Abstract
Aim
To assess the diagnostic gain of transrectal real-time elastography (RTE) compared to transrectal B-mode ultrasonography (US) in the detection of tumors in patients suspected of having prostate cancer.
Materials and methods
Eighty-four patients suspected of having prostate cancer on the basis of clinical and biochemical evaluation underwent transrectal US, RTE and transperineal prostate biopsy.
Results
Biopsy was considered the gold standard. Analysis related to the total number of patients showed a B-mode US sensitivity of 56%, specificity 80%, positive predictive value (PPV) 70% and negative predictive value (NPV) 67%. Analysis related to the total number of biopsy cores showed sensitivity 33%, specificity 92%, PPV 69% and NPV 73%. In the patient-related analysis, RTE sensitivity was 51%, specificity 75%, PPV 64% and NPV 64%, while the core-related analysis showed sensitivity 36%, specificity 93%, PPV 72% and NPV 74%. Comparison of B-mode US and RTE diagnostic accuracy in the detection of tumors located in the peripheral zone of the prostate gland showed a significant difference. Analysis related to the total number of biopsy cores harvested in the peripheral zone of the prostate gland showed a B-mode US sensitivity of 48%, specificity 81%, PPV 75% and NPV 58%, whereas RTE achieved the following values: sensitivity 66%, specificity 78%, PPV 77%, and NPV 67%.
Conclusions
RTE is a valid addition to B-mode US, and RTE reached a higher accuracy than B-mode US in the evaluation of the peripheral zone of the prostate gland and in the selection of appropriate biopsy sites.
Keywords: Prostate cancer, Ultrasonography, Real-time elastography
Sommario
Scopo
Verificare il guadagno diagnostico dell'elastosonografia transrettale real-time (RTE) rispetto alla ecografia transrettale B-mode nel rilievo del tumore prostatico in una popolazione di pazienti con sospetto di neoplasia.
Materiali e metodi
Ottantaquattro pazienti con sospetto clinico-laboratoristico di tumore prostatico sono stati valutati mediante ecografia transrettale, elastosonografia e biopsia transperineale.
Risultati
L'esame bioptico è stato considerato lo standard di riferimento. Nella valutazione per paziente, la sensibilità dell'ecografia B-mode è stata del 56%; la specificità dell'80%; il valore predittivo positivo (VPP) del 70%, il valore predittivo negativo (VPN) del 67%. Nella valutazione sul totale dei prelievi bioptici la sensibilità è stata del 33%, la specificità del 92%, il VPP del 69%, il VPN del 73%. La RTE ha ottenuto i seguenti risultati nella valutazione per paziente: sensibilità 51%, specificità 75%, VPP 64% e VPN 64%. Nella valutazione sul totale dei prelievi bioptici: sensibilità 36%, specificità 93%, VPP 72%, VPN 74%. Confrontando i valori di accuratezza dell'ecografia B-mode e della RTE per i tumori della zona periferica, è stata trovata una differenza significativa. Nella valutazione sul totale dei prelievi bioptici della zona periferica la sensibilità dell'ecografia B-mode è stata del 48%, la specificità dell'81%, il VPP del 75%, il VPN del 58%. La RTE ha ottenuto i seguenti valori: sensibilità 66%, specificità 78%, VPP 77%, VPN 67%.
Conclusioni
La RTE rappresenta un valore aggiunto all'ecografia B-mode. Ha presentato un'accuratezza superiore rispetto all'esame B-mode nella valutazione della prostata periferica e nella possibilità di indirizzare i prelievi bioptici.
Introduction
Prostate adenocarcinoma is the most frequent malignant tumor affecting adult males in the industrialized world [1]. Prostate-specific antigen (PSA) evaluation and transrectal ultrasound (TRUS) examination have led to a remarkable increase in the early diagnosis of this tumor unlike digital rectal exploration (DRE), which has a low sensitivity [2–4]. Evaluation of PSA level has a reduced specificity, as 20–50% of patients affected by benign prostatic hypertrophy present elevated PSA levels ranging from 4 to10 ng/ml, while 25–45% of patients who have localized prostate cancer present normal PSA levels [5]. Sensitivity of PSA evaluation ranges from 70% to 80% if cut-off value is set at 4 ng/ml [6] but it increases to 93–100% if cut-off value is reduced to 2.5 ng/ml. According to some studies, 65–82% of carcinomas elude diagnosis if cut-off value is 4 ng/ml [7,8]. Since TRUS was introduced in the clinical practice in 1971, it has become the most frequently used imaging technique in the evaluation of prostatic pathologies. Its role in the evaluation of prostate volume and as a biopsy guidance is still unchallenged. However, the accuracy of TRUS in the detection and staging of tumors is more controversial. TRUS can detect hypoechoic lesions which have a high probability of being malignant, particularly in the peripheral zone of the prostate gland, but not all hypoechoic lesions are tumors and not all tumors are hypoechoic [9]. The American Cancer Society (ACS) recommends yearly DRE and PSA evaluations after the age of 50 [10]. Widespread screening means that numerous patients with elevated tumor marker values undergo TRUS examination, and the number of prostatic biopsies is steadily increasing. However, positive biopsy results due to carcinoma are not frequent, particularly in patients with PSA values 4–10 ng/ml, and there is therefore a need to reduce the number of unnecessary biopsies.
According to recent research, magnetic resonance (MR) using an endorectal coil is significantly more accurate in the diagnosis of carcinoma than DRE and TRUS [11,12]. However, the indication for MR examination using an endorectal coil is still controversial, and according to some authors this method cannot be included in the standard protocol for the evaluation of patients who have prostate carcinoma [13]. At present, the main role of MR examination is locoregional staging of the disease (extracapsular involvement, invasion of seminal vesicles) where MR is more accurate than TRUS [14]. Elastography is a US technique [15] which permits in vivo evaluation of the mechanical properties of the soft tissue in terms of elasticity.
The aim of this study was to assess the diagnostic gain of transrectal real-time elastography (RTE) compared to transrectal B-mode ultrasonography (US) in the detection of prostate tumors in patients suspected of having prostate cancer and to identify a repeatable and reproducible elastographic semiological pattern.
Materials and methods
The present study was conducted in two phases. Initially a group of healthy volunteers underwent TRUS and RTE to establish a basic elastographic semiological pattern. Subsequently we studied a group of patients suspected of having prostate cancer on the basis of clinical and biochemical evaluation. Ethical approval was granted by the Medical Research Ethics Committee of our institution, and informed consent was obtained from all patients. According to the current privacy law, all patients were guaranteed privacy-protection and a proper use of personal data.
TRUS and RTE were performed at the same sitting using US equipment Hitachi EUB 8500 Logos (Hitachi Medical Systems, Tokyo, Japan) and a biplane transrectal probe, broadband convex and linear (3.38–11 MHz).
During RTE examination, prostate compression was applied by the probe through the anterior wall of the rectum. In the evaluation of the deformation and thereby the elasticity of the soft tissues, we used dedicated software CAM (Combined Autocorrelation Method) with an algorithm which provided immediate evaluation of the degree of distortion. The elastogram was displayed over the B-mode image in a color scale: red (elastic), green (intermediate) and blue (anelastic) tissue. An indicator showed in real time the correct performance of the examination through a scale of values from 1 to 5. These values indicate if the compression applied by the probe is sufficient to guarantee an exact evaluation of the tissue elasticity. The quality of the examination was evaluated and considered acceptable only if the elastographic image was continuously displayed over the B-mode image with the illuminated indicator showing a value between 4 and 5.
From October 2005 to January 2006, we evaluated 30 male patients (age range 29–37 years) whose clinical history and clinical examination were negative for urological and urinary tract pathologies. These patients were referred to our department for TRUS evaluation in connection with infertility assessment; in all patients the examination was completed with RTE. All patients had a normal prostate volume (about 20 ml); the organ was symmetrical, and the seminal vesicles showed no central or peripheral calcifications which could have altered the elasticity of the prostate.
B-mode examination consisted of sagittal and transversal scans of the prostate with calculation of weight and volume. At RTE, 4 elastograms were obtained on each side, plus one on the midsagittal plane and 3 on the axial plane (one at the base, one intermediate and one at the top). RTE performed on the healthy controls evidenced a higher elasticity, elastic (red) and intermediate (green) in the peripheral zone of the prostate as compared to other areas of the gland. Denonvillier's fascia was generally elastic (red). Analysis of elastographic findings obtained in the healthy controls permitted identification of 3 basic elastographic patterns (Figs. 1–3). In the peripheral zone of the prostate which is more elastic than the central-transition zone, only 2 patterns were identified: type A pattern (Fig. 1): elastic (red) and intermediate (green) tissue quite equally distributed, and type B pattern (Fig. 2): mainly intermediate (green) tissue with small areas of elastic (red) and anelastic (blue) tissue. The areas of different elasticity presented a linear or winding morphology and never appeared distinctly nodular. The elastographic pattern of the central-transition zone of the prostate was more heterogeneous. In addition to the dominant type A and B patterns, we identified a type C pattern with a prevalence of anelastic (blue) tissue mixed with some intermediate and elastic tissue (Fig. 3). In the central-transition zone of the prostate, the distribution of elasticity was more heterogeneous; in most cases the morphology of the anelastic tissue was not winding or linear, but mainly micronodular.
Fig. 1.
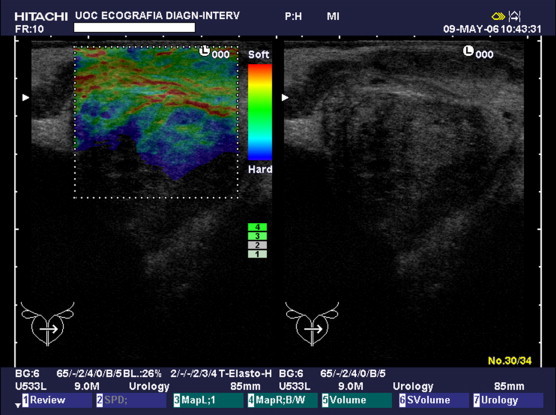
Type A pattern. Presence of elastic (red) and intermediate (green) tissue with a linear, winding morphology.
Fig. 2.
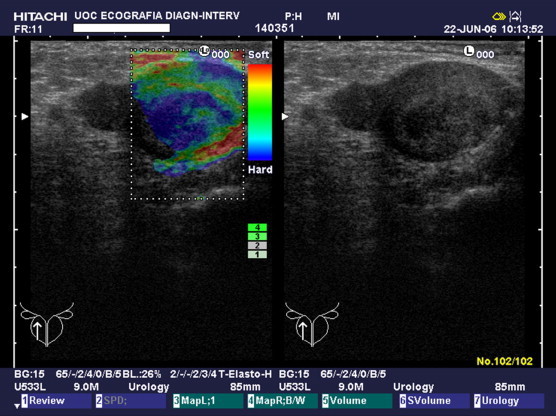
Type B pattern. Mainly intermediate (green) tissue with small areas of elastic (red) and anelastic (blue) tissue.
Fig. 3.
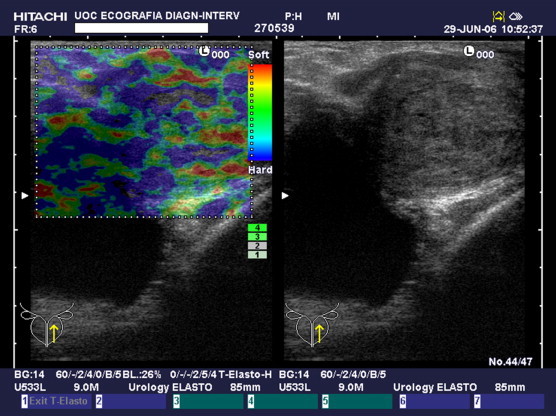
Type C pattern. Prevalence of anelastic (blue) tissue and some intermediate and elastic tissue with a prevalence of anelastic (blue) tissue and some small areas of intermediate and elastic tissue.
In the second phase of the study, from February 2006 to May 2007, we examined 84 consecutive male patients (mean age 61.3 years; age range 49–78) suspected of having prostate cancer on the basis of clinical and biochemical evaluation. Forty-three patients presented increased PSA values (mean value 5.4 ng/ml) but normal DRE outcome; 31 patients presented increased PSA values (mean value 9.4 ng/ml) and suspicious DRE outcome; 10 patients had normal PSA values (<4 ng/ml) but suspicious DRE outcome. None of the patients had a previous clinical history of prostate pathologies. All patients underwent TRUS, RTE and US-guided transperineal prostate biopsy at the same sitting. All procedures were carried out by a radiologist with a longstanding experience in transrectal prostate US and US-guided biopsy.
B-mode US was carried out with the patient in decubitus knee-chest position. The prostate and the seminal vesicles were examined on sagittal and axial scans, and prostate weight and volume were calculated. The patient was also examined for hypoechoic–anechoic areas or masses, local bulging or asymmetric formations visible on both scan planes, particularly in the posterior-peripheral zone of the prostate.
For each patient, 10 standard images were stored on photographic film and on the hard disk of the US scanner pertaining to the sagittal sections of the area in which biopsy was subsequently performed (5 images per side: 3 of the peripheral part of the prostate and 2 of the transition zone) and according to findings suspicious for neoplasm. Each B-mode image was judged negative or positive for suspected prostate cancer. RTE was then carried out with the patient still in decubitus knee-chest position. Elastograms were obtained on the axial and transversal planes; 10 elastographic sagittal standard images were stored analogous to the ones taken at the B-mode US study in the area where biopsy was subsequently carried out. In all patients whose B-mode US examination revealed a suspicious area, an elastogram was obtained also on the longitudinal plane. Each elastographic image was judged negative or positive for suspected prostate cancer. Persisting evidence of an anelastic (blue) area with a clear-cut margin and oval or nodular morphology, on at least 3 consecutive elastograms of elevated quality (the indicator showing 4 or 5) were considered indicative of prostate cancer. Mean time required for the RTE examination was approximately 6 min, never more than 10 min. With the patient still in decubitus knee-chest position, US-guided transperineal biopsy was performed. Local anesthesia was administered under US-guidance using lidocaine (2%) percutaneously injected through the perineal floor reaching the prostatic capsule: 5–6 cc on each side. After positioning an insertion needle (14 G × 140 mm, Biomedical, Florence, Italy), a standard 10 core biopsy was performed harvesting 5 samples per side using an automatic biopsy pistol (guillotine-type Biomedical, Florence, Italy) (Fig. 4). Targeted biopsy was also carried out on the suspicious areas identified at B-mode US and RTE. After biopsy, the patient was administered antibiotic prophylaxis: ciprofloxacine 500 mg per day for 2 days. Outcome of histological examination was considered gold standard in the evaluation of B-mode US and RTE accuracy.
Fig. 4.
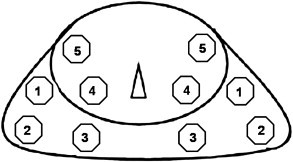
Transperineal biopsy scheme; 10 core biopsy with 5 samples obtained from each side.
Results
None of the patients presented early or late complications originating from the prostate biopsy. In 84 patients, 894 biopsy cores were harvested: 840 were taken according to the random biopsy protocol and 54 were targeted (34 targeted to areas judged suspicious at B-mode US and 20 to areas judged suspicious at RTE). Histological examination revealed the presence of neoplastic tissue in at least one biopsy core in 39/84 patients (46%) (Table 1). The remaining patients had atrophy of the prostate gland, chronic prostatitis or low-grade PIN (Prostatic Intraepithelial Neoplasia); there were no cases of high-grade PIN.
Table 1.
Total number of cores and biopsy outcome.
| Total number of cores | Tumor-positive | |
|---|---|---|
| Random biopsy cores | 840 | 267 |
| Targeted biopsy cores | ||
| B-mode US | 34 | 22 |
| Elastography | 20 | 16 |
| Total cores | 894 | 305 |
| Total biopsies | 894 | 302 |
| Patients | 84 | 39 |
Sensitivity and specificity of B-mode US and RTE were calculated, related to both the total number of patients and to the total number of biopsy cores.
In a total of 39 patients who had neoplastic tissue in at least one sample, 22 were judged as positive (suspected neoplasm) at B-mode US and 17 were judged as negative. Of the 45 patients who did not have prostate cancer, 9 were judged as positive at B-mode US and 36 were judged as negative (Table 2). In the patient-related analysis, sensitivity of B-mode US was 56%, specificity 80%, positive predictive value (PPV) 70%, negative predictive value (NPV) 67% and diagnostic accuracy 69%. In the core-related analysis, sensitivity of B-mode US was 33%, specificity 92%, PPV 69%, NPV 73% and diagnostic accuracy 72%.
Table 2.
Outcome of B-mode US related to the number of patients and to the number of biopsy cores (the latter in parenthesis).
| Positive biopsy | Negative biopsy | Total | |
|---|---|---|---|
| B-mode positive | 22 (101) | 9 (45) | 31 (146) |
| B-mode negative | 17 (201) | 36 (547) | 53 (748) |
| Total | 39 (302) | 45 (592) | 84 (894) |
In a total of 39 patients who had neoplastic tissue in at least one sample, 20 were judged as positive (suspected neoplasm) at RTE (Fig. 5) and 19 were judged as negative. Of the 45 patients who did not have prostate cancer, 11 were judged as positive at RTE and 34 were judged as negative (Table 3). In the patient-related analysis of RTE, sensitivity was 51%, specificity 75%, PPV 64%, NPV 64% and diagnostic accuracy 64%. In the core-related analysis, sensitivity of RTE was 36%, specificity 93%, PPV 72%, NPV 74% and diagnostic accuracy 74%.
Fig. 5.
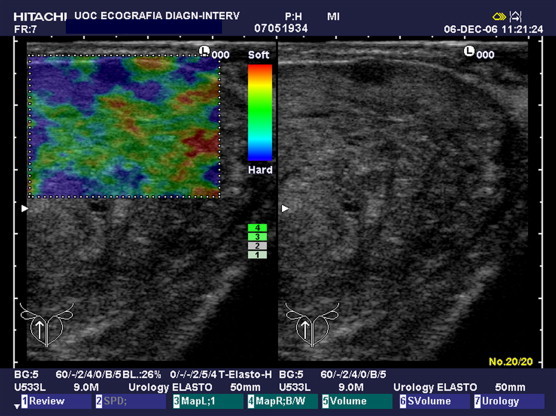
Peripheral tumor detected at B-mode US and at elastography.
Table 3.
Outcome of elastography related to the number of patients and to the number of biopsy cores (the latter in parenthesis).
| Positive biopsy | Negative biopsy | Total | |
|---|---|---|---|
| Elastography positive | 20 (110) | 11 (41) | 31 (151) |
| Elastography negative | 19 (192) | 34 (551) | 53 (743) |
| Total | 39 (302) | 45 (592) | 84 (894) |
In 3 of the 39 patients who had prostate cancer, B-mode US outcome was normal, and a correct diagnosis was made only on the basis of targeted biopsy carried out according to RTE outcome (Fig. 6). In 12 patients, harvesting of additional cores based on suspicious elastographic findings resulted in the detection of a more elevated number of neoplastic lesions. One patient would have eluded diagnosis of prostate cancer on the basis of random biopsy and B-mode US alone, as diagnosis was achieved only by targeted biopsy based on RTE outcome.
Fig. 6.
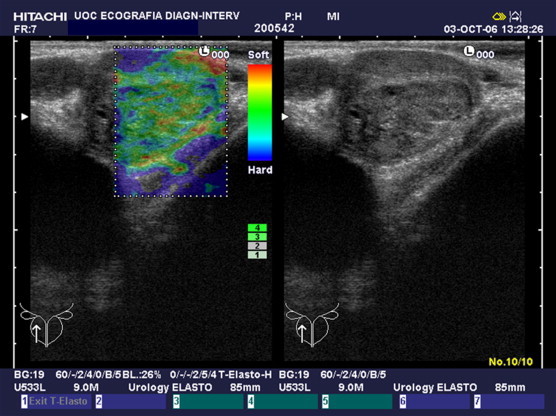
Peripheral tumor detected at elastography; B-mode US appearance is normal.
In 20 (51%) of the 39 patients who had neoplastic tissue in at least one sample, neoplasm was diagnosed in biopsy cores harvested in both the peripheral and the transition zone; 17 (44%) only in samples harvested in the peripheral zone; 2 (5%) only in samples harvested in the central-transition zone. Biopsy cores harvested in the peripheral zone of the prostate were in total 542: 514 random and 28 targeted; of these, 17 were performed on the basis of suspicious findings at B-mode US and 11 on the basis of suspicious findings at RTE. Sensitivity of B-mode US in the study of tumors located in the peripheral zone of the prostate was 48%, specificity 81%, PPV 75%, NPV 58% and diagnostic accuracy 64%. In the study of lesions located only in the posterior zone of the prostate, RTE reached a sensitivity of 66%, specificity 78%, PPV 77%, NPV 67% and diagnostic accuracy 72%. A comparison of B-mode US and RTE exclusively in the detection of tumors located in the peripheral zone (Tables 4 and 5), RTE showed a higher sensitivity and diagnostic accuracy than B-mode US. Analysis of RTE outcomes showed that an elevated number of false-positive and false-negative results occurred in connection with tumors located in the central zone of the prostate.
Table 4.
Outcome of B-mode US related to the number of cores harvested in the posterior-peripheral zone of the prostate.
| Positive biopsy | Negative biopsy | Total | |
|---|---|---|---|
| B-mode positive | 140 | 46 | 186 |
| B-mode negative | 147 | 209 | 356 |
| Total | 287 | 255 | 542 |
Table 5.
Outcome of elastography related to the number of cores harvested in the posterior-peripheral zone of the prostate.
| Positive biopsy | Negative biopsy | Total | |
|---|---|---|---|
| Elastography positive | 192 | 55 | 247 |
| Elastography negative | 95 | 200 | 295 |
| Total | 287 | 255 | 542 |
Discussion
TRUS is the most frequently used imaging technique in the detection of prostate carcinoma. The results obtained in our study in terms of sensitivity and specificity of B-mode US are similar to those reported in the literature.
Specificity of TRUS is quite elevated and a hypoechoic lesion in the peripheral zone of the prostate has a great probability of being a tumor, although not all hypoechoic lesions are tumors, as prostatitis and focal atrophy can appear hypoechoic [9]. Sensitivity of TRUS is less elevated and the main US finding in neoplasm is a hypoechoic area. Other diagnostic signs such as capsular bulging, asymmetry, loss of demarcation between the peripheral zone and the transition zone can be difficult to detect. Furthermore, a number of prostate cancers are totally isoechoic and therefore not detectable at B-mode US examination. Shinohara et al. compared US appearance to final diagnosis and found that 60–75% of tumors are hypoechoic, 25–40% are isoechoic and 1–2% are hyperechoic [16]. These differences in echogenicity are probably due to different cellular compositions, the dimensions of the tumor, association with benign prostatic hypertrophy and fibrotic changes [17]. Because of the low sensitivity and specificity, TRUS cannot be a screening tool in itself although this method is used as guidance for biopsy. However, more imaging techniques have been developed in order to improve the sensitivity and specificity of TRUS. Tumor growth is characterized by neoangiogenesis which can be evaluated using color Doppler (CD) technique [18] which has improved the accuracy of B-mode US in the detection of prostate carcinoma [19–21]. However, CD presents certain limitations as prostate cancer is not particularly vascularized [19]; there are significant similarities between inflammatory and neoplastic changes and both pathologies are characterized by hypervascularization at CD [19]. Some studies suggest that CD improves the detection of glandular areas with a high Gleason score [22] and that blood flow detectable at CD in tumors of the prostate and the adjacent capsule correlates with the grading and staging [23]. Power Doppler (PD) is considered sufficiently sensitive in the detection of slow venous flow in neoplastic lesions of the prostate and more sensitive than CD in the detection of blood flow in the new tumor vessels [24]. Various studies have evaluated the clinical use of PD in the study of prostate cancer [25,26] concluding that PD is useful in the characterization of hypoechoic areas of the posterior-peripheral zone. Sakarya et al. concluded that PD increases the sensitivity of TRUS and helps identifying adequate biopsy sites [27]. Okihara et al. established that PD reaches an elevated NPV (99%) and is useful for selecting patients who should undergo biopsy [28].
However, both prostate carcinoma and prostatitis can show increased vascularization at PD, and a blood flow pattern specific for carcinoma has not been identified [29]. A comparison between PD and CD has shown that PD is more sensitive in the detection of hypervascular lesions, reaching a sensitivity of 98% [30]. CD and PD cannot show microvessels whose density increases in the carcinoma, but US contrast agent administration can improve the detection of small caliber vessels with a slow blood flow [31]. Contrast enhanced Doppler US increases prostate carcinoma detection rate [32]. Targeted biopsy harvesting biopsy cores in the contrast enhanced area permits significantly increased carcinoma detection rates [32–34]. Mitterberger et al. [35] stated that contrast enhanced biopsy can identify carcinomas with higher Gleason scores than systematic biopsy permitting a more exact prognosis and adequate treatment of aggressive carcinomas. Harmonic grey-scale US provides a better temporal and spatial resolution than contrast enhanced Doppler US and a better visualization of prostate carcinoma. Contrast enhanced US increases sensitivity of B-mode US in the detection of neoplastic lesions, as it permits targeted biopsy. However, focal enhancement appears also in areas affected by benign prostate hyperplasia [36].
Prostate carcinoma is characterized by increased cell density and consequently reduced tissue elasticity, and a significant difference in elasticity between normal and neoplastic prostate tissue has been reported [37]. Cochlin et al. [38] examined anatomic specimens using RTE to detect prostate carcinoma reporting a sensitivity of 51% and a specificity of 83% related to the number of patients, and a sensitivity of 31% and a specificity of 82% related to the number of biopsy cores. Tsutsumi et al. [39] stated that RTE identified neoplastic lesions in 84% of patients who had carcinoma, concluding that a combination of RTE and B-mode US provides an increased tumor detection rate. In a study carried out by König et al. [40], RTE associated with B-mode US provided an elevated sensitivity in detecting carcinoma, thus identifying neoplastic lesions in 84.1% of patients who had carcinoma. A recent study [41] reported a RTE sensitivity of 87% and specificity of 92% combined with particularly high detection rates in carcinomas affecting the upper lobe of the prostate (100%) and in the mid-glandular region (94%). The role of RTE in targeted biopsy was investigated by Pallwein et al. [42], who compared RTE guided targeted biopsy and systematic biopsy in 230 patients. Detection rates obtained in targeted and systematic biopsy were not significantly different when related to the number of patients, whereas the detection rate related to the number of cores was significantly higher in targeted biopsy than in systematic biopsy. A combination of the two biopsy techniques can improve carcinoma detection rates, and RTE guided targeted biopsy can reduce the number of core biopsies.
In our study RTE reached a higher diagnostic accuracy than B-mode US. As regards the tumor site, Tsutsumi et al. [39] compared histological outcomes with elastographic images concluding that RTE achieved good detection rates in tumors located in the anterior zone yielding a sensitivity of 94% and PPV of 83% compared to a sensitivity and PPV of 76% and 72%, respectively, in tumors located in the mid-glandular region and of 57% and 70%, respectively, in tumors located in the posterior region. According to the authors, the low sensitivity in carcinomas located in the posterior region could be caused by the fact that the probe easily dislocates posterior lesions. Other authors have observed that RTE is more sensitive than other imaging modalities in the detection of carcinomas located in the anterior zone [43]. Magnetic resonance (MR) is less sensitive in detecting carcinomas located in the anterior zone because of the distance between the tumor site and the coil [11]. In our study, analysis of results related to the tumor site showed that RTE reached a higher sensitivity, PPV and NPV than B-mode US in the detection of peripheral prostate tumors: 66% vs 48%, 77% vs 75% and 67% vs 58%, respectively. This is explained by the fact that the peripheral zone near the rectum is more accessible; in this area RTE detected a significant number of carcinomas which went undetected at B-mode US. In a normal prostate, the peripheral zone remains elastic also in elderly patients, and a carcinoma which is more rigid than the surrounding parenchyma is more easily identified at RTE. The transition zone is a difficult site from an elastographic point of view because of hypertrophy, as a hypertrophic prostate is less elastic and can mimic (or hide) a carcinoma. When the anelastic areas identified at RTE are associated with hypertrophic nodules at B-mode US, elastogram reading is not problematic, whereas it is difficult to interpret a nodular anelastic area in the central and transition zone of the prostate when no hypertrophic nodules are detected at B-mode US. In the central and transition zone of the prostate there are sometimes heavy calcifications caused by chronic inflammation which significantly reduce the elasticity of the area. In our patient population, RTE detected 3 cases of neoplasm which appeared normal at B-mode US, depicting anelastic areas in the posterior-peripheral zone of the prostate. In these three patients, the anelastic nodules were situated in zones which underwent random biopsy so they would in any case have been detected, but RTE permitted a significant improvement of the US examination. In one patient diagnosis was achieved only by targeted core biopsy on the basis of RTE outcome; in this case random biopsy could have yielded a negative result. RTE reached a more elevated NPV than B-mode US: in fact, a peripheral prostate with no anelastic areas is probably not neoplastic.
Gleason score assigned to biopsy specimens indicates the risk of extracapsular extension of the disease thereby guiding the therapeutic management of the patient [44]. As regards correlation between RTE and Gleason score, Tsutsumi et al. [39] observed that RTE reaches a higher detection rate in tumors with low Gleason scores. In contrast, Sumura et al. [43] achieved an elevated detection rate in tumors with high Gleason scores. In our study, the biopsy cores obtained in suspicious areas identified at RTE were assigned a Gleason score higher than or identical to the score assigned to the other cores.
Among the disadvantages of RTE we consider the reduced reliability in the evaluation of the central and transition zones of the prostate and the fact that RTE is static, as only one section can be studied at a time, whereas B-mode US is a dynamic examination which allows a full exploration of the gland by quick and continuous passages from one plane to another. The limitations of RTE are linked to the dimensions of the neoplastic lesions, presence of benign prostatic hyperplasia, reduced elasticity of the tissues in chronic inflammatory pathologies developing stromal hyperplasia and fibrosis, presence of stiffness artifacts in the lateral regions of the elastogram and in deeper lesions.
RTE does not permit identification of small neoplastic lesions, although further development of the method/equipment may soon permit identification of small anelastic areas of about 5 mm [41]. Tilting of the probe can be useful to overcome lateral stiffness artifacts, but elimination of deep stiffness artifacts is difficult considering that RTE permits adequate evaluation of elasticity only to a depth of about 5 cm [41]. Some artifacts which appear in the elastogram can be useful; soft rim artifacts surrounding the prostate are useful for evaluating extracapsular extension [41]. Miyanaga et al. [45] compared RTE to DRE and TRUS and reported that RTE identified neoplastic lesions (areas of less elasticity than the surrounding prostate) in 93% of patients who had carcinoma, compared to 59% identified at DRE and 55% at TRUS. Sumura et al. [43] compared RTE, DRE, TRUS, CD and MR, and RTE reached a higher detection rate than the other imaging modalities.
Conclusions
The role of RTE is to integrate US by providing a “second reading” in cases where B-mode US outcome is suspicious for cancer and to increase the accuracy of random biopsy in cases where US outcome is negative. The results of our study show that RTE achieves a higher accuracy compared to B-mode US in the evaluation of the peripheral zone of the prostate and as guidance for biopsy. This accuracy makes it possible to identify a greater number of tumoral lesions and lesions with high Gleason scores and to make the correct management decisions.
Conflict of interest statement
The authors have no conflict of interest.
References
- 1.Bray F., Sankila R., Ferlay J., Parkin D.M. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38(1):99–166. doi: 10.1016/s0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- 2.Men S., Cakar B., Conkbayir I., Hekimoglu B. Detection of prostatic carcinoma: the role of TRUS, TRUS guided biopsy, digital rectal examination, PSA and PSA density. J Exp Clin Cancer Res. 2001;20(4):473–480. [PubMed] [Google Scholar]
- 3.Scardino P.T. Early detection of prostate cancer. Urol Clin North Am. 1989;16(4):635–655. [PubMed] [Google Scholar]
- 4.Fair W.R. Carcinoma of the prostate: current thoughts on diagnosis and staging. Surg Clin North Am. 1982;62(6):1085–1099. doi: 10.1016/s0039-6109(16)42886-0. [DOI] [PubMed] [Google Scholar]
- 5.Catalona W.J., Smith D.S., Ratliff T.L. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 6.Wilson S.S., Crawford E.D. Screening for prostate cancer: current recommendations. Urol Clin North Am. 2004;31(2):219–226. doi: 10.1016/j.ucl.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Catalona W.J., Richie J.P., Ahmann F.R. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6630 men. J Urol. 1994;151(5):1283–1290. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 8.Punglia R.S., D'Amico A.V., Catalona W.J. Effect of verification bias on screening for prostate cancer by measurement of prostate-specific antigen. N Engl J Med. 2003;349(4):225–242. doi: 10.1056/NEJMoa021659. [DOI] [PubMed] [Google Scholar]
- 9.Engelbrecht M.R., Barentsz J.O., Jager G.J. Prostate cancer staging using imaging. BJU Int. 2000;86(Suppl. 1):123–134. doi: 10.1046/j.1464-410x.2000.00592.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith R.A., Cokkinides V., Eyre H.J. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J Clin. 2007;57(2):90–104. doi: 10.3322/canjclin.57.2.90. [DOI] [PubMed] [Google Scholar]
- 11.Mullerad M., Hricak H., Kuroiwa K. Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J Urol. 2005;174:2158–2163. doi: 10.1097/01.ju.0000181224.95276.82. [DOI] [PubMed] [Google Scholar]
- 12.Sala E., Eberhardt S.C., Akin O. Endorectal MR imaging before salvage prostatectomy: tumor localization and staging. Radiology. 2006;238:176–183. doi: 10.1148/radiol.2381052345. [DOI] [PubMed] [Google Scholar]
- 13.Jager G.J., Severens J.L., Thornbury J.R. Prostate cancer staging: should MR imaging be used? – a decision analytic approach. Radiology. 2000;215(2):445–451. doi: 10.1148/radiology.215.2.r00ap09445. [DOI] [PubMed] [Google Scholar]
- 14.Presti J.C., Hricak H., Narayan P.A. Local staging of prostatic carcinoma: comparison of transrectal sonography and endorectal MR imaging. AJR Am J Roentgenol. 1996;166(1):103–108. doi: 10.2214/ajr.166.1.8571856. [DOI] [PubMed] [Google Scholar]
- 15.Ophir J., Cespedes I., Ponnekanti H., Yazdi Y., Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13(2):111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 16.Shinohara K., Wheeler T.M., Scardino P.T. The appearance of prostate cancer on transrectal ultrasonography: correlation of imaging and pathological examinations. J Urol. 1989;142(1):76–82. doi: 10.1016/s0022-5347(17)38666-4. [DOI] [PubMed] [Google Scholar]
- 17.Rifkin M.D., McGlynn E.T., Choi H. Echogenicity of prostate cancer correlated with histologic grade and stromal fibrosis: endorectal US studies. Radiology. 1989;170(2):549–552. doi: 10.1148/radiology.170.2.2643148. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3(2):65–71. [PubMed] [Google Scholar]
- 19.Rifkin M.D., Sudakoff G.S., Alexander A.A. Prostate: techniques, results, and potential applications of color Doppler US scanning. Radiology. 1993;186(2):509–513. doi: 10.1148/radiology.186.2.7678467. [DOI] [PubMed] [Google Scholar]
- 20.Newman J.S., Bree R.L., Rubin J.M. Prostate cancer: diagnosis with color Doppler sonography with histologic correlation of each biopsy site. Radiology. 1995;195(1):86–90. doi: 10.1148/radiology.195.1.7534429. [DOI] [PubMed] [Google Scholar]
- 21.Patel U., Rickards D. The diagnostic value of colour Doppler flow in the peripheral zone of the prostate with histological correlation. Br J Urol. 1994;74(5):590–595. doi: 10.1111/j.1464-410x.1994.tb09189.x. [DOI] [PubMed] [Google Scholar]
- 22.Cornud F., Belin X., Piron D. Color Doppler-guided prostate biopsies in 591 patients with an elevated serum PSA level: impact on Gleason score for nonpalpable lesions. Urology. 1997;49(5):709–715. doi: 10.1016/S0090-4295(96)00632-2. [DOI] [PubMed] [Google Scholar]
- 23.Ismail M., Gomella L.G., Alexander A.A. Color Doppler sonography of the prostate. Tech Urol. 1997;3(3):140–146. [PubMed] [Google Scholar]
- 24.Rubin J.M., Bude R.O., Carson P.L., Bree R.L., Adler R.S. Power Doppler US. A potentially useful alternative to mean frequency-based color Doppler US. Radiology. 1994;190:853–856. doi: 10.1148/radiology.190.3.8115639. [DOI] [PubMed] [Google Scholar]
- 25.Sauvain J.L., Palascak P., Bremon J.M. Power Doppler ultrasonography and hypoechoic nodules of the peripheral prostate: perspectives and limitations. J Radiol. 1997;78(7):491–497. [PubMed] [Google Scholar]
- 26.Cho J.Y., Kim S.H., Lee S.E. Diffuse prostatic lesions: role of color Doppler and power Doppler ultrasonography. J Ultrasound Med. 1998;17(5):283–287. doi: 10.7863/jum.1998.17.5.283. [DOI] [PubMed] [Google Scholar]
- 27.Sakarya M.E., Arslan H., Unal O., Atilla M.K., Aydin S. The role of power Doppler ultrasonography in the diagnosis of prostate cancer: a preliminary study. Br J Urol. 1998;82(3):386–388. doi: 10.1046/j.1464-410x.1998.00753.x. [DOI] [PubMed] [Google Scholar]
- 28.Okihara K., Kojima M., Nakanouchi T., Okada K., Miki T. Transrectal power Doppler imaging in the detection of prostate cancer. BJU Int. 2000;85(9):1053–1057. doi: 10.1046/j.1464-410x.2000.00663.x. [DOI] [PubMed] [Google Scholar]
- 29.Downey D.B. Power Doppler in prostate cancer. Curr Opin Urol. 1997;7:93–99. [Google Scholar]
- 30.Giesen R.J., Huynen A.L., de la Rosette J.M.C.H. Ultrasonic computer imaging of the prostate; correlation between longitudinal and transverse texture descriptions. Eur J Ultrasound. 1998;2:145–149. [Google Scholar]
- 31.Forsberg F., Merton D.A., Liu J.B., Needleman L., Goldberg B.B. Clinical applications of ultrasound contrast agents. Ultrasonics. 1998;36(1–5):695–701. doi: 10.1016/s0041-624x(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 32.Roy C., Buy X., Lang H., Saussine C., Jacqmin D. Contrast enhanced color Doppler endorectal sonography of prostate: efficiency for detecting peripheral zone tumors and role for biopsy procedure. J Urol. 2003;170(1):69–72. doi: 10.1097/01.ju.0000072342.01573.8d. [DOI] [PubMed] [Google Scholar]
- 33.Frauscher F., Klauser A., Volgger H. Comparison of contrast enhanced color Doppler targeted biopsy with conventional systematic biopsy: impact on prostate cancer detection. J Urol. 2002;167(4):1648–1652. [PubMed] [Google Scholar]
- 34.Pelzer A., Bektic J., Berger A.P. Prostate cancer detection in men with prostate specific antigen 4 to 10 ng/ml using a combined approach of contrast enhanced color Doppler targeted and systematic biopsy. J Urol. 2005;173(6):1926–1929. doi: 10.1097/01.ju.0000158444.56199.03. [DOI] [PubMed] [Google Scholar]
- 35.Mitterberger M., Pinggera G., Horninger W. Comparison of contrast enhanced colour Doppler targeted biopsy to conventional systematic biopsy: impact on Gleason score. J Urol. 2007;178(2):464–468. doi: 10.1016/j.juro.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 36.Halpern E.J., Ramey J.R., Strup S.E. Detection of prostate carcinoma with contrast-enhanced sonography using intermittent harmonic imaging. Cancer. 2005;104(11):2373–2383. doi: 10.1002/cncr.21440. [DOI] [PubMed] [Google Scholar]
- 37.Krouskop T.A., Younes P.S., Srinivasan S., Wheeler T., Ophir J. Differences in the compressive stress–strain response of infiltrating ductal carcinomas with and without lobular feature implications for mammography and elastography. Ultrasound Imaging. 2003;25(3):162–170. doi: 10.1177/016173460302500304. [DOI] [PubMed] [Google Scholar]
- 38.Cochlin D.L., Ganatra R.H., Griffiths D.F. Elastography in the detection of prostatic cancer. Clin Radiol. 2002;57(11):1014–1020. doi: 10.1053/crad.2002.0989. [DOI] [PubMed] [Google Scholar]
- 39.Tsutsumi M., Miyagawa T., Matsumura T. The impact of real-time tissue elasticity imaging (elastography) on the detection of prostate cancer: clinicopathological analysis. Int J Clin Oncol. 2007;12:250–255. doi: 10.1007/s10147-007-0669-7. [DOI] [PubMed] [Google Scholar]
- 40.König K., Scheipers U., Pesavento A., Lorenz A., Ermert H., Senge T. Initial experiences with real-time elastography guided biopsies of the prostate. J Urol. 2005;174(1):115–157. doi: 10.1097/01.ju.0000162043.72294.4a. [DOI] [PubMed] [Google Scholar]
- 41.Pallwein L., Mitterberger M., Struve P. Real-time elastography for detecting prostate cancer: preliminary experience. BJU Int. 2007;100(1):42–46. doi: 10.1111/j.1464-410X.2007.06851.x. [DOI] [PubMed] [Google Scholar]
- 42.Pallwein L., Mitterberger M., Struve P. Comparison of sonoelastography guided biopsy with systematic biopsy: impact on prostate cancer detection. Eur Radiol. 2007;17(9):2278–2285. doi: 10.1007/s00330-007-0606-1. [DOI] [PubMed] [Google Scholar]
- 43.Sumura M., Shigeno K., Hyuga T. Initial evaluation of prostate cancer with real-time elastography based on step-section pathologic analysis after radical prostatectomy: a preliminary study. Int J Urol. 2007;14(9):811–816. doi: 10.1111/j.1442-2042.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 44.Beerlage H.P., de Reijke T.M., de la Rosette J. Considerations regarding prostate biopsies. Eur Urol. 1998;34(4):303–312. doi: 10.1159/000019746. [DOI] [PubMed] [Google Scholar]
- 45.Miyanaga N., Akaza H., Yamakawa M. Tissue elasticity imaging for diagnosis of prostate cancer: a preliminary report. Int J Urol. 2006;13:1514–1518. doi: 10.1111/j.1442-2042.2006.01612.x. [DOI] [PubMed] [Google Scholar]


