Abstract
Background
Our unit adopted the single administration of cefepime as the initial treatment for febrile episodes in neutropenic patients with hematological malignancies. However, recently, cefepime-resistant gram-negative bacteremia, including those with extended-spectrum β-lactamase (ESBL)-producers, was frequently observed in these patients. Therefore, we instituted a rotation of primary antibiotics for febrile neutropenic patients in an attempt to control antibiotic resistance.
Methods
This prospective trial was performed from August 2008 through March 2011 at our unit. After a pre-intervention period, in which cefepime was used as the initial agent for febrile neutropenia, 4 primary antibiotics, namely, piperacillin-tazobactam, ciprofloxacin, meropenem, and cefepime, were rotated at 1-month intervals over 20 months. Blood and surveillance cultures were conducted for febrile episodes, in order to assess the etiology, the resistance pattern (particularly to cefepime), and the prognosis.
Results
In this trial, 219 patients were registered. A 65.9% reduction in the use of cefepime occurred after the antibiotic rotation. In the surveillance stool cultures, the detection rate of cefepime-resistant gram-negative isolates, of which ESBL-producers were predominant, declined significantly after the intervention (8.5 vs 0.9 episodes per 1000 patient days before and after intervention respectively, P<0.01). Interestingly, ESBL-related bacteremia was not detected after the initiation of the trial (1.7 vs 0.0 episodes per 1000 patient days before and after intervention respectively, P<0.01). Infection-related mortality was comparable between the 2 periods.
Conclusions
We implemented a monthly rotation of primary antibiotics for febrile neutropenic patients. An antibiotic heterogeneity strategy, mainly performed as a cycling regimen, would be useful for controlling antimicrobial resistance among patients treated for febrile neutropenia.
Introduction
Febrile neutropenia is one of the most serious adverse events in patients with hematological malignancies and chemotherapy. A prompt initiation of empirical antibiotic therapy is favorable for patients with febrile neutropenia, regardless of the detection of bacteremia. Based on guidelines [1], [2], our unit had adopted single administration of cefepime, a fourth-generation cephalosporin, as the initial treatment for febrile episodes in neutropenic patients. However, recently, cefepime-resistant gram-negative bacteremia, including those with extended-spectrum β-lactamase (ESBL)-producers, was frequently seen in these patients [3]. ESBL-producing bacteria have been frequently detected not only in our hematology unit, but in all the units at our hospital. Therefore, we considered rotating primary antibiotics for the treatment of febrile neutropenic episodes as a measure to suppress the antibiotic resistance seen in our unit.
Most antibiotic rotation trials, known as cycling therapies, have been conducted in adult intensive-care units (ICUs), where infectious agents are exposed to heavy antimicrobial pressure. Many cycling therapies implemented in clinical settings have been reported to date. Most of these trials were conducted in order to reduce the selective pressure of antibiotic-resistant gram-negative bacteria, which was a major concern for patient prognosis in an ICU setting. The efficacy of antibiotic cycling on the recovery of antimicrobial susceptibility and patient mortality is controversial. Several trials have successfully reported a recovery of susceptibility to specific antibiotics or a reduction in detection rates of multidrug-resistant bacteria [4]–[9]. Moreover, some studies have shown a significant decrease in mortality as a result of the trial [4], [5]. However, other trials did not report recovery of susceptibility to a given antibiotic after antibiotic rotation [10], [11]. Due to the contradictory results of several trials, no conclusion has been reached about the benefits of antibiotic rotation on controlling antimicrobial resistance [12]–[14].
Cycling therapies have been instituted as the primary treatment for febrile neutropenic patients in hematological units [15]–[19]. Most of these attempts were focused on controlling antibiotic resistance among gram-negative bacteria [16]–[19]. From these studies, no conclusive findings have been obtained on the efficacy of antibiotic rotation strategies in patients with febrile neutropenia. Reasons for this include the following: (1) most studies did not clearly show a significant decrease in the frequency of antibiotic-resistant bacteria after intervention, and (2) some studies implemented the antibiotic cycling program in patients receiving fluoroquinolone prophylaxis, which is known to dramatically change the etiology and resistance pattern of infectious agents recovered from patients with febrile neutropenia [20], [21]. Therefore, precise evaluation of the benefits of cycling therapy on suppressing antibiotic resistance in a program of antibiotic prophylaxis is difficult.
For the abovementioned reasons, the efficacy of antibiotic cycling in reducing antibiotic resistance among patients with hematological malignancies, as well as those in ICUs, has not been determined. However, patients with febrile neutropenia, which often develops in those with hematological malignancies, are expected to apply for antibiotic rotation strategy in controlling antibiotic resistance. Febrile neutropenia is a unique infectious disease. Broad-spectrum antibiotics are often administered to febrile neutropenic patients for long periods, without the identification of the causative bacteria, until the neutrophil count recovers. A few classes of antimicrobial agents are recommended as the first line of treatment for febrile neutropenic patients. As a result, overuse of some antibiotics in a small population increases the risk of selecting antibiotic-resistant bacteria. Therefore, if cycling therapy reduces the selection of antibiotic-resistant bacteria, this therapeutic regimen may be beneficial in patients with febrile neutropenia following hematological malignancies.
We prospectively conducted a systematic cycling program for initial use of antibiotics in febrile neutropenic patients in our unit. After a prospective observational period of cefepime administration, 4 antibiotics were rotated monthly over 20 months without quinolone prophylaxis. We observed a significant decrease in the rates of antibiotic resistance. This is the first study to show a significant reduction in antibiotic resistance in a hematology and febrile neutropenia setting after use of antibiotic cycling. Further discussion on the benefits and difficulties of introduction of antibiotic rotation strategies is warranted in hematological settings.
Methods
Patients and Ethics Statement
This study was conducted in a single hematological unit with 37-beds at the Hara-Sanshin Hospital. The study was prospectively conducted from August 2008 to March 2011. The protocol was approved, through the ethics review process, by the Institutional Review Board of the Hara-Sanshin Hospital. Written informed consent was obtained from all registered patients before the study protocol was implemented, in order to publish these case details. Infection control measures, including hand-washing promotion and isolation procedures, were maintained at the same intensity before and after the initiation of the study.
Enrollment
The study protocol was applied for patients with febrile neutropenia who had provided informed consent. The febrile neutropenia defined in this study needs to fulfill both of fever and neutropenia as follows: (1) fever was defined as a single axillary temperature >38.0°C or a temperature of >37.5°C sustained over a 1-h period, and (2) neutropenia was defined as an absolute neutrophil count (ANC) of <1,000 cells/mm3 or an ANC that was expected to decrease to <1,000 cells/mm3 during the following 48 h. Exclusion criteria included treatment for allogeneic hematopoietic stem cell transplantation (HSCT), evidence of hepatic and/or renal dysfunctions (defined as a serum transaminase level of more than 3 times the upper limit of the normal range or as a serum creatinine level of more than 1.5 times the upper limit of the normal range), and a history of hypersensitivity to β-lactam antimicrobial agents. Patients receiving an allogeneic HSCT were excluded from the trial, because a confirmative diagnosis of febrile neutropenia is often difficult to make due to the presence of other causative factors such as graft-versus-host disease and engraftment syndrome. The routine use of fluoroquinolone prophylaxis for neutropenic patients was discontinued in January 2006 at our unit.
Treatment protocol
From August 2008 to July 2009, cefepime was used as the initial agent for treating febrile neutropenia in the registered patients. Next, 4 primary antibiotics, namely, piperacillin-tazobactam, ciprofloxacin, meropenem, and cefepime, were rotated at 1-month intervals over 20 months from August 2009 to March 2011. Ciprofloxacin was adopted as a rotated agent in addition to the 3 other antibiotics recommended for febrile neutropenia [22]. Most cycling studies conducted in ICU settings have used fluoroquinolones as one of the rotated agents because quinolones are highly effective against gram-negative bacteria. In addition, no prophylaxis with quinolones was performed during the study. There is no recommendation for the optimal duration of antibiotic cycling. Although many studies have rotated antibiotics every 3 months, significant evidence on the benefits of cycling therapy has not been obtained from this duration. Some groups have reported that monthly rotation has a positive effect on suppressing antibiotic resistance [7], [8]. Theoretically, a shorter interval of antibiotic cycling is expected to have a lower selection pressure on resistant bacteria [23]. Considering the results to date, monthly cycling was adopted in this study.
The Standard dosage of antibiotics rotated in this study was piperacillin-tazobactam 4.5 g intravenous (i.v.) every 8 h, ciprofloxacin 0.3 g i.v. every 12 h, meropenem 0.5–1 g i.v. every 8 h, and cefepime 2 g i.v. every 12 h. Meropenem (2 g/day) was administered in unequally divided doses. A daily dose of ciprofloxacin, meropenem and cefepime was the maximum dosage approved for them in Japan. The administration of antibiotics was continued until recovery of neutrophil counts and/or resolution of infection. When causative bacteria were isolated from a sample of culture, antimicrobial therapy was adjusted according to the antibiotic resistance pattern obtained. The degrees of consumption of rotated antibiotics were compared before and after the intervention, using the defined daily dose (DDD) per 1000 patient days as an index for the evaluation. The DDD of each antibiotic is defined by the World Health Organization.
Microbiology
When the registered neutropenic patients had fever defined as above, blood and stool samples were collected. Several samples were obtained from the same patient and treated as independent results. If multiple organisms were detected from a single sample, they were counted and analyzed as independent isolates. An automated blood culture system (BACTEC) was used for each test. Stool samples were cultured, chiefly using 5% Sheep Blood Agar medium (BD) and CHROMagar Candida medium (BD). The species were identified using the Vitek system (bioMerieux Japan Ltd., Tokyo, Japan). Antibiotic susceptibilities were determined by the breakpoints standardized by the Clinical and Laboratory Standards Institute (CLSI; formerly the NCCLS) [24]. The screening and confirmation tests for ESBL and metallo-β-lactamase were conducted according to the recommendation of the CLSI [24]. In addition, β-lactamase producers were confirmed using a Cica β test I/MBL kit (Kanto Chemical Co. Ltd., Tokyo, Japan). Clostridium difficile toxin A and B were examined in stool samples, using a TOX A/B QUIK CHEK kit (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan).
Statistical analysis
In the comparisons between patients receiving pre-intervention and those receiving the antibiotic rotation trial, categorical variables were analyzed using a Fisher's exact test, and continuous variables were compared using the Mann-Whitney U test. P<0.05 was considered to be statistically significant. All statistical calculations were performed using the SAS software (SAS Institute, Inc., Cary, NC, USA).
Results
Characteristics of patients registered for antibiotic rotation therapy
In this study, 219 patients were enrolled. Seventy-one patients were registered with 2350 patient days before the antibiotic rotation. After the initiation of cycling therapy, 148 patients were recorded, accounting for 4592 patient days. Some patients were enrolled multiple times for sequential chemotherapies. The basic characteristics of the registered patients were found to be different before and after the rotation, as shown in Table 1. The multinational association for supportive care in cancer (MASCC) score was comparable between the 2 groups, suggesting that both groups were at a similar risk for serious complications and death [22].
Table 1. Characteristics of patients before and after intervention.
| Variable | Before, n = 71 | After, n = 148 | P |
| Age, mean years±SD (range) | 62.0±10.6 (40–82) | 59.0±17.0 (17–87) | 0.86 |
| Male sex | 58 (81.7) | 95 (64.2) | <0.05 |
| Malignant disease | |||
| Leukemia | 27 (38.0) | 78 (52.7) | <0.05 |
| Lymphoma | 20 (28.2) | 46 (31.1) | 0.75 |
| MDS | 7 (9.9) | 9 (6.1) | 0.40 |
| Multiple myeloma | 17 (23.9) | 7 (4.7) | <0.01 |
| Other | 0 (0.0) | 8 (5.4) | 0.06 |
| Therapy for hematological disorders | |||
| Chemotherapy | 61 (85.9) | 136 (91.9) | 0.23 |
| Autologous HSCT | 9 (12.7) | 8 (5.4) | 0.10 |
| Therapy for infectious diseases | |||
| Use of G-CSF | 41 (57.7) | 71 (48.0) | 0.20 |
| Use of Antimycotic drugs | 54 (76.1) | 84 (56.8) | <0.01 |
| Intravenous hyperalimentation catheter | 49 (69.0) | 115 (77.7) | 0.19 |
| Neutrophil cell count (/uL), mean | 299.5±326.0 (0–968) | 155.7±258.2(0–1270) | <0.05 |
| number ± SD (range) | |||
| MASCC score ± SD (range) | 21.3±1.8 (16–24) | 21.4±1.4 (17–23) | 0.94 |
P-value shows statistical comparison for each variable between patients receiving pre-intervention and those receiving intervention.
MDS, myelodysplastic syndromes;HSCT, hematopoietic stem cell transplantation.
G-CSF, granulocyte colony-stimulating factor; MASCC, multinational association for supportive care in cancer.
Effects of antibiotic rotation on the etiology of bacteremic and colonized isolates
Before the initiation of the cycling therapy, 23 bacterial strains were isolated from the blood cultures of the patients, with a total of 71 episodes of febrile neutropenia (Table 2). During cycling therapy, 33 bacteria were recovered from blood culture isolates associated with 148 episodes of febrile neutropenia (Table 2). After the antibiotic rotation trial, the incidence of gram-negative isolates decreased, but this decrease was not statistically significant. Gram-positive organisms were detected comparatively in both the periods. Methicillin-resistant Staphylococcus aureus (MRSA) was first detected after the intervention. The incidence of Enterococcus species was similar in both periods. Vancomycin-resistant enterococci (VRE) were not isolated in either of the 2 periods.
Table 2. Effect of antibiotic rotation on the etiology of bacteremic isolates.
| Before cycling | After cycling | ||||
| Organism | No. of isolates | Rate | No. of isolates | Rate | P |
| Gram-negative | |||||
| Escherichia coli | 6 | 2.6 | 7 | 1.5 | 0.38 |
| Pseudomonas aeruginosa | 4 | 1.7 | 3 | 0.7 | 0.24 |
| Klebsiella pneumoniae | 3 | 1.3 | 3 | 0.7 | 0.41 |
| Other | 0 | 0.0 | 1 | 0.2 | 1.00 |
| Gram-negative, total | 13 | 5.5 | 14 | 3.1 | 0.15 |
| Gram-positive | |||||
| Staphylococcus species, total | 7 | 3.0 | 14 | 3.1 | 1.00 |
| Coagulase negative staphylococci | 6 | 2.6 | 11 | 2.4 | 1.00 |
| Staphylococcus aureus | 1 | 0.4 | 1 | 0.2 | 1.00 |
| Enterococcus species, total | 2 | 0.9 | 2 | 0.4 | 0.61 |
| Enterococcus faecium | 2 | 0.9 | 1 | 0.2 | 0.27 |
| Enterococcus faecalis | 0 | 0.0 | 1 | 0.2 | 1.00 |
| Streptococcus species, total | 1 | 0.4 | 3 | 0.7 | 1.00 |
| Viridans Group streptococci | 1 | 0.4 | 3 | 0.7 | 1.00 |
| Gram-positive, total | 10 | 4.3 | 19 | 4.1 | 1.00 |
| Other | 0 | 0.0 | 0 | 0.0 | 1.00 |
| Isolates, total | 23 | 9.8 | 33 | 7.2 | 0.26 |
Rate indicates number of positive cultures per 1000 patient days.
P-value shows statistical comparison for each variable between patients receiving pre-intervention and those receiving intervention.
Acquisition of VRE, as a causative and/or colonized organism, has been reported after antibiotic rotation in both ICUs and hematological units [17], [18], [25]. This implies that antibiotic cycling for preventing multidrug-resistant gram-negative infections affects the entire bacterial flora of patients receiving the therapy. Therefore, stool cultures were performed not only to examine the colonization of multidrug-resistant gram-negative bacteria, but also to observe changes in the enteric bacterial flora of the patients after antibiotic rotation. As shown in Table 3, the detection rate of Escherichia coli isolates significantly decreased after antibiotic rotation. The incidence of gram-positive isolates was similar in both periods. MRSA was recovered from enteric cultures as well as blood cultures after antibiotic rotation. The incidence of a whole of Enterococcus species was comparable in both periods; however, the number of Enterococcus faecium isolated significantly increased after the antibiotic rotation. The colonization of VRE was not seen in either of the 2 periods. Candida species were detected more frequently after the cycling therapy.
Table 3. Effect of antibiotic rotation on the etiology of stool isolates.
| Before cycling | After cycling | ||||
| Organism | No. of isolates | Rate | No. of isolates | Rate | P |
| Gram-negative | |||||
| Escherichia coli | 45 | 19.2 | 53 | 11.5 | <0.05 |
| Klebsiella pneumoniae | 19 | 8.1 | 52 | 11.3 | 0.26 |
| Pseudomonas aeruginosa | 5 | 2.1 | 4 | 0.9 | 0.18 |
| Other | 20 | 8.5 | 48 | 10.5 | 0.52 |
| Gram-negative, total | 89 | 37.9 | 157 | 34.2 | 0.34 |
| Gram-positive | |||||
| Staphylococcus species, total | 46 | 19.6 | 76 | 16.6 | 0.39 |
| Coagulase negative staphylococci | 46 | 19.6 | 74 | 16.1 | 0.33 |
| Staphylococcus aureus | 0 | 0.0 | 2 | 0.4 | 0.55 |
| Enterococcus species, total | 66 | 28.1 | 129 | 28.1 | 1.00 |
| Enterococcus faecium | 7 | 3.0 | 33 | 7.2 | <0.05 |
| Enterococcus faecalis | 29 | 12.3 | 55 | 12.0 | 0.91 |
| Streptococcus species, total | 17 | 7.2 | 34 | 7.4 | 1.00 |
| Viridans Group streptococci | 17 | 7.2 | 26 | 5.7 | 0.42 |
| Other | 7 | 3.0 | 26 | 5.7 | 0.14 |
| Gram-positive, total | 136 | 57.9 | 265 | 57.7 | 1.00 |
| Other | 2 | 0.9 | 16 | 3.5 | <0.05 |
| Isolates, total | 227 | 96.6 | 438 | 95.4 | 0.86 |
Rate indicates number of positive cultures per 1000 patient days.
P-value shows statistical comparison for each variable between patients receiving pre-intervention and those receiving intervention.
Antimicrobial susceptibilities of gram-negative isolates from patients with bacteremia and colonization
As the purpose of this study was to examine the susceptibility pattern of multidrug-resistant gram-negative organisms after the intervention, we analyzed the antibiotic sensitivity of gram-negative isolates, rather than gram-positive isolates. The incidence of bacteremic gram-negative isolates resistant to the cycled antibiotics was compared between the 2 periods (Table 4). In the period before the cycling therapy, 6 of the 13 gram-negative isolates were resistant to cefepime. Approximately 70% of the cefepime-resistant isolates were found to be ESBL-producing strains. For the duration of the cycling therapy, 1 of 14 gram-negative isolates was resistant to cefepime and this isolate was identified as P. aeruginosa (Table 4). ESBL-producing organisms were not detected during the cycling therapy. The detection rates of cefepime-resistant and ESBL-producing isolates after the antibiotic rotation were significantly lower than those before the rotation.
Table 4. Antibiotic resistance of bacteremic isolates before and after intervention.
| CFPM-resistant | |||||||
| Total No. | Rate (n) of isolates | Rate (n) of isolates | Rate (n) of isolates | Rate (n) of isolates | Rate (n) of isolates | ||
| Organism | of isolates | resistant to CFPM | producing ESBL | resistant to MEPM | resistant to PIPC/TAZ | resistant to CPFX | |
| Gram-negative, total | Before | 13 | 2.6 (6)a | 1.7 (4)b | 0.0 (0) | 0.0 (0) | 2.1 (5)c |
| After | 14 | 0.2 (1)a | 0.0 (0)b | 0.0 (0) | 0.0 (0) | 0.4 (2)c | |
Rate indicates number of positive cultures per 1000 patient days.
P-value shows statistical comparison for each variable between patients receiving pre-intervention and those receiving intervention.
p = 0.007,
p = 0.013,
p = 0.048.
CFPM, cefepime; ESBL, extended-spectrum β-lactamase; MEPM, meropenem; PIPC/TAZ, piperacillin-tazobactam; CPFX, ciprofloxacin.
The frequencies of gram-negative isolates resistant to the cycled antibiotics recovered from stool cultures both before and after antibiotic rotation are shown in Table 5. For the period before antibiotic rotation, 20 of 89 gram-negative isolates, including 15 of 45 E. coli isolates and 3 of 19 K. pneumoniae, were resistant to cefepime and most of them were ESBL-producers. Other gram-negative isolates resistant to cefepime were Citrobacter koseri, which produce ESBL. For the period of antibiotic rotation, 4 of the 157 gram-negative isolates were ESBL-producing E. coli isolates that were cefepime-resistant. No resistance to cefepime was detected from organisms other than E. coli. The rates of cefepime-resistant and ESBL-producing gram-negative isolates, including E. coli and K. pneumoniae strains, obtained after antibiotic cycling were significantly lower than those obtained before the cycling.
Table 5. Antibiotic resistance of stool isolates before and after intervention.
| CFPM-resistant | |||||||
| Total No. | Rate (n) of isolates | Rate (n) of isolates | Rate (n) of isolates | Rate (n) of isolates | Rate (n) of isolates | ||
| Organism | of isolates | resistant to CFPM | producing ESBL | resistant to MEPM | resistant to PIPC/TAZ | resistant to CPFX | |
| Gram-negative, total | Before | 89 | 8.5 (20)a | 7.7 (18)b | 0.0 (0) | 0.0 (0) | 14.9 (35)c |
| After | 157 | 0.9 (4)a | 0.9 (4)b | 0.0 (0) | 0.0 (0) | 5.7 (26)c | |
| Escherichia coli | Before | 45 | 6.4 (15)d | 5.5 (13)e | 0.0 (0) | 0.0 (0) | 14.0 (33)f |
| After | 53 | 0.9 (4)d | 0.9 (4)e | 0.0 (0) | 0.0 (0) | 5.0 (23)f | |
| Klebsiella pneumoniae | Before | 19 | 1.3 (3)g | 1.3 (3)h | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| After | 52 | 0.0 (0)g | 0.0 (0)h | 0.0 (0) | 0.0 (0) | 0.7 (3) | |
| Pseudomonas aeruginosa | Before | 5 | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| After | 4 | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | |
| Other | Before | 20 | 0.9 (2) | 0.9 (2) | 0.0 (0) | 0.0 (0) | 0.9 (2) |
| After | 48 | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | |
Rate indicates number of positive cultures per 1000 patient days.
P-value shows statistical comparison for each variable between patients receiving pre-intervention and those receiving intervention.
p<0.0001,
p<0.0001,
p<0.01.
p<0.0001,
p<0.001,
p<0.001,
p<0.05,
p<0.05.
CFPM, cefepime; ESBL, extended-spectrum β-lactamase; MEPM, meropenem; PIPC/TAZ, piperacillin-tazobactam; CPFX, ciprofloxacin.
Antibiotic use
The dosages of the rotated antibiotics used within our unit were calculated by including not only the registered patients but also the other non-registered patients for the duration of this study. As shown in Table 6, the DDD per 1000 patient days of cefepime was 211 and 72 before and after the intervention, respectively. Thus, the unit-wide cefepime use reduced by 65.9% after the antibiotic rotation trial. The reduction rate of cefepime use remained stable throughout the period of the cycling program (Table 6).
Table 6. Unit-wide antibiotic use shown as defined daily dose.
| Before cycling | After cycling | ||||||
| 1st-5th | 1st | 2nd | 3rd | 4th | 5th | ||
| Antibiotic | Aug/08-Jul/09 | Aug/09-Mar/11 | Aug/09-Nov/09 | Dec/09-Mar/10 | Apr/10-Jul/10 | Aug/10-Nov/10 | Dec/10-Mar/11 |
| CFPM | 211 | 72 | 81 | 51 | 60 | 93 | 77 |
| MEPM | 98 | 82 | 89 | 92 | 80 | 72 | 79 |
| PIPC/TAZ | 149 | 543 | 472 | 587 | 406 | 617 | 632 |
| CPFX | 5 | 30 | 27 | 39 | 26 | 19 | 38 |
Each number is shown to defined daily dose per 1000 patient days.
CFPM, cefepime; MEPM, meropenem; PIPC/TAZ, piperacillin-tazobactam; CPFX, ciprofloxacin.
In several cases, an inadequate antibiotic therapy was administered due to antibiotic resistance. Antibiotics were then changed. During the pre-intervention period, in which cefepime was used, 6 isolates of cefepime-resistant gram-negative bacteria, comprising 4 cases of ESBL-producers and 2 cases of P. aeruginosa, were detected from blood culture. After the intervention trial, 1 isolate of ciprofloxacin-resistant E. coli was detected when ciprofloxacin was used as an initial antibiotic. An empirical change to the initial antibiotic therapy was carried out due to persistent fever; this was done without a microbiological isolation. The antibiotic change was applied to 14 cases and 38 cases, before and after the trial, respectively.
Mortality
The antibiotic agents adopted in this study were well tolerated, regardless of the intervention. Although clinical and laboratory abnormalities, including skin rash, diarrhea, and elevation of serum transaminase level, were observed, these occurred at a frequency of less than 5% and were not severe. C. difficile-associated diarrhea was observed in 2 patients receiving cefepime or piperacillin-tazobactam for the duration of the trial. We recorded 2 deaths (2.8%) during the pre-cycling period and 4 (2.7%) during the cycling period (P = 1.0). One patient died during the trial due to possible septic shock caused by E. coli. No infection-related death was seen before cycling therapy.
Discussion
In this trial, cefepime administration, as a result, dramatically declined at a unit-wide level after antibiotic rotation (Table 6). Thus, the antibiotic rotation designed to decrease the antibiotic pressure due to the dominant use of cefepime led to a unit-wide reduction in cefepime use. This means that antimicrobial use for febrile neutropenia has a huge impact on antibiotic intake throughout a hematological unit. Further to the revised guideline [22], piperacillin-tazobactam was newly recommended as a first-line agent for febrile neutropenia, in addition to cephalosporins and carbapenems. The increase in the number of the initial agents recommended for febrile neutropenia, with different antibiotic mechanisms, significantly contributes to decreasing the dominant use of a particular agent. The introduction of piperacillin-tazobactam also facilitated the initiation of this antibiotic rotation trial.
The primary purpose of this study was to investigate whether antibiotic rotation, when used as the first line of treatment for febrile neutropenia, contributes to controlling the spread of antibiotic resistance; a significant reduction in the incidence of cefepime-resistant and ESBL-producing organisms was observed after antibiotic rotation (Table 4 and 5). The dosage of cefepime used throughout our unit decreased to approximately 30% after the initiation of antibiotic rotation (Table 6). The constant use of broad-spectrum cephalosporins is known to be associated, as an independent risk factor, with the emergence of ESBL-producing bacteria [26]. In addition, the restricted use of cephalosporins was reported to reduce the detection rates of ESBL-producing organisms [27]–[30]. Interestingly, a trial of cephalosporin restriction, conducted by Rahal et al. showed a significant decrease in ESBL-producing Klebsiella infection and colonization within 1 year [27]. Although the period of the intervention was relatively short, the antibiotic rotation trial, followed by a decline in cephalosporin consumption, may be related to the successful control of multidrug-resistant bacteria.
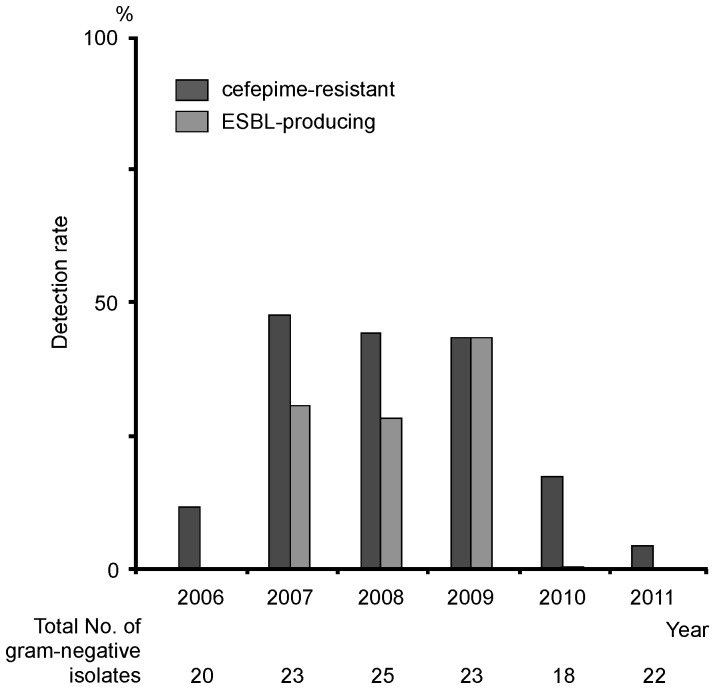
There is a concern regarding the quality of this trial. A control group, to be compared to the enrolled group, was not included in our study. To split a single unit into 2 sections was needed in order to set up a definite control group. In this case, the control group included patients that were under continuous antibiotic pressure induced by the dominant use of cefepime. Finally, we abandoned preparing a control group due to concerns about feasibility. Retrospectively, we tried to analyze the detection rates of cefepime-resistant bacteremic organisms throughout our unit, including in registered and non-registered patients. As shown in Figure 1, the frequency of cefepime-resistant bacteremia, including ESBL-related bacteremia, dramatically decreased after antibiotic rotation. A unit-wide reduction in cefepime use may lead to a unit-wide decrease in the susceptibility of antibiotic resistance. We think that this finding further supports the positive impact of antibiotic rotation in controlling antimicrobial resistance.
Figure 1. Unit-wide frequency of cefepime-resistant and extended-spectrum β-lactamase producing strains in bacteremic isolates obtained from patients with febrile neutropenia.
The detection rates of cefepime-resistant and extended-spectrum β-lactamase (ESBL)-related bacteremia were retrospectively analyzed throughout a hematological unit at the Hara-Sanshin Hospital. The frequency (%) indicates the rate of cefepime-resistant and ESBL-producing isolates obtained from total gram-negative bacteremic isolates. The antibiotic rotation trial was conducted from August 2009 to March 2011.
Antibiotic rotation may affect the etiology of bacteria detected, for not only gram-negative but also gram-positive organisms. In antibiotic cycling trials conducted for febrile neutropenic patients with hematological malignancies, an increase in the incidence of Enterococcus sp., as an agent of infection and colonization, has been observed [16]–[18], and the emergence of VRE has especially been of concern [17], [18]. Craig et al. reported that the detection rates of vancomycin-resistant E. faecium dramatically increased, in the form of bacteremic isolates, after adoption of antibiotic rotation [17]. Antibiotic cycling may be related to the appearance of VRE in ICU settings as well as in hematological units [25]. In this study, the number of Enterococcus isolates detected from blood did not increase after antibiotic rotation; however, a stool culture showed a significant increase in E. faecium after antibiotic rotation (Table 2 and 3). No VRE isolates were detected in this trial. The use of antibiotics with anaerobic activity may be related to the acquisition of VRE [17], [18], [25]. In our trial, piperacillin-tazobactam and meropenem, 2 of the 4 antimicrobials adopted, are active against anaerobic bacteria. The lack of detection of VRE in our unit may be due to the epidemiology of VRE. The environment around our hospital maintains a low prevalence of VRE among the E. faecium isolates found here. Thus, if VRE is epidemic in a certain area, colonization pressure on VRE may be higher after a trial of antibiotic rotation. Further studies will be necessary to confirm this hypothesis.
In this trial, ciprofloxacin was used as the initial agent for the treatment of febrile neutropenia. This selection was based on ICU-oriented regimens, which focus on the control of multidrug-resistant gram-negative bacteria, such as P. aeruginosa. A guideline issued by the IDSA dose not recommend monotherapy with ciprofloxacin as an initial treatment for febrile neutropenia [22]. Intriguingly, E. coli isolates resistant only to fluoroquinolones, including ciprofloxacin and levofloxacin, were frequently detected in this study, even though the routine use of quinolone prophylaxis was avoided in febrile neutropenic patients. The epidemic spread of quinolone-resistant E. coli is characteristic as a local factor. Considering these results, it may be difficult, in our institution to use fluoroquinolones as an initial monotherapy for febrile neutropenia, even if the quinolones have anti-pseudomonal activity.
Whether antibiotic cycling strategies contribute to decreased mortality has not been addressed. However, some studies reported improved prognosis after antibiotic rotation [4], [5]. No studies have shown a decline in mortality in cycling trials for febrile neutropenic patients with hematological malignancies. Our study showed no difference in mortality before and after implementation of antibiotic rotation. This may be partially due to the low mortality of patients undergoing therapy before antibiotic rotation; these patients received cefepime as an initial treatment for febrile neutropenia. ESBL-producing organisms were frequently recovered from blood cultures before antibiotic rotation was performed in our unit. In most cases of patients with ESBL-producing isolates, a prompt antibiotic change to carbapenems resulted in successful treatment and good prognosis [3]. The clinical outcome of bacteremia caused by ESBL-producing organisms remains controversial [26]; however, inappropriate use of empirical antibiotics for ESBL-related bacteremia possibly results in high mortality [31]–[33]. A consistent decline in ESBL-related bacteremia may contribute to a decreased risk for fatality over a longer observation period.
We implemented an antibiotic rotation protocol conducted in our institution, in which 4 antibiotics were rotated at 1-month intervals. Multiple compliance issues with the protocol were appropriately overcome by the presence of properly educated staff, including physicians, nurses, pharmacists, and laboratory technicians. However, due to the limited feasibility of such a protocol, it will be difficult to adopt such a cycling program widely, even if antibiotic resistance could be controlled by this rotation strategy. The most important conclusion of this study is not that this cycling regimen is needed to control antimicrobial resistance, but that the maintenance of “antibiotic heterogeneity” may be effective in reducing the selection pressure on antibiotic resistance. Antibiotic heterogeneity can be achieved by antibiotic mixing, as well as antibiotic cycling [14]. Bergstrom et al theoretically estimated that antibiotic mixing may be more beneficial than antibiotic cycling [23]. In addition, a new strategy of Periodic Antibiotic Monitoring and Supervision (PAMS) has been recently proposed for controlling antimicrobial resistance [34]. If an antibiotic rotation strategy is not feasible in any given situation, agents recommended for the treatment of febrile neutropenia should be constantly monitored and used as evenly as possible to control antibiotic resistance.
Some groups have performed antibiotic rotation trials in febrile neutropenic patients with hematological malignancies [16]–[19]; however, few cycling protocols have been designed with a regimen that rotates recommended agents for febrile neutropenia monotherapy without quinolone prophylaxis. No study had definitely shown a significant reduction in the incidence of particular antibiotic-resistant bacteria among febrile neutropenic patients after antibiotic cycling. Our trial suggests a positive impact of antibiotic rotation on the control of antimicrobial resistance. In this study, the use of carbapenems as an initial treatment for febrile neutropenia did not induce the emergence of either Stenotrophomonas maltophilia or metallo-β-lactamase-producing bacteria. Treatment with all the first line antibiotics recommended for febrile neutropenia was well tolerated in our trial. The strategy of “antibiotic heterogeneity” would be useful for the control of antimicrobial resistance, particularly in the treatment of febrile neutropenia, in which long-term administration of broad-spectrum antibiotics is often needed.
Acknowledgments
We thank the 38 staff members of the hematological unit who collected samples for blood and stool cultures, the laboratory technicians who facilitated the biochemical and microbiological tests, and the pharmacists who collected data regarding consumption of antimicrobial agents.
Funding Statement
The authors have no support or funding to report.
References
- 1. Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, et al. (2002) 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34: 730–751. [DOI] [PubMed] [Google Scholar]
- 2. Link H, Bohme A, Cornely OA, Hoffken K, Kellner O, et al. (2003) Antimicrobial therapy of unexplained fever in neutropenic patients–guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO), Study Group Interventional Therapy of Unexplained Fever, Arbeitsgemeinschaft Supportivmassnahmen in der Onkologie (ASO) of the Deutsche Krebsgesellschaft (DKG-German Cancer Society). Ann Hematol 82 Suppl 2: S105–117. [DOI] [PubMed] [Google Scholar]
- 3. Chong Y, Yakushiji H, Ito Y, Kamimura T (2010) Cefepime-resistant Gram-negative bacteremia in febrile neutropenic patients with hematological malignancies. Int J Infect Dis 14 Suppl 3: e171–175. [DOI] [PubMed] [Google Scholar]
- 4. Kollef MH, Ward S, Sherman G, Prentice D, Schaiff R, et al. (2000) Inadequate treatment of nosocomial infections is associated with certain empiric antibiotic choices. Crit Care Med 28: 3456–3464. [DOI] [PubMed] [Google Scholar]
- 5. Raymond DP, Pelletier SJ, Crabtree TD, Gleason TG, Hamm LL, et al. (2001) Impact of a rotating empiric antibiotic schedule on infectious mortality in an intensive care unit. Crit Care Med 29: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 6. Gruson D, Hilbert G, Vargas F, Valentino R, Bui N, et al. (2003) Strategy of antibiotic rotation: long-term effect on incidence and susceptibilities of Gram-negative bacilli responsible for ventilator-associated pneumonia. Crit Care Med 31: 1908–1914. [DOI] [PubMed] [Google Scholar]
- 7. Barie PS, Hydo LJ, Shou J, Larone DH, Eachempati SR (2005) Influence of antibiotic therapy on mortality of critical surgical illness caused or complicated by infection. Surg Infect (Larchmt) 6: 41–54. [DOI] [PubMed] [Google Scholar]
- 8. Bennett KM, Scarborough JE, Sharpe M, Dodds-Ashley E, Kaye KS, et al. (2007) Implementation of antibiotic rotation protocol improves antibiotic susceptibility profile in a surgical intensive care unit. J Trauma 63: 307–311. [DOI] [PubMed] [Google Scholar]
- 9. Raineri E, Crema L, Dal Zoppo S, Acquarolo A, Pan A, et al. (2010) Rotation of antimicrobial therapy in the intensive care unit: impact on incidence of ventilator-associated pneumonia caused by antibiotic-resistant Gram-negative bacteria. Eur J Clin Microbiol Infect Dis 29: 1015–1024. [DOI] [PubMed] [Google Scholar]
- 10. Warren DK, Hill HA, Merz LR, Kollef MH, Hayden MK, et al. (2004) Cycling empirical antimicrobial agents to prevent emergence of antimicrobial-resistant Gram-negative bacteria among intensive care unit patients. Crit Care Med 32: 2450–2456. [DOI] [PubMed] [Google Scholar]
- 11. Evans HL, Milburn ML, Hughes MG, Smith RL, Chong TW, et al. (2005) Nature of gram-negative rod antibiotic resistance during antibiotic rotation. Surg Infect (Larchmt) 6: 223–231. [DOI] [PubMed] [Google Scholar]
- 12. Brown EM, Nathwani D (2005) Antibiotic cycling or rotation: a systematic review of the evidence of efficacy. J Antimicrob Chemother 55: 6–9. [DOI] [PubMed] [Google Scholar]
- 13. Kollef MH (2006) Is antibiotic cycling the answer to preventing the emergence of bacterial resistance in the intensive care unit? Clin Infect Dis 43 Suppl 2: S82–8. [DOI] [PubMed] [Google Scholar]
- 14. Bal AM, Kumar A, Gould IM (2010) Antibiotic heterogeneity: from concept to practice. Ann N Y Acad Sci 1213: 81–91. [DOI] [PubMed] [Google Scholar]
- 15. Bradley SJ, Wilson AL, Allen MC, Sher HA, Goldstone AH, et al. (1999) The control of hyperendemic glycopeptide-resistant Enterococcus spp. on a haematology unit by changing antibiotic usage. J Antimicrob Chemother 43: 261–266. [DOI] [PubMed] [Google Scholar]
- 16. Dominguez EA, Smith TL, Reed E, Sanders CC, Sanders WE Jr (2000) A pilot study of antibiotic cycling in a hematology-oncology unit. Infect Control Hosp Epidemiol 21: S4–8. [DOI] [PubMed] [Google Scholar]
- 17. Craig M, Cumpston AD, Hobbs GR, Devetten MP, Sarwari AR, et al. (2007) The clinical impact of antibacterial prophylaxis and cycling antibiotics for febrile neutropenia in a hematological malignancy and transplantation unit. Bone Marrow Transplant 39: 477–482. [DOI] [PubMed] [Google Scholar]
- 18. Cadena J, Taboada CA, Burgess DS, Ma JZ, Lewis JS 2nd, et al. (2007) Antibiotic cycling to decrease bacterial antibiotic resistance: a 5-year experience on a bone marrow transplant unit. Bone Marrow Transplant 40: 151–155. [DOI] [PubMed] [Google Scholar]
- 19. Hashino S, Morita L, Kanamori H, Takahata M, Onozawa M, et al. (2011) Clinical impact of cycling the administration of antibiotics for febrile neutropenia in Japanese patients with hematological malignancy. Eur J Clin Microbiol Infect Dis 31: 173–178. [DOI] [PubMed] [Google Scholar]
- 20. Ramphal R (2004) Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis 39 Suppl 1: S25–31. [DOI] [PubMed] [Google Scholar]
- 21. Chong Y, Yakushiji H, Ito Y, Kamimura T (2011) Clinical impact of fluoroquinolone prophylaxis in neutropenic patients with hematological malignancies. Int J Infect Dis 15: e277–781. [DOI] [PubMed] [Google Scholar]
- 22. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, et al. (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis 52: e56–93. [DOI] [PubMed] [Google Scholar]
- 23. Bergstrom CT, Lo M, Lipsitch M (2004) Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc Natl Acad Sci U S A 101: 13285–13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards (2004) Performance Standards for Antimicrobial Susceptibility Testing. 14th Informational Supplement. Wayne, Pennsylvania: NCCLS
- 25. Puzniak LA, Mayfield J, Leet T, Kollef M, Mundy LM (2001) Acquisition of vancomycin-resistant enterococci during scheduled antimicrobial rotation in an intensive care unit. Clin Infect Dis 33: 151–157. [DOI] [PubMed] [Google Scholar]
- 26. Ramphal R, Ambrose PG (2006) Extended-spectrum beta-lactamases and clinical outcomes: current data. Clin Infect Dis 42 Suppl 4: S164–172. [DOI] [PubMed] [Google Scholar]
- 27. Rahal JJ, Urban C, Horn D, Freeman K, Segal-Maurer S, et al. (1998) Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 280: 1233–1237. [DOI] [PubMed] [Google Scholar]
- 28. Lee SO, Lee ES, Park SY, Kim SY, Seo YH, et al. (2004) Reduced use of third-generation cephalosporins decreases the acquisition of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol 25: 832–837. [DOI] [PubMed] [Google Scholar]
- 29. Brahmi N, Blel Y, Kouraichi N, Lahdhiri S, Thabet H, et al. (2006) Impact of ceftazidime restriction on gram-negative bacterial resistance in an intensive care unit. J Infect Chemother 12: 190–194. [DOI] [PubMed] [Google Scholar]
- 30. Kim JY, Sohn JW, Park DW, Yoon YK, Kim YM, et al. (2008) Control of extended-spectrum {beta}-lactamase-producing Klebsiella pneumoniae using a computer-assisted management program to restrict third-generation cephalosporin use. J Antimicrob Chemother 62: 416–421. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez-Bano J, Navarro MD, Romero L, Muniain MA, de Cueto M, et al. (2006) Bacteremia due to extended-spectrum beta -lactamase-producing Escherichia coli in the CTX-M era: a new clinical challenge. Clin Infect Dis 43: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez-Bano J, Picon E, Gijon P, Hernandez JR, Ruiz M, et al. (2010) Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis 50: 40–48. [DOI] [PubMed] [Google Scholar]
- 33. Gudiol C, Calatayud L, Garcia-Vidal C, Lora-Tamayo J, Cisnal M, et al. (2010) Bacteraemia due to extended-spectrum beta-lactamase-producing Escherichia coli (ESBL-EC) in cancer patients: clinical features, risk factors, molecular epidemiology and outcome. J Antimicrob Chemother 65: 333–341. [DOI] [PubMed] [Google Scholar]
- 34. Takesue Y, Nakajima K, Ichiki K, Ishihara M, Wada Y, et al. (2010) Impact of a hospital-wide programme of heterogeneous antibiotic use on the development of antibiotic-resistant Gram-negative bacteria. J Hosp Infect 75: 28–32. [DOI] [PubMed] [Google Scholar]