Abstract
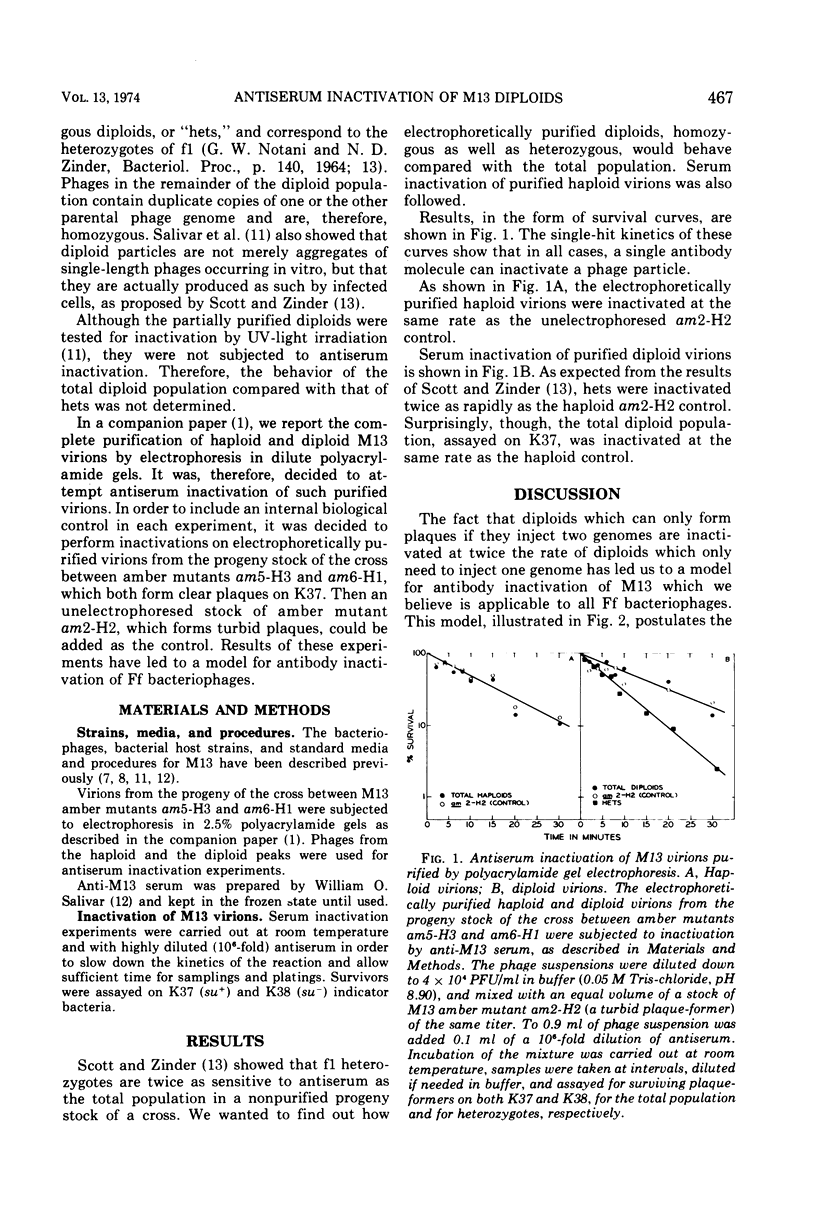
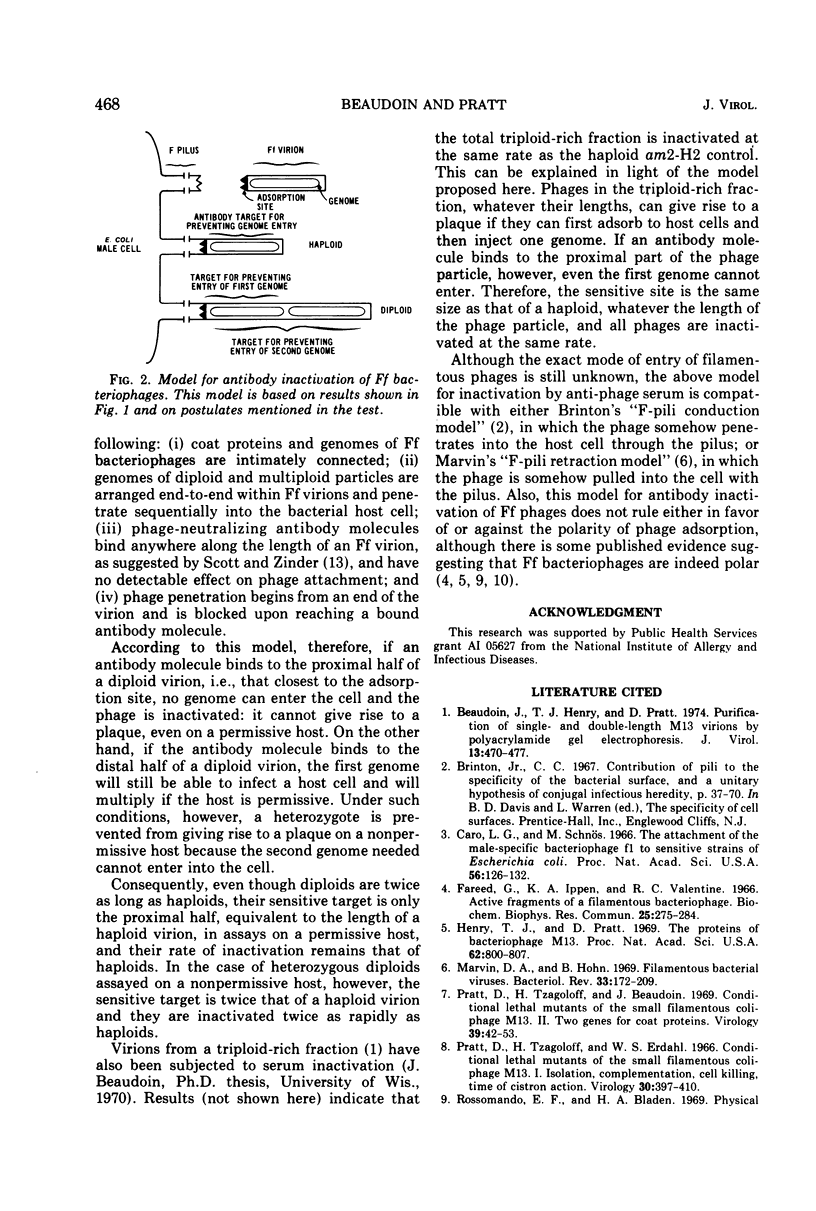
Antiserum inactivation experiments were carried out on electrophoretically purified diploid virions from a cross between two complementing amber mutants of phage M13. The total (homozygous plus heterozygous) diploid population, assayed on a permissive host where only one genome is needed for plaque formation, was inactivated at the same rate as haploids. Heterozygous diploids, assayed on a nonpermissive host, where both genomes are needed for plaque formation, were twice as sensitive as haploids and the total diploid population. These results have led us to propose a model for serum inactivation of the F-specific filamentous phages. According to this model, phage-neutralizing antibodies attach anywhere along the length of the phage and allow the phage to penetrate only up to the first bound antibody molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudoin J., Henry T. J., Pratt D. Purification of single- and double-length M13 virions by polyacrylamide gel electrophoresis. J Virol. 1974 Feb;13(2):470–477. doi: 10.1128/jvi.13.2.470-477.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G., Ippen K. A., Valentine R. C. Active fragments of a filamentous bacteriophage. Biochem Biophys Res Commun. 1966 Nov 11;25(3):275–284. doi: 10.1016/0006-291x(66)90772-8. [DOI] [PubMed] [Google Scholar]
- Henry T. J., Pratt D. The proteins of bacteriophage M13. Proc Natl Acad Sci U S A. 1969 Mar;62(3):800–807. doi: 10.1073/pnas.62.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Beaudoin J. Conditional lethal mutants of the small filamentous coliphage M13. II. Two genes for coat proteins. Virology. 1969 Sep;39(1):42–53. doi: 10.1016/0042-6822(69)90346-8. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Erdahl W. S. Conditional lethal mutants of the small filamentous coliphage M13. I. Isolation, complementation, cell killing, time of cistron action. Virology. 1966 Nov;30(3):397–410. doi: 10.1016/0042-6822(66)90118-8. [DOI] [PubMed] [Google Scholar]
- Rossomando E. F., Bladen H. A. Physical changes associated with heating bacteriophage f1. Virology. 1969 Dec;39(4):921–924. doi: 10.1016/0042-6822(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Rossomando E. F., Zinder N. D. Studies on the bacteriophage fl. I. Alkali-induced disassembly of the phage into DNA and protein. J Mol Biol. 1968 Sep 28;36(3):387–399. doi: 10.1016/0022-2836(68)90163-0. [DOI] [PubMed] [Google Scholar]
- SALIVAR W. O., TZAGOLOFF H., PRATT D. SOME PHYSICAL-CHEMICAL AND BIOLOGICAL PROPERTIES OF THE ROD-SHAPED COLIPHAGE M13. Virology. 1964 Nov;24:359–371. doi: 10.1016/0042-6822(64)90173-4. [DOI] [PubMed] [Google Scholar]
- Salivar W. O., Henry T. J., Pratt D. Purification and properties of diploid particles of coliphage M13. Virology. 1967 May;32(1):41–51. doi: 10.1016/0042-6822(67)90250-4. [DOI] [PubMed] [Google Scholar]



