S., Heffner J.E. Interventional radiology of the chest: image-guided percutaneous drainage of pleural effusions, lung abscess, and pneumothorax. AJR Am J Roentgenol. 1995;164:581–588. doi: 10.2214/ajr.164.3.7863875. [DOI] [PubMed] [Google Scholar]
- 11.Sperandeo M., Sperandeo G., Varriale A. Contrast-enhanced ultrasound (CEUS) for the study of peripheral lung lesions: a preliminary study. Ultrasound Med Biol. 2006;32:1467–1472. doi: 10.1016/j.ultrasmedbio.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Shuder G., Isringhaus H., Kubale B. Endoscopic ultrasonography of the mediastinum in the esophageal varices by means of endoscopic ultrasonography. Scand J Gastroenterol. 1986;21:74–77. [Google Scholar]
- 13.Hurter T., Hanrath P. Endobronchial sonography: feasibility and preliminary result. Thorax. 1992;47:565–567. doi: 10.1136/thx.47.7.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker H.D., Lam S. Future diagnostic procedures: EBUS. Chest Surg Clin N Am. 1996;6:363–380. [PubMed] [Google Scholar]
- 15.Lichtenstein D., Meziere G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;15:940–946. doi: 10.1007/s001340050771. [DOI] [PubMed] [Google Scholar]
- 16.Lo Giudice V., Granieri S., Corcioni B. Uno studio preliminare sulla applicabilità degli ultrasuoni nella valutazione della patologia interstiziale polmonare. Giornale Italiano Ecografia. 2004;7:267–272. [Google Scholar]
- 17.Soldati G. Lung sonography: artifact, movement or echotexture? Giornale Italiano Ecografia. 2001;4:329–338. [Google Scholar]
- 18.Soldati G., Rossi M. Wet and dry lungs: a useful sonographic distinction. Crit Care. 1999;3(Suppl. 1):61. [Google Scholar]
- 19.Soldati G., Bergamini C. Chest sonography for extravascular lung water. Am J Cardiol. 2005;96:322–323. doi: 10.1016/j.amjcard.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Sperandeo M. L'utilità dell'ecografia nello studio della patologia toracica. Giornale Italiano di Ultrasonografia. 1991;12:19–21. [Google Scholar]
- 21.Sperandeo M. Ecografia del torace. CIC; Roma: 2004. Tecniche e metodiche – Anatomia; pp. 3–7. [Google Scholar]
- 22.Zompatori M. L'ecografia nello studio della patologia toracica. Radiol Med. 1992;84:10–23. [Google Scholar]
- 23.Soldati G., Copetti R. Medico Scientifiche; Torino: 2006. Ecografia toracica. pp. 1–64. [Google Scholar]
- 24.Ghigi T., Pirronti T., Zompatori M. vol. 2. Liviana Medicina; 1994. Richiami anatomici e anatomia ecografia. (Torace). 2–7. [Google Scholar]
- 25.Krejci C.S., Trent E.J., Dubinsky T. Thoracic sonography. Respir Care. 2001;46:932–939. [PubMed] [Google Scholar]
- 26.Lo Giudice V., Bruni A., Noto A. Il ruolo dell'ultrasonografia nella patologia toracica. Rass Mal App Resp. 2000;15:477–482. [Google Scholar]
- 27.Hearth F.J., Ernest A., Becker H.D. Endobronchial ultrasound (EBUS) guided transbronchial lung biopsy (TBBX) in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002;20:972–975. doi: 10.1183/09031936.02.00032001. [DOI] [PubMed] [Google Scholar]
- 28.Falcone F., Fois F., Grosso D. Endobronchial ultrasound. Respiration. 2003;70:179–184. doi: 10.1159/000070066. [DOI] [PubMed] [Google Scholar]
- 29.Hearth F.J., Becker H.D., Ernest A. A conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest. 2004;125:322–325. doi: 10.1378/chest.125.1.322. [DOI] [PubMed] [Google Scholar]
- 30.Hearth F.J., Lunn W., Becker H.D. Transbronchial vs transesophageal ultrasound-guided aspiration of enlarged mediastinal lymph nodes. Am J Respir Crit Care Med. 2005;171:1164–1167. doi: 10.1164/rccm.200411-1560OC. [DOI] [PubMed] [Google Scholar]
- 31.Fraser R.D., Colman N., Müller N.L. Synopsis of diseases of the chest. Elsevier; 2005. Pare: chronic interstitial lung disease; pp. 480–569. [Google Scholar]
- 32.Thannickal V.J., Wells A.U. Classification of interstitial pneumonias. Am J Respir Crit Care Med. 2006;173:141–142. doi: 10.1164/rccm.2510004. [DOI] [PubMed] [Google Scholar]
- 33.Talmadge E.K.J. Approach to the patient with interstitial lung disease. UpToDate. 2001 [Google Scholar]
- 34.Antó J.M., Cullinan P. Classification and epidemiology of interstitial lung diseases: concepts, methods and critical reflections. Eur Respir J. 2001;18:101S–106S. [PubMed] [Google Scholar]
- 35.Brown K.K., Schwarz M.I. Classifying interstitial lung disease: remembrance of things past. Chest. 2006;130:1289–1291. doi: 10.1378/chest.130.5.1289. [DOI] [PubMed] [Google Scholar]
- 36.American Thoracic Society, European Respiratory Society International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 37.Soldati G. Ecografia polmonare: artefatto, movimento o ecostruttura? Giornale Italiano di Ecografia. 2001;4:329–338. [Google Scholar]
- 38.Lichtenstein D., Meziere G., Biderman P. The comet tail artifact. An ultrasound sign of alveolar–interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–1646. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 39.Lichtenstein D., Meziere G., Biderman P. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26:1434–1440. doi: 10.1007/s001340000627. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenstein D., Lascols N., Prin S. The lung pulse: an early ultrasound sign of complete atelectasis. Intensive Care Med. 2003;29:2187–2192. doi: 10.1007/s00134-003-1930-9. [DOI] [PubMed] [Google Scholar]
- 41.Barlett J.G., Doell S.F., Mandel L.A. Guidelines from the Infections Diseases Society of America. Practice guidelines for the management of CAP in adults. Clin Infect Dis. 2000;31:347–382. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyashita N., Niki Y., Nakajima N. Prevalence of asymptomatic infection with Chlamydia in subjectively healthy adults. Chest. 2000;119:1416–1419. doi: 10.1378/chest.119.5.1416. [DOI] [PubMed] [Google Scholar]
- 43.Bosio A. Polmonite da Chlamidya Pn. How & Why in Medicine. 2006 [Maggio(Anno XVIII)] [Google Scholar]
- 44.Sénac J.P., Vernhet-Kovacsik H., Bousquet C. Is it possible to diagnose interstitial pneumonia only with lung imaging? Rev Mal Respir. 2006;23(4 Pt 2):10S92–10S96. [PubMed] [Google Scholar]
- 45.Bourke S.J. Interstitial lung disease: progress and problems. Postgrad Med J. 2006;82(970):494–499. doi: 10.1136/pgmj.2006.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King T.E. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 2005;172:268–279. doi: 10.1164/rccm.200503-483OE. [DOI] [PubMed] [Google Scholar]
- 47.Gharib A.M., Stern E.J. Radiology of pneumonia. Med Clin North Am. 2001;85:1461–1491. doi: 10.1016/s0025-7125(05)70391-6. [DOI] [PubMed] [Google Scholar]
- 48.Reittner P., Muller L.N., Heyneman L. Mycoplasma pneumoniae pneumonia: radiographic and high resolution CT features in 28 patients. AJR Am J Roentgenol. 2000;174:37–41. doi: 10.2214/ajr.174.1.1740037. [DOI] [PubMed] [Google Scholar]
- 49.Genereux G., Stillwell G.A. Bacterial infections of the lung. Semin Roentgenol. 1980;15:9–16. doi: 10.1016/0037-198x(80)90035-8. [DOI] [PubMed] [Google Scholar]
- 50.Kim E.A., Lee K.S., Primack S.L. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics. 2002;22:S137–S149. doi: 10.1148/radiographics.22.suppl_1.g02oc15s137. [DOI] [PubMed] [Google Scholar]
- 51.Wohlgenannt S., Gehmacher O., Mathis G. Sonography findings in interstitial lung diseases. Ultraschall Med. 2001;22:27–31. doi: 10.1055/s-2001-11252. [DOI] [PubMed] [Google Scholar]
- 52.Reissig A., Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease: the role of comet tail artifacts. J Ultrasound Med. 2003;22:173–180. doi: 10.7863/jum.2003.22.2.173. [DOI] [PubMed] [Google Scholar]
- 53.Jambrik Z., Monti S., Coppola V. Usefulness of ultrasound lung comets as a non radiologic sign of extravascular lung water. Am J Cardiol. 2004;93:1265–1270. doi: 10.1016/j.amjcard.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Agricola E., Bove T., Oppizzi M. Ultrasound comet-tail images: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–1695. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 55.Shevland J.E., Hirleman M.T., Hoang K.A. Lobar collapse in the surgical intensive care unit. Br J Radiol. 1983;56:531–534. doi: 10.1259/0007-1285-56-668-531. [DOI] [PubMed] [Google Scholar]
- 56.Beydon L., Saada M., Liu N. Can portable chest x-ray examination accurately diagnose lung consolidation after major abdominal surgery? A comparison with computed tomography scan. Chest. 1992;102:1697–1703. doi: 10.1378/chest.102.6.1697. [DOI] [PubMed] [Google Scholar]
- 57.Yang P.C., Luh K.T., Chang D.B. Ultrasonographic evaluation of pulmonary consolidations. Am Rev Respir Dis. 1992;146:756–762. doi: 10.1164/ajrccm/146.3.757. [DOI] [PubMed] [Google Scholar]
- 58.Dorne H.L. Differentiation of pulmonary parenchymal consolidation from pleural disease using the sonographic fluid bronchogram. Radiology. 1986;158:41–42. doi: 10.1148/radiology.158.1.3510027. [DOI] [PubMed] [Google Scholar]
- 59.American Medical Association. Privileging for ultrasound imaging. Available from: http://www.ana-assn.org.
- 60.Beaulieu Y., Marik P.E. Bedside ultrasonography in the ICU. Chest. 2005;128:881–895. doi: 10.1378/chest.128.2.881. [DOI] [PubMed] [Google Scholar]
- 61.Avruch L., Cooperberg P.L. The ring down artefact. J Ultrasound Med. 1985;4:21–28. doi: 10.7863/jum.1985.4.1.21. [DOI] [PubMed] [Google Scholar]
- 62.Sani S., Bolognesi L., Vivaldi I. Segni ecografici di polmonite interstiziale. Giornale Italiano di Ecografia. Sett. 1998;156 [da Abstract Congresso Nazionale SIUMB 1998] [Google Scholar]
- 63.Lo Giudice V. Gli ultrasuoni nella diagnosi delle interstiziopatie. Giornale Italiano di Ecografia. Sett. 2001:205–206. [da Abstract Congresso Nazionale SIUMB 2001] [Google Scholar]
- 64.Lichtenstein D., Peyrouset O. Is lung ultrasound superior to CT? The example of a CT occult necrotizing pneumonia. Intensive Care Med. 2006;32:334–335. doi: 10.1007/s00134-005-0004-6. [DOI] [PubMed] [Google Scholar]
- 65.Lichtenstein D.A., Lasscols N., Meziere G. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004;30:276–281. doi: 10.1007/s00134-003-2075-6. [DOI] [PubMed] [Google Scholar]
- 66.Lichtenstein D.A., Goldestein I., Mourgeon E. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Lichtenstein D.A. Pont-of-care ultrasound: infection control in the intensive care unit. Crit Care Med. 2007;35:S262–S267. doi: 10.1097/01.CCM.0000260675.45549.12. [DOI] [PubMed] [Google Scholar]
- 68.Lichtenstein D.A. Ultrasound in the management of thoracic disease. Crit Care Med. 2007;35:S250–S261. doi: 10.1097/01.CCM.0000260674.60761.85. [DOI] [PubMed] [Google Scholar]


