Abstract
Introduction
To illustrate the lesions detected with transrectal ultrasound (TRUS) in patients with hematospermia.
Material and methods
This study included 74 male patients (25–73 years old) affected by hematospermia. Clinical history was obtained and all patients underwent rectal examination as well as TRUS examination in both axial and coronal planes to evaluate the prostate, ejaculatory ducts and seminal vesicles. Biopsy was performed in 10 patients.
Results
Abnormalities were detected in 59 patients. Calculi (n = 20) were seen within the prostate, seminal vesicles and along the course of the ejaculatory ducts. Chronic prostatitis (n = 14) appeared as hyperechoic and hypoechoic areas within the prostate with capsule thickening suggesting seminal vesiculitis (n = 8). Granulomatous prostatitis (n = 3) appeared as hyperechoic and calcified areas scattered within the prostate and the seminal vesicles. Hypoechoic focal lesions and heterogeneous texture were seen in prostate cancer (n = 5). Utricular cysts (n = 3) appeared as small midline lesions, and Mullerian duct cysts (n = 8) appeared as larger midline cysts protruding above the prostate. Ejaculatory duct cysts (n = 4) appeared as thick walled cystic lesions along the course of the ejaculatory duct. Seminal vesicle cysts were detected in 2 patients.
Conclusion
Our conclusion is that TRUS is a safe, non-invasive technique which can be used to detect lesions of the prostate, seminal vesicles and the ejaculatory ducts in patients with hematospermia.
Keywords: Hematospermia, Ultrasound, Prostatitis
Sommario
Introduzione
Illustrare le lesioni identificate con ecografia prostatica transrettale in pazienti con ematospermia.
Materiali e metodi
Il presente studio include 74 pazienti di sesso maschile (25–73 anni) con ematospermia. Tutti i pazienti successivamente sono stati sottoposti a esame ecografico prostatico transrettale sui piani assiale e coronale della prostata, dei dotti eiaculatori e delle vescichette seminali dopo aver effettuato l'anamnesi e l'esplorazione rettale. La biopsia è stata effettuata in 10 pazienti.
Risultati
In 59 pazienti sono stati identificati reperti patologici. Calcoli (n = 20) sono stati identificati entro il parenchima ghiandolare prostatico, nelle vescichette seminali e lungo il decorso dei dotti eiaculatori. La prostatite cronica (n = 14) si presentava con un'immagine iperecogena con aree ipoecogene del parenchima ghiandolare con ispessimento della capsula che si associava con vesciculite seminale (n = 8). La prostatite granulomatosa (n = 3) appariva iperecogena con alcune regioni calcifiche distribuite nella prostata e nelle vescichette seminali. Nei casi di tumore della prostata sono stati identificate lesioni focali ipoecogene e ecostruttura eterogenea (n = 5). La cisti utriculare (n = 3) era una lesione della linea mediana e le cisti dei Dotti Mulleriani (n = 8) apparivano come una cisti della linea centrale più grande che protrudeva sopra la prostata. Le cisti dei dotti eiaculatori (n = 4) apparivano come lesioni con pareti ispessite lungo il decorso del dotto eiaculatorio e le cisti vescicali sono state identificate in 2 pazienti.
Conclusioni
Concludiamo che l'ecografia transrettale è una tecnica non invasiva che può essere usata per identificare lesioni della prostata, delle vescichette seminali e dei dotti eiaculatori in pazienti con ematospermia.
Introduction
Hematospermia represents 1% of all andrological and urological symptoms. The international nomenclature of human semen parameters defines hematospermia as the presence of fresh or altered blood in the ejaculate that appears brown. There are several reasons why hematospermia may occur. It may be caused by inflammation, neoplastic formations or obstructive cystic lesions along the course of the ejaculatory ducts, and it may also be idiopathic. Differentiation between the different causes is extremely important in order to plan adequate treatment in these patients. Hematospermia is mainly of inflammatory origin in young patients, but in older patients, it is usually due to a benign or malignant prostatic tumor [1–4].
Non-invasive imaging is essential in the diagnostic work-up of men with hematospermia. Different imaging modalities have been used in the diagnosis. CT scan is not helpful due to poor visualization of the distal duct system [4]. MR imaging using an endorectal coil can depict the distal duct system, but it is expensive [5–7]. Transrectal ultrasound (TRUS) is more accurate in the visualization of the distal duct system and the various abnormalities that can provide diagnosis and etiological factors of hematospermia [8–12].
The aim of this work is to illustrate the lesions which were detected using TRUS in patients with hematospermia.
Material and methods
This study included 74 male patients aged from 25–73 years (mean age = 36) who complained of recurrent hematospermia over the past 2–15 months (mean = 8 months). A thorough clinical history was obtained and all patients underwent rectal examination of the prostate and seminal vesicle for swellings, nodules, and tenderness. Laboratory tests were expressed prostatic secretions (EPS) and semen analysis. Semen was tested for peroxidase-positive white blood cells (WBCs), fructose level and for bacterial cultures using suitably prepared semen dilutions. Prostatic specific antigen (PSA) was evaluated in 10 patients. The study was performed according to the ethical principles for medical research contained in the declaration of Helsinki, and informed consent was obtained from all patients.
TRUS examination was performed (AU5, Esaote Biomedica, Italy) using a high frequency (7.5 MHz) bi-plane endorectal probe. All examinations were carried out by a radiologist with 15 years' experience in TRUS examinations. Patients were instructed to self-administer a cleansing enema the night before the examination. Examination was performed with the bladder half-full and the patient in left lateral decubitus position and the hips fully flexed. The prostate, ejaculatory ducts and seminal vesicles were examined in axial and sagittal planes. TRUS guided biopsy was performed in 10 patients who had elevated PSA value. Core biopsy was performed after positioning of an insertion needle (14G-140 mm, Biomedical, Florence, Italy) using an automatic biopsy pistol (guillotine-type Biomedical, Florence, Italy). After biopsy, the patient was administered antibiotic prophylaxis: ciprofloxacin 500 mg per day for 3 days. Final diagnosis was made on the basis of typical sonographic appearance and site of calculi and cystic lesions. Chronic prostatitis was confirmed by pus cells in the semen analysis and EPS. Prostate cancer and tuberculous prostatitis were confirmed by TRUS guided biopsy.
Results
The following causes of hematospermia were revealed: calculi within the prostate, ejaculatory ducts and seminal vesicles (n = 20); chronic prostatitis (n = 14) associated with seminal vesiculitis (n = 8); granulomatous prostatitis (n = 3); prostate cancer (n = 5); cystic lesions located at the midline (n = 11), in paramedian location along the course of the ejaculatory duct (n = 4) or in the seminal vesicle (n = 2). In 15 patients TRUS revealed no abnormality.
Calculi appeared as bright echogenic foci with posterior acoustic shadows within the prostate (n = 13), the ejaculatory ducts (n = 7) and in the seminal vesicle (n = 3). Calculi were either diffuse or segmental (Fig. 1). They were associated with increased wall thickness of the ejaculatory duct in 6 patients.
Fig. 1.
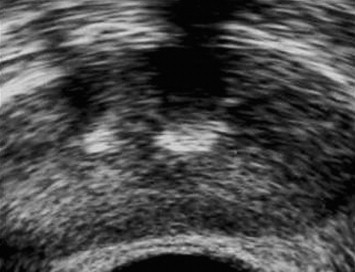
Calculi of the prostate. Axial TRUS image shows two calculi with bright echogenic texture and posterior acoustic shadow in both ejaculatory ducts.
Chronic prostatitis appeared as multiple hyperechoic areas associated with hypoechoic areas scattered within the prostatic parenchyma and around the ejaculatory ducts in 11 patients. It was associated with enlargement of both seminal vesicles and hypo and hyperechoic areas in 8 patients and dilated thick walled ejaculatory duct in 4 patients (Fig. 2). A hypoechoic lesion was seen in the periurethral region in 3 patients (Fig. 3). The prostate was normal size but with irregular, capsular thickening.
Fig. 2.
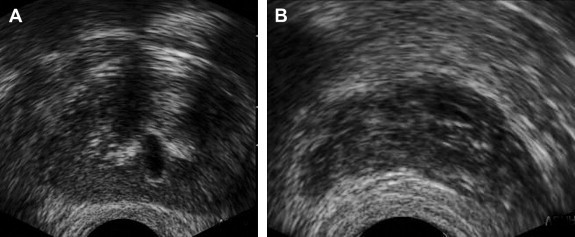
Chronic prostatitis. (A) Axial TRUS image shows multiple discrete hyperechoic areas in the periurethral region associated with dilated and thickened wall of the ejaculatory duct. (B) Axial TRUS image demonstrates mildly enlarged seminal vesicles with a few discrete hyperechoic regions.
Fig. 3.
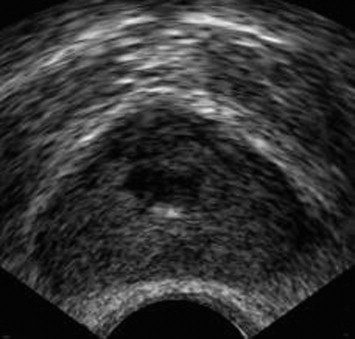
Chronic prostatitis. Axial TRUS image shows a hypoechoic area within the periurethral region.
Granulomatous prostatitis was detected in 3 patients. Tuberculous prostatitis (n = 2) appeared as multiple discrete hyperechoic areas and multiple discrete areas of calcification scattered within the prostate in all affected patients (Fig. 4). Bilharzial prostatitis was associated with enlargement of both seminal vesicles and discrete areas of calcification in one patient.
Fig. 4.
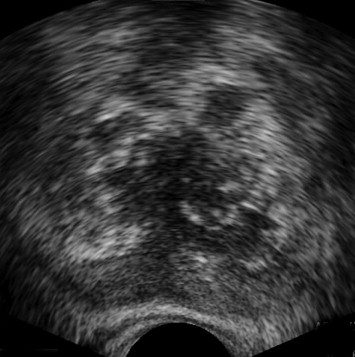
Tuberculous prostatitis. Axial TRUS image shows multiple discrete hyperechoic areas and multiple discrete areas of calcification scattered within the prostate.
Prostate carcinoma appeared as a hypoechoic focal lesion in the peripheral zone of the prostate in 3 patients, while 2 patients presented heterogeneous texture of the prostate (Fig. 5).
Fig. 5.
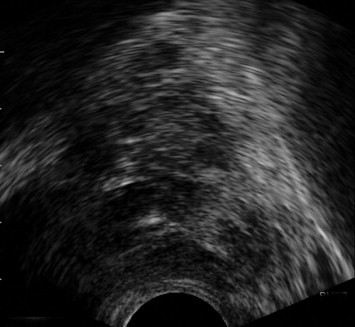
Cancer prostate. Axial TRUS image shows multiple discrete hypoechoic focal lesions scattered within the prostate mainly in the peripheral zone.
Cysts were midline (n = 11) or laterally located (n = 6). Median cysts included utricular and Mullerian duct cysts; paramedian cysts included seminal vesicle cysts and ejaculatory duct cysts. Utricular cysts (n = 3) appeared as small tubular median cystic lesions at the level of the veromontanum (Fig. 6). They were thin walled with no areas of calcification or coarse echoes. Mullerian duct cysts (n = 8) were large midline cystic lesions, cone-shaped with downward apex (n = 6) or rounded (n = 2) (Fig. 7). Ejaculatory duct cysts (n = 4) appeared as thin or thick walled elliptical cysts along the course of the ejaculatory duct (Fig. 8). Seminal vesicle cysts (n = 2) appeared as multiple small sized cysts within the seminal vesicle.
Fig. 6.
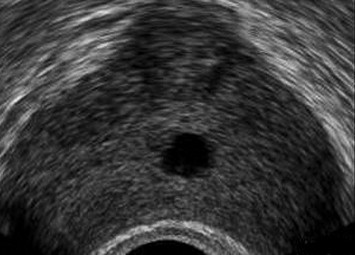
Utricular cyst. Axial TRUS image shows a small sized, tubular, thin walled midline cyst.
Fig. 7.
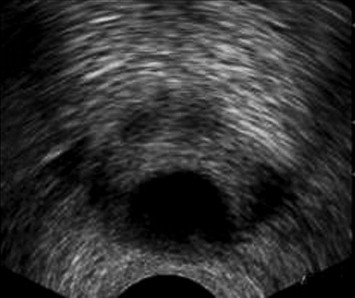
Mullerian duct cyst. Axial TRUS image shows awell-defined, large, thin walled midline cyst. (B) Sagittal midline TRUS image shows that the cyst has a cone-shaped appearance.
Fig. 8.
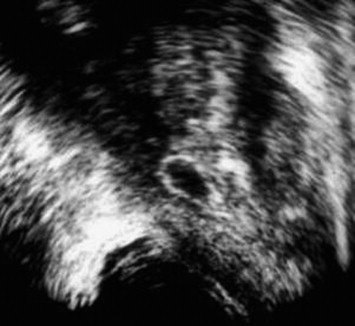
Ejaculatory duct cyst. Axial TRUS image shows a thick walled cyst located along the course of the ejaculatory duct slightly off the midline.
Discussion
In this study, the commonest cause of hematospermia was calculi associated with chronic prostatitis. Hematospermia can occur for many reasons such as infectious or inflammatory disorders, prostate cancer or cystic lesions, or it may be idiopathic. Several studies of hematospermia carried out using TRUS have revealed that the commonest cause of hematospermia is prostate calculi. Other causes include cysts, chronic prostatitis, prostatic hypertrophy and malignant lesions [8–12].
At TRUS, prostatic calculi appear as well circumscribed focal foci of increased echogenicity with or without posterior acoustic shadowing, situated in the prostate gland or seminal tract. More than 50% of prostatic calculi consist partly of urine components thus suggesting reflux phenomena, and seminal vesicle calculi may therefore also be caused in part by a similar reflux phenomenon or may be due to stasis and consequent concretion of complex fluid and debris [4,13].
Patients affected by chronic prostatitis present diffuse, focal or multifocal hypoechoic lesions in the peripheral zone. These hypoechoic lesions are most commonly multifocal patchy areas but may also involve confluent areas of the peripheral zone. A hypoechoic rim along the outer periphery of the prostate has also been described in patients with chronic prostatitis, and histological analysis showed correlation with the degree of stromal fibrosis. Inflammatory infiltration rarely appears as a focal hypoechoic lesion similar to prostate carcinoma; however, capsule deformity is uncommon and there is no capsular interruption. High-density echoes represent corpora amylacea deposition, and hypoechoic areas represent fibrosis and inflammation. Chronic prostatitis may be associated with seminal vesiculitis. The seminal vesicles are enlarged with septal thickening and hyperechoic areas up to 20% of affected patients [14–16].
In mycobacterial infection, US shows multiple dense areas of calcification of the prostate gland, seminal vesicles and urinary bladder wall which may be associated with hypoechoic regions or abscess formation. The infection is a result of upper urinary tract seeding or primary genital tuberculosis [17,18]. Bilharzial infection of the prostate gland and seminal vesicles should be suspected when calcification of the prostate and seminal vesicles is detected at TRUS and associated with multiple echogenic foci in the prostate with occasional dilation of the ejaculatory duct or seminal vesicle due to distal obstruction caused by fibrosis. Up to 58% of men living in endemic areas are affected by Bilharzial infection of the prostate and seminal vesicles [19]. In this study, granulomatous lesions due to tuberculosis and bilharziasis of the prostate and seminal vesicle were associated with hematospermia.
Prostate cancer is the commonest cause of cancer in men. The association between prostatic cancer and hematospermia has been established. Prostate cancer most commonly arises from the peripheral zone of the prostate gland and, occasionally, from the central zone. On TRUS, prostate carcinoma is most often hypoechoic relative to the normal peripheral zone but may sometimes be isoechoic or even hyperechoic. Asymmetry in prostate size, particularly in the peripheral zone, capsular distortion, and loss of differentiation between the central gland and the peripheral zone may also be seen [20,21].
Utricular and Mullerian duct cysts typically occur in the midline of the prostate gland where ejaculatory duct cysts usually occur in paramedian location along the expected course of the ejaculatory duct. Mullerian duct cysts are often teardrop shaped and may extend beyond the posterosuperior margin of the prostate gland, particularly when they are large, whereas utricular cysts tend to be confined within the prostate gland. Mullerian duct cysts do not normally communicate with the urethra or ejaculatory duct, whereas utricular cysts may present this characteristic [22–26].
Ejaculatory duct cysts appear as simple or minimally complex cysts along the course of the ejaculatory duct, which may or may not contain calculi. Seminal vesicle cysts appear as rounded or oval anechoic lesions within the seminal vesicle, often displaying the urinary bladder. Simple cysts are anechoic, thin walled, rounded or oval in shape, whereas complex cysts show increased echogenicity due to proteinaceous or hemorrhagic contents and may furthermore present internal septations, be thick walled and contain calculi [22,27,28].
We concluded that TRUS is a safe, inexpensive, non-invasive, radiation-free imaging technique which can be used to detect lesions of the prostate, seminal vesicles and the ejaculatory ducts in patient with hematospermia.
Conflict of interest statement
The authors have no conflict of interest.
References
- 1.Ahmad I., Krishna N. Hemospermia. J Urol. 2007;177:1613–1618. doi: 10.1016/j.juro.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Kumar P., Kapoor S., Nargund V. Hematospermia – a systematic review. Ann R Coll Surg Engl. 2006;88:339–342. doi: 10.1308/003588406X114749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munkelwitz R., Krasnokutsky S., Lie J., Shah S.M., Bayshtok J., Khan S.A. Current perspectives on hematospermia: a review. J Androl. 1997;18:6–14. [PubMed] [Google Scholar]
- 4.Torigian D.A., Ramchandani P. Hematospermia: imaging findings. Abdom Imaging. 2007;32:29–49. doi: 10.1007/s00261-006-9013-3. [DOI] [PubMed] [Google Scholar]
- 5.Maeda H., Toyooka N., Kinukawa T., Hattori R., Furukawa T. Magnetic resonance images of haematospermia. Urology. 1993;41:499–504. doi: 10.1016/0090-4295(93)90519-g. [DOI] [PubMed] [Google Scholar]
- 6.Cho I.R., Lee M.S., Rha K.H., Hong S.J., Park S.S., Kim M.J. Magnetic resonance imaging in hemospermia. J Urol. 1997;157:258–262. [PubMed] [Google Scholar]
- 7.Lencioni R., Ortori S., Cioni D., Morelli G., Ceretti E., Cosottini M. Endorectal coil MR imaging findings in hemospermia. Magma. 1999;8:91–97. doi: 10.1007/BF02590525. [DOI] [PubMed] [Google Scholar]
- 8.Etherington R.J., Clements R., Griffiths G.J., Peeling W.B. Transrectal ultrasound in the investigation of haemospermia. Clin Radiol. 1990;41:175–177. doi: 10.1016/s0009-9260(05)80962-6. [DOI] [PubMed] [Google Scholar]
- 9.Amano T., Kunimi K., Ohkawa M. Transrectal ultrasonography of the prostate and seminal vesicles with hemospermia. Urol Int. 1994;53:139–142. doi: 10.1159/000282655. [DOI] [PubMed] [Google Scholar]
- 10.Worischeck J.H., Parra R.O. Chronic hematospermia: assessment by transrectal ultrasound. Urology. 1994;43:515–520. doi: 10.1016/0090-4295(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 11.Yagci C., Kupeli S., Tok C., Fitoz S., Baltaci S., Gogus O. Efficacy of transrectal ultrasonography in the evaluation of hematospermia. Clin Imaging. 2004;28:286–290. doi: 10.1016/S0899-7071(03)00157-8. [DOI] [PubMed] [Google Scholar]
- 12.Jianquan Z. Diagnosis and therapeutics of the causative diseases for hemospermia on transrectal ultrasound. Ultrasound Med Biol. 2006;32:S249. [Google Scholar]
- 13.Singh I., Sharma N., Singh N., Gangas R. Hematospermia (ejaculatory duct calculus) – an unusual cause. Int Urol Nephrol. 2003;35:517–518. doi: 10.1023/b:urol.0000025638.79357.fd. [DOI] [PubMed] [Google Scholar]
- 14.Langer J., Cornud F. Inflammatory disorders of the prostate and the distal genital tract. Radiol Clin North Am. 2006;44:665–677. doi: 10.1016/j.rcl.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Neil F., Wasserman Prostatitis: clinical presentations and transrectal ultrasound findings. Semin Roentgenol. 1999;34:325–337. doi: 10.1016/s0037-198x(99)80009-1. [DOI] [PubMed] [Google Scholar]
- 16.Doble A., Carter S. Ultrasonographic findings in prostatitis. Urol Clin North Am. 1989;16:763–772. [PubMed] [Google Scholar]
- 17.Das K.M., Indudhara R., Vaidyanathan S. Sonographic features of genitourinary tuberculosis. AJR Am J Roentgenol. 1992;158:327–329. doi: 10.2214/ajr.158.2.1729792. [DOI] [PubMed] [Google Scholar]
- 18.Tamsel S., Killi R., Ertan Y., Demirpolat G. A rare case of granulomatous prostatitis caused by Mycobacterium tuberculosis. J Clin Ultrasound. 2007;35:58–61. doi: 10.1002/jcu.20251. [DOI] [PubMed] [Google Scholar]
- 19.Vilana R., Corachan M., Gascon J., Valls E., Bru C. Schistosomiasis of the male genital tract: transrectal sonographic findings. J Urol. 1997;158:1491–1493. [PubMed] [Google Scholar]
- 20.Ozden E., Gogus C., Karamursel T., Baltaci S., Kupeli S., Gogus O. Transrectal sonographic features of prostatic intraepithelial neoplasia: correlation with pathologic findings. J Clin Ultrasound. 2005;33:5–9. doi: 10.1002/jcu.20080. [DOI] [PubMed] [Google Scholar]
- 21.Littrup P., Bailey S. Prostate cancer: the role of transrectal ultrasound and its impact on cancer detection and management. Radiol Clin North Am. 2000;38:87–113. doi: 10.1016/s0033-8389(05)70151-2. [DOI] [PubMed] [Google Scholar]
- 22.Ngheim H., Kellman G., Sandberg S., Craig B. Cystic lesions of the prostate. Radiographics. 1990;10:635–650. doi: 10.1148/radiographics.10.4.1696019. [DOI] [PubMed] [Google Scholar]
- 23.Hamper U.M., Epstien J.L., Sheth S., Walsh P.C., Sanders R.C. Cystic lesions of the prostate gland, a sonographic pathologic correlation. J Ultrasound Med. 1990;9:395–402. doi: 10.7863/jum.1990.9.7.395. [DOI] [PubMed] [Google Scholar]
- 24.Moukaddam H., Haddad M., ELSayyed K., Wazzan W. Diagnosis and treatment of midline prostatic cyst. Clin Imaging. 2003;27:44–46. doi: 10.1016/s0899-7071(02)00484-9. [DOI] [PubMed] [Google Scholar]
- 25.Furuya S., Kato H. A clinical entity of cystic dilatation of the utricle associated with hemospermia. J Urol. 2005;174:1039–1042. doi: 10.1097/01.ju.0000169494.48968.aa. [DOI] [PubMed] [Google Scholar]
- 26.Verma S., Shetty B., Kanth L. A boy with acute urinary retention: a Mullerian duct remnant. Eur Radiol. 2006;16:1401–1403. doi: 10.1007/s00330-005-0116-y. [DOI] [PubMed] [Google Scholar]
- 27.Ng W.T., Kong C.K. Ejaculatory duct cyst versus Müllerian duct cyst. Urology. 1994;43:273–274. doi: 10.1016/0090-4295(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 28.Keenan J.F., Rifkin M.D. Ultrasonographic diagnosis of seminal vesicle cysts in a patient with polycystic kidney disease. J Ultrasound Med. 1995;15:343–344. doi: 10.7863/jum.1996.15.4.343. [DOI] [PubMed] [Google Scholar]


