Abstract
Purpose
To evaluate the response of breast cancers to neoadjuvant chemotherapy (NAC) with second-generation contrast-enhanced ultrasound (CEUS) and magnetic resonance (MR).
Materials and Methods
We studied 16 women aged 33–74 years (mean, 53 years; median, 38 years) with locally advanced breast carcinoma or large operable breast cancer (>2 cm; T2–T4, N0–N3, M0) that had been detected by mammography, conventional ultrasonography, and biopsy. CEUS (with SonoVue, 5 ml) and MR (with Gd-DTPA; 0.2 mM/kg) were performed under blinded conditions before, during, and after 6–8 cycles of NAC. Lesions were measured and time/signal intensity (T/SI) curves were calculated during both the examinations. The data obtained were analyzed in light of the results of surgical pathology.
Results
Six patients had complete responses manifested by the disappearance of enhancement at both CEUS and MR. Six others had partial responses (reduction of lesion enhancement >50%). In 5/6, T/SI curves obtained with CEUS and MR were both indicative of malignancy (flat curves at CEUS, type I curves at MR); the sixth had a discontinuous curve at CEUS and a type II curve at MR. Four patients had lesional enhancement reductions of <50%. In 3, concordant pictures emerged from the analysis of T/SI curves (discontinuous curves in CEUS, type II and III curves in MR); the fourth had a flat CEUS curve and a type I MR curve. Responses to NAC classified on the basis of MR and CEUS findings showed good correlation with the pathological response.
Conclusions
T/SI curves recorded during CEUS correlate with those obtained during MR and may be a valid index of response to the therapy.
Keywords: Breast ultrasonography, Breast cancer, Contrast media, Contrast-enhanced US, MRI
Sommario
Scopo
Valutare la risposta alla chemioterapia neoadiuvante (NAC) in pazienti con carcinoma della mammella confrontando l'ecografia con mezzo di contrasto di seconda generazione (CEUS) con la risonanza magnetica (RM).
Materiali e metodi
Sedici pazienti (età fra 33 e 74 anni; media di 53 anni; mediana 38 anni) affette da carcinoma mammario localmente avanzato o operabile ma superiore a 2 cm (T2-T4; N0-N3; M0), già diagnosticato con mammografia, ecografia convenzionale e biopsia, sono state monitorate con CEUS (con SonoVue, 5 ml) e RM (con Gd-DTPA, 0,2 mM/kg). Le indagini sono state effettuate in cieco: prima di iniziare il trattamento, dopo 4 cicli di terapia e prima dell'intervento chirurgico. Con entrambe le metodiche sono state calcolate le dimensioni delle lesioni e le curve tempo/intensità di segnale (T/IS). I dati ottenuti sono stati confrontati con l'esame patologico eseguito al termine della terapia sul pezzo operatorio.
Risultati
Sei su 16 pazienti hanno mostrato sia alla CEUS sia alla RM una risposta completa alla NAC con scomparsa di enhancement. In 6 su 16 pazienti si è riscontrata una riduzione parziale dell'enhancement delle lesioni (>50%) con curve T/IS concordanti fra CEUS e RM in cinque casi (in particolare curve piatte alla CEUS e di tipo I alla RM); nel sesto caso la curva è stata di tipo indeterminato sia alla CEUS sia alla RM. Quattro su 16 pazienti hanno presentato una riduzione dell'enhancement delle lesioni <50% con curve T/IS concordanti in tre casi (curve irregolari alla CEUS e di tipo II e III alla RM); nel quarto caso la curva è stata piatta alla CEUS e di tipo I alla RM. Si è osservata una buona corrispondenza fra la risposta al trattamento valutata con CEUS e RM e quella patologica.
Conclusioni
I risultati ottenuti dimostrano che le curve T/IS della CEUS correlano con quelle calcolate con RM e sembrano essere un valido indice di risposta alla terapia.
Introduction
Recent advances in the field of neoadjuvant chemotherapy (NAC) for breast cancer have increased the frequency of tumor regression [1]. The data in the literature shows rates of complete pathological responses that sometimes exceeds 60% [1,2]. This finding is closely related to favorable long-term outcomes, and it is a valuable end point [3].
Survival is similar whether chemotherapy is given before or after surgery, but the neoadjuvant approach reduces the need for mastectomy and provides an estimate of the short-term response to treatment [3,4]. Primary chemotherapy could be used as an in vivo measure of responsiveness to treatment. Therefore, the correct analysis of the presence of residual disease after NAC would allow more effective approaches for treatment and more specific therapeutic strategies.
The objectives of breast imaging are based on the availability of incorporating technology, which achieves both accurate and effective monitoring of tumor response at the same time. Mammography and conventional ultrasonography have limited value in this setting. The information they provide on the tumor response is usually based on changes in size, morphology, and echo patterns [5]. In fact, correlation between clinical, mammographic, conventional ultrasonographic, and histopathological findings is observed in only 50% of all cases [6].
The purpose of our study was to determine whether the pathological responsiveness of breast carcinoma to NAC can be assessed with real-time contrast-enhanced ultrasonography (CEUS) with a second-generation contrast agent (SonoVue™, Bracco, Milan, Italy) and with dynamic contrast-enhanced magnetic resonance (MR) imaging performed after i.v. injection of gadopentetate dimeglumine (Gd-DTPA).
Materials and methods
Ethical approval for this study was granted by the institutional review board, and informed consent was obtained from all patients. From January 2005 through February 2007, 16 women aged 33–74 years (mean, 53 years; median 38 years) received NAC for pathologically confirmed, locally advanced breast carcinoma or large operable breast cancer (>2 cm; T2–T4; N0–N3; M0). The tumors had been previously diagnosed by mammography and conventional ultrasonography. Each patient received 6–8 cycles of NAC (which was largely based on anthracyclines and taxanes). Clinical assessment and imaging studies (mammography, CEUS, and MR) were performed at the time of diagnosis, after 4 cycles of chemotherapy, and before surgery.
All lesions were detected by mammography performed with highly specialized equipment and a standardized technique that involves mediolateral and craniocaudal projections. The exam can be completed with additional projections (lateromedial) and microfocus magnification. Suspicious findings include spiculated lesions, architectural distortion of the glandular parenchyma, asymmetric densities, and microcalcifications.
Ultrasonography
The ultrasound examination was carried out with high-frequency linear probes (10–13 MHz) with Technos Esaote equipment. The breast was initially examined with conventional B-mode ultrasonography to identify the lesion. This was followed by a real-time CEUS examination using a low mechanical index (0.14), a power setting of 36 KPa, and a gain of 160. The contrast agent, SonoVue™ (Bracco, Milan, Italy), was administered through an 18-gauge catheter placed in an antecubital vein. The agent was injected rapidly as a single intravenous bolus (one 4.8 ml vial), and the line was flushed with 5 ml of normal saline (0.9% NaCl). The microcirculation of the lesion was then examined. Images were recorded with the clip function for 3 min using the Contrast Tuned Imaging technique, which detects the signals generated by the microbubbles. It was also possible to make qualitative evaluations based on changes observed in the intensity of intralesional ultrasound signals.
During the post-processing analysis, we noted the numerical values reflecting the mean grey-scale signal intensity recorded in different regions of interest (ROI) within the lesions, and time/signal intensity (T/SI) curves were plotted (similar to those obtained during MR). Contrast-enhancement kinetics were quantitatively analyzed with QONTRAST software (AMID-Bracco, Milan, Italy) capable of generating T/SI curves from temporal sequences of images (Fig. 1). The curves were classified according to their shapes, “flat” curves reflecting benign lesions and “irregular” curves malignancy [7] (Fig. 2).
Fig. 1.
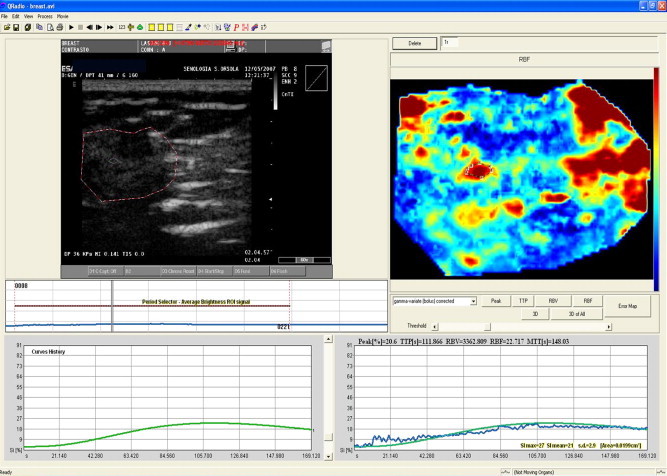
Contrast-enhanced US with kinetic analysis. Qontrast software calculates changes in the signal intensity of pixel (every second), producing color maps of the perfusion of the lesion. T/SI curves are plotted based on values recorded within a selected ROI.
Fig. 2.
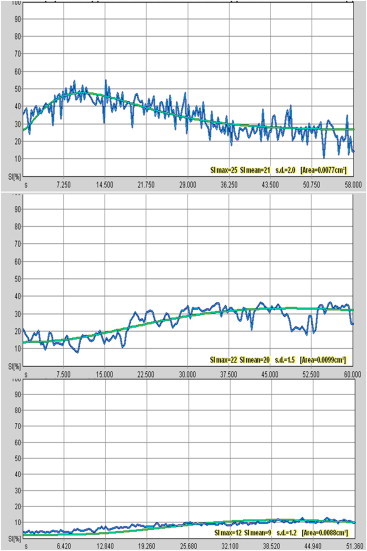
Analysis of CEUS enhancement kinetics. Top: Irregular T/SI curve (early peak of enhancement followed by “wash out”). Middle: Indeterminate T/SI curve (reduction of the slope of enhancement with a lower peak). Bottom: Flat T/SI curves at CEUS, which show a gradual increase in signal intensity.
Magnetic resonance imaging
MR imaging was performed with a 1.5 T Signa tomographer (GE Healthcare) with a bilateral breast coil. A bolus of paramagnetic contrast medium (Gd-DTPA; dose: 0.2 mmol/kg) was injected at a rate of 2 ml/s and the line flushed with 15 ml of normal saline. The MR imaging protocol included axial breath-hold T2-weighted images (TR, 5200.0 ms; TE 40.0 ms), coronal T1-weighted 3D fast spoiled-gradient-echo images (TR automatic, TE: minimum, flip angle 40°, BV 62.50 Kz, a variable field of view, slice thickness 3.6 mm with a ZIP × 2, matrix 416 × 224, NEX 1): 1 pre-contrast and 5 post-contrast acquisitions with a delay time of 30 s.
The post-processing analysis included subtractions and generation of dynamic T/SI curves of enhancement with the Functool Algorithm. Two radiologists who were unaware of the final pathologic results measured the dimensions of the lesions (2 main diameters, according to World Health Organization, WHO criteria) and analyzed the ROI-based T/SI curve (enhancement rates and classification of curves as type I, II, or III, according to Kuhl et al. [8]).
Surgical pathology
All imaging findings were compared with the results of the pathological examination carried out after the last cycle of chemotherapy on the surgical specimen. The histological response to the chemotherapy was evaluated with the Miller and Payne grading system (Tumor Response Grade, TRG [9]). This is a 5-point scale, which is based on reductions in tumor cellularity, as shown below:
-
-
Grade 1: Some alteration in individual malignant cells but no reduction in overall cellularity.
-
-
Grade 2: Minor loss of tumor cells (reduction of ≤30%) but overall cellularity still high.
-
-
Grade 3: 30–90% reduction in tumor cells.
-
-
Grade 4: >90% reduction of tumor cells.
-
-
Grade 5: Complete absence of identifiable cancerous cells in sections from the tumor site (Ductal carcinoma in situ may be present).
To quantify the correlation between CEUS or MR findings and the TRG, Spearman's coefficient (r values) were calculated.
Results
The mean size of the lesions evaluated with CEUS and MR was 3.3 cm (range 2.5–5.0 cm). The kinetic analysis of intralesional contrast enhancement during MR showed T/SI curves with malignant characteristics in all 16 cases: type II in 5 (31.2%) lesions, type III in 11 (68.7%). Kinetic analysis with CEUS confirmed malignant curves (irregular) in all cases.
CEUS and MR enhancement values after the fourth cycle of chemotherapy revealed an initial reduction of the maximum tumor diameter (mean 35% with respect to baseline) with malignant indeterminate T/SI curves in 12 of the 16 patients. In the other 4 patients, there was no reduction in tumor size and no changes in either the CEUS or MR contrast-enhancement kinetics.
In 6 of the 12 patients who had responses after 4 cycles of NAC, analysis of the lesion before surgery showed a complete absence of contrast enhancement. The other 6 had reductions in tumor size of >50% with similar T/SI curves in CEUS and MR (in 5 cases, flat in CEUS and type I in MR; in 1 case malignant undetermined characteristics with both imaging examinations).
In the 4 patients who were non-responders after 4 cycles of NAC, concordant results were obtained with CEUS and MR: reductions in tumor size of <50% associated with malignant T/SI curves (irregular in CEUS, type II–III in MR) in 3 cases and benign curves (flat in CEUS, type I in MR) in the fourth.
The morphological analysis showed two patterns of response to the NAC. Pattern 1 (Shrinkage) was characterized by concentric reduction in the size of the lesion. Nine of the 16 patients had this type of response. Pattern 2 (Fragmentation) was characterized by fibrosis and necrosis with islands of residual tumor (7/16 patients).
Histopathological examination of the tumor after surgical excision revealed 13 cases of invasive ductal carcinoma, 2 cases of invasive ductal-lobular carcinoma, and 1 case of undetermined carcinoma.
In 5 of the 6 patients whose lesions displayed no enhancement at all in either CEUS and MR, the Miller and Payne TRG was 4, and the sixth had a TRG of 3. Six patients with partial imaging responses had a TRG of 3. The 4 non-responders had TRGs of 2 (n = 3) or 1 (n = 1).
Correlation between MR and TGR was 88% (Spearman's r value = 0.88; p value = 0.000) while the correlation between CEUS and TGR was 86% (Spearman's r value = 0.86; p value = 0.000).
Discussion
Neoadjuvant chemotherapy is an excellent experimental approach for the treatment of breast cancer because it provides an estimate of the short-term responsiveness of the tumor to the chosen treatment. Complete pathological responses have been shown to be a predictor of a favorable long-term outcome [4].
Our study presents an imaging evaluation of response to neoadjuvant chemotherapy: functional imaging examinations (CEUS and MR) are correlated with pathological data.
For the detection of treatment-induced changes in the size of breast lesions, CEUS and MR are more sensitive than conventional imaging modalities (mammography and B-mode ultrasonography), which are more susceptible to the confounding effects of breast edema and fibrosis, a common effect of chemotherapy [10].
According to the literature, mammography and B-mode ultrasonography display low accuracy in the measurement of tumor size during NAC, and the values obtained with these modalities correlate with MR findings in only 53% of cases [6,11,12].
In the present series, the average reduction in tumor size observed at the end of NAC with mammography was 44% vs. 73% with B-mode US and 81% with CEUS and MR.
CEUS and MR allow a more accurate assessment (morphological and functional) of the response of breast carcinoma to chemotherapy. Unlike postoperative fibrosis, the fibrosis induced by chemotherapy consists of hyaline tissue, which is not enhanced after the injection of paramagnetic contrast medium [10,12].
The appraisal of tumor response to chemotherapy by comparing CEUS and MR findings obtained after the fourth cycle of NAC displayed statistically significant correlation in both the dimensional and kinetic analyses (Spearman's coefficient = 0.98; p value = 0.000).
Based on tumor sizes documented at the end of NAC, we classified 6 of our patients as responders (tumor volume reductions of close to 100%), 6 as partial responders (reductions of 50–65%), and 4 as non-responders (reductions of <50%) (see Figs. 3–10).
Fig. 3.
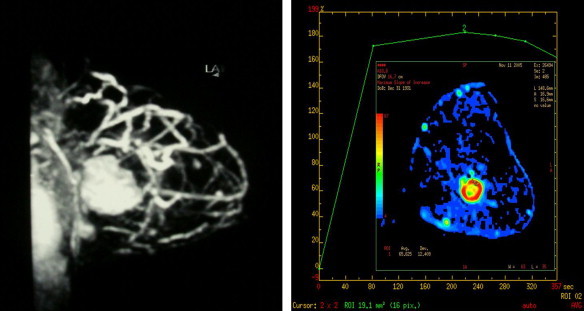
MR findings in responders to NAC. Before chemotherapy, the examination reveals a large breast lesion with a type II T/SI curve.
Fig. 4.
MR findings in the same case shown in Fig. 3 after 4 cycles of NAC (left) and right before surgery (right). Absence of pathological contrast enhancement.
Fig. 5.
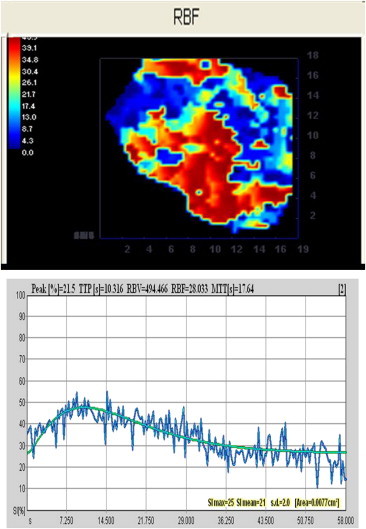
CEUS findings in the same case shown in Fig. 3. Before treatment, the predominance of red in the map reflects high intralesional contrast enhancement (regional blood flow, RBF), and the T/SI curve is irregular with wash out.
Fig. 6.
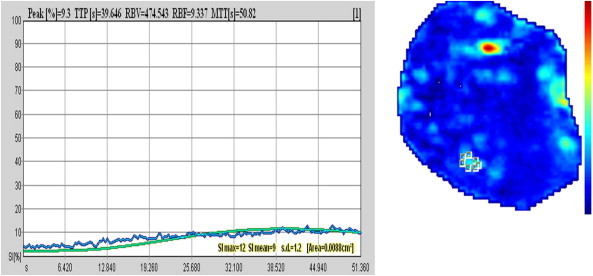
CEUS findings in the case shown in Fig. 5 after 6 cycles of NAC. The pathologic contrast enhancement has disappeared completely.
Fig. 7.
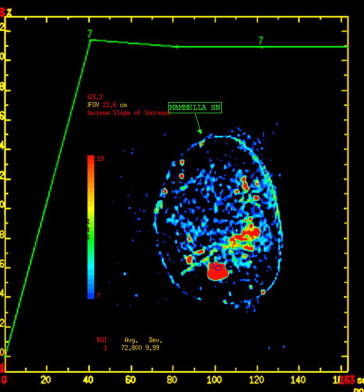
MR findings in a non-responder. The pre-NAC examination yielded a type II curve with plateau.
Fig. 8.
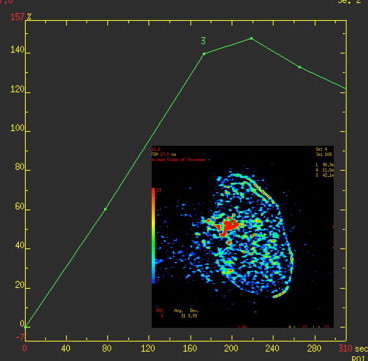
MR findings in the non-responder shown in Fig. 7. Persistence of enhancement after NAC.
Fig. 9.
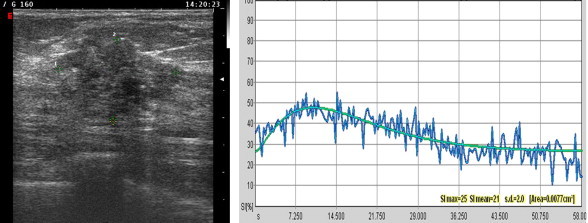
CEUS findings in a non-responder. The pretreatment T/SI curve is irregular.
Fig. 10.
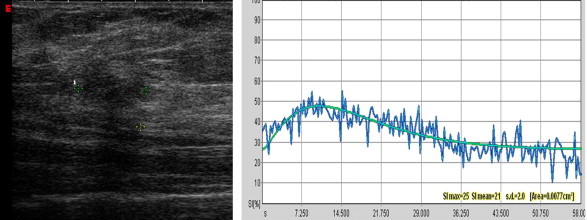
CEUS findings in the non-responder shown in Fig. 9. Persistence of enhancement after treatment with an irregular T/SI curve.
In both the responder and non-responder groups, lesion sizes measured with CEUS at the mid-point and end of NAC were slightly smaller than those measured with MR, especially in cases with the fragmented response pattern. This discrepancy probably reflects the higher spatial, temporal, and contrast resolution of MR (compared with CEUS) and differences between the two modalities in the distribution of contrast medium within the interstitial fluid compartment [13].
In the analysis of enhancement kinetics, MR and CEUS findings displayed good agreement. In fact, in our experience, MR T/SI curves suggestive of malignancy (types II-III) are correlated with “irregular” curves at CEUS. The latter are characterized by an early peak of enhancement (>40%) within 1 min after injection of the contrast medium and a subsequent loss (>10%) of signal intensity or “wash out”. Curves indicative of a benign lesion at MR are associated with “flat” curves at CEUS, which show a gradual increase of signal intensity, especially in the late post-contrast period (Fig. 2).
All of the women (12/16) who showed a response after the fourth cycle of NAC had “indeterminate” curves in CEUS and in MR. This type of curve is characterized by a reduction of the slope of enhancement with a lower peak than that observed at the beginning of the therapy; the peak of enhancement was always >30% (Fig. 2). This curve suggests an initial response to NAC associated with residual disease and posttreatment modifications. Analysis of the T/SI curve may be useful during follow-up for the early evaluation of the response of breast cancer to the NAC.
We compared the dynamic enhancement parameters of CEUS and MR with TGR: the correlation between the TGR showed good correlation with the dynamic enhancement parameters recorded with MR (Spearman's r value = 0.88; p value = 0.000) and with CEUS (Spearman's r value = 0.86; p value = 0.000). Therefore, in our study, both imaging tools provided highly accurate predictions of the pathological response. CEUS and MR are reliable tools for the early evaluation of the response to chemotherapy in women with breast carcinoma.
However, chemotherapy often produces morphological changes in the breast that can lead to false negative or false positive findings. In 1 of the women we studied, the tumor sites was characterized by the complete absence of contrast enhancement on CEUS and on MR (complete response), but pathological analysis of surgical specimens detected the persistence of tumor cells (TRG 3). This patient showed a fragmented pattern of response to the NAC, which is characterized by the presence of areas of fibrosis and necrosis mixed with islands of residual tumor (fragmented pattern), and image-based detection of residual disease is more difficult in these cases [14].
Our results show that CEUS findings correlate well with those of MR, and they seem to be promising tools for evaluating the response of breast cancer lesions to NAC.
However, our study has certain limitations, such as the small number of the patients and the complex reproducibility of the post-processing evaluation, and our findings need to be confirmed in a larger patient population. US and MR contrast agents also differ from one another in terms of their blood–tissue distributions: microbubbles are confined to the vascular compartment while gadolinium diffuses into the interstitial fluid [13]. These differences should be remembered when comparing these techniques although they are more evident during the late post-contrast period (after the first minute).
Conclusion
The characteristics of dynamic lesion enhancement during CEUS correlate with MR findings, and they seem to be a good predictor of the response to neoadjuvant chemotherapy for locally advanced breast carcinoma or large operable breast cancer (>2 cm). Both imaging methods accurately predict the outcome of the surgical pathology examination in terms of the presence or absence of residual disease. These findings suggest that, when MR is not available, CEUS may be a potentially valid alternative for assessing the response to NAC of breast cancers.
Conflict of interest
The authors have no conflict of interest.
Acknowledgements
The authors would like to thank Dr. Maurizio Rossi and Dr. Francesca Magri of Bracco Imaging and Davide Bonfiglioli of Esaote Biomedica.
Footnotes
SIUMB 2007 – Award for the best oral communication presented at the 19th National Congress of the SIUMB.
References
- 1.Kanazawa T., Akashi-Tanaka S., Iwamoto E. Diagnostic of complete response to neoadjuvant chemotherapy using diagnostic imaging in primary breast cancer patients. The Breast Journal. 2005;11(5):311–316. doi: 10.1111/j.1075-122X.2005.00003.x. [DOI] [PubMed] [Google Scholar]
- 2.Budzar A.U., Hunt K., Smith T. Significantly higher pathological complete remission (PCR) rate following neoadjuvant therapy with trastuzumab(H), paclitaxel(P), and anthracycline-containing chemotherapy(CT): initial results of a randomised trial in operable breast cancer(BC) with HER/2 positive disease. Journal of Clinical Oncology. 2004;22(July 15 Suppl.):520. [Google Scholar]
- 3.Charfare H., Limongelli S., Purushotham A.D. Neoadjuvant chemotherapy in breast cancer. British Journal of Surgery. 2005;92:14–23. doi: 10.1002/bjs.4840. [DOI] [PubMed] [Google Scholar]
- 4.Smith I.E., Chua S. ABC of breast diseases Medical treatment of early breast cancer. IV: neoadjuvant treatment. BMJ. 2006;332:223–224. doi: 10.1136/bmj.332.7535.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grandinetti M.L., Cossu E., Simonetti G. Chemioterapia Neoadiuvante. In: Del Maschio A., Pozzi Mucelli R., editors. Mezzi di contrasto in Risonanza Magnetica. 2nd ed. 2003. pp. 199–206. Poletto. [Google Scholar]
- 6.Herrada J., Iyer R.B., Atkinson E.N. Relative value of physical examination, mammography and breast sonography in evaluating the size of the primary tumor and regional lymph node metastases in women receiving neoadjuvant chemotherapy for locally advanced breast carcinoma. Clinical Cancer Research. 1997;3:1565–1569. [PubMed] [Google Scholar]
- 7.Ricci P., Cantisani V., Ballesio L. Benign and malignant breast lesions: efficacy of real time contrast-enhanced ultrasound vs. magnetic resonance imaging. Ultrashall in Medicine. 2006;27:57–62. doi: 10.1055/s-2006-927226. [DOI] [PubMed] [Google Scholar]
- 8.Kuhl C.K., Mielcareck P., Klaschik S. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101–110. doi: 10.1148/radiology.211.1.r99ap38101. [DOI] [PubMed] [Google Scholar]
- 9.Ogston K., Miller I., Payne S. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. The Breast. 2003;12:320–327. doi: 10.1016/s0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 10.Rieber A., Brambs H.J., Gabelmann A. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eurpean Radiology. 2002 July;12(17):1711–1719. doi: 10.1007/s00330-001-1233-x. [DOI] [PubMed] [Google Scholar]
- 11.Trecate G., Vernaghi D. Risonanza Magnetica della mammella. Idelson Gnocchi; 2006. I tumori mammari localmente avanzati; pp. 25–31. [Google Scholar]
- 12.Harms S.E. Staging for breast cancer treatment. In: Stark D.D., Bradley W.J., editors. Magnetic resonance imaging. 3rd ed. Mosby; 1999. pp. 321–333. [Google Scholar]
- 13.Greis C. Technical rewiew of contrast-enhamced ultrasonography. In: Greis C., Jedrzejczyk M., editors. Contrast enhancement ultrasound in general imaging. Springer; 2005. pp. 1–8. [Google Scholar]
- 14.Thibault F., Nos C., Meunier M. MRI of surgical planning in patients with breast cancer who undergo preoperative chemotherapy. AJR. 2004;183:1159–1168. doi: 10.2214/ajr.183.4.1831159. [DOI] [PubMed] [Google Scholar]


