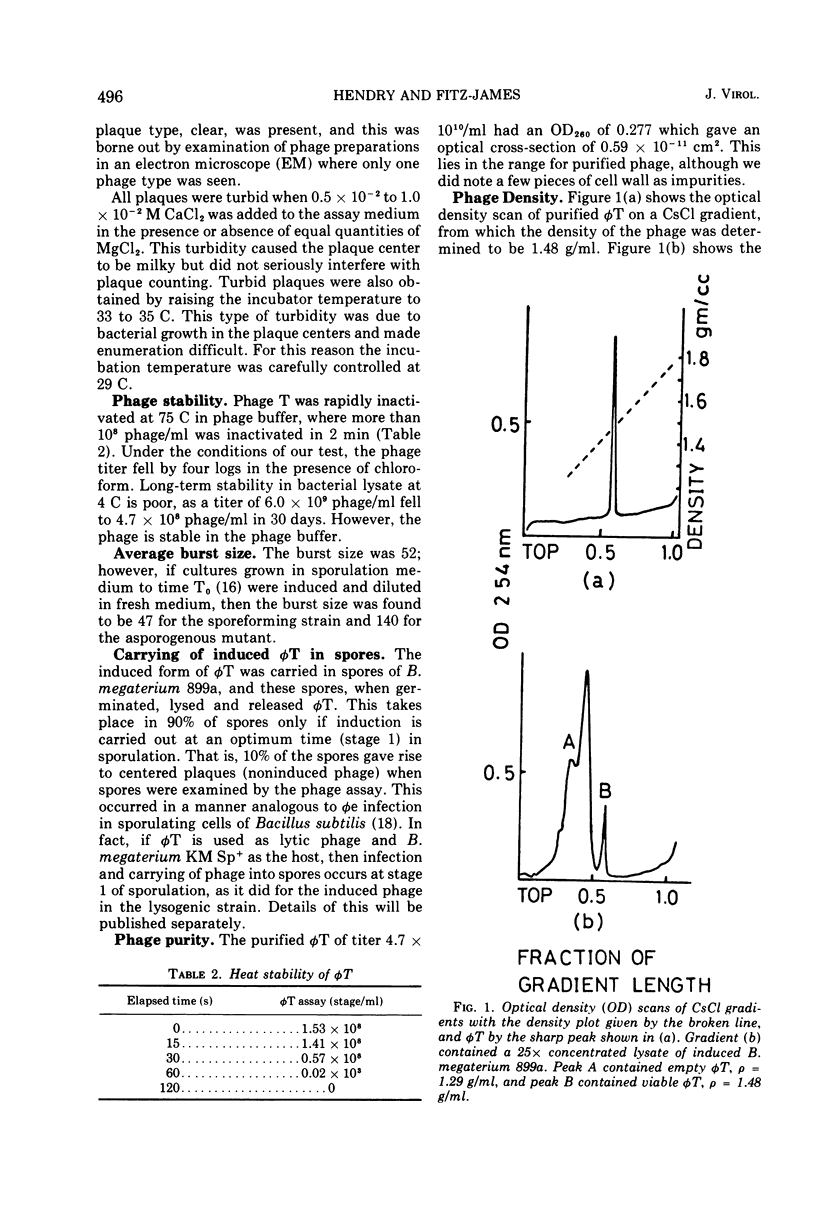
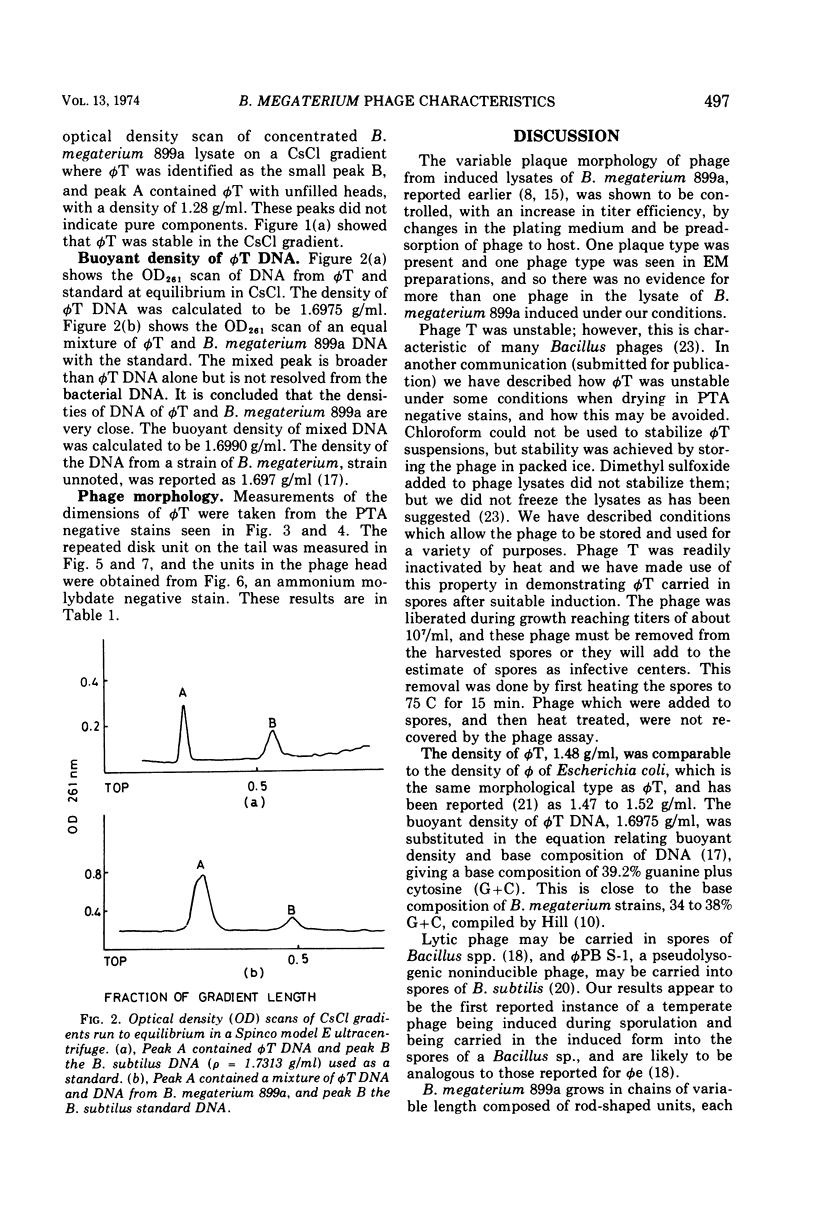
Abstract
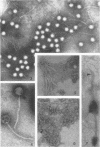
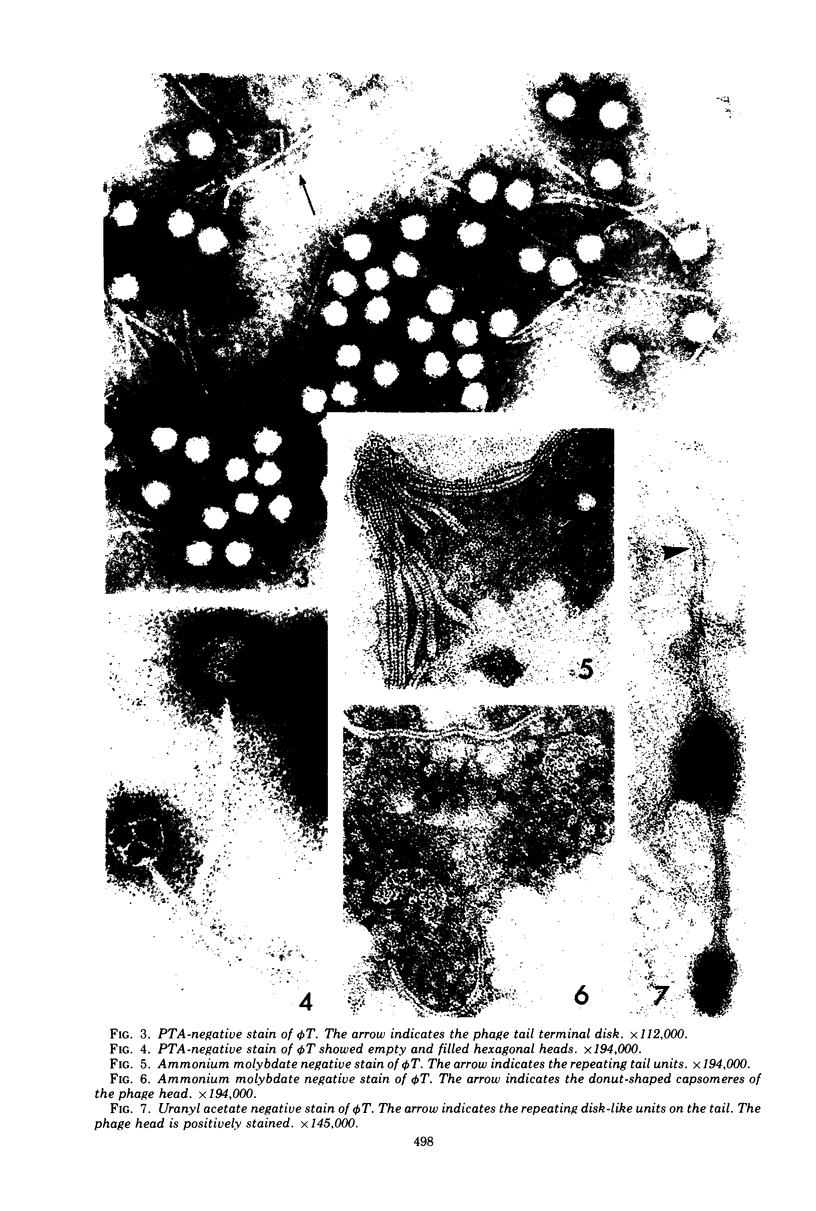
Phage T was the only phage observed in lysates of Bacillus megaterium 899a induced with mitomycin C, 0.35 μg/ml. The phage adsorbed slowly to its host in nutrient agar, giving rise to plaques of varying sizes and turbidity. Only clear plaques were observed when the phage and host cells were preincubated in an adsorption buffer and plated under optimum conditions. Plaque turbidity was caused by either the addition of 0.5 × 10−2 to 1.0 × 10−2 M CaCl2 to the phage assay medium, or by raising the incubation temperature to 34 C. Phage T purified on a CsCl gradient had a density of 1.48 g/ml in CsCl and the extracted phage DNA had a buoyant density in CsCl of 1.6975 g/ml, equivalent to 38.2% guanine plus cytosine. The phage was rapidly inactivated at 75 C and was unstable in the presence of chloroform at 4 C, but it was stable in buffer stored in ice. When stage I sporulating cells were induced with mitomycin C, phage were carried into spores which when germinated lyse with the release of φ T. The burst size on induction of early-log vegetative cells was 52, whereas the burst size of induced T0 sporulating cells, diluted in fresh medium, was 47 for a sporulating strain and 140 for an asporogenous mutant. A typical phage T had a long, noncontracting tail 240 nm long, 9 to 11 nm wide, with a repeating disk unit along the tail, 4 nm in size center to center. The tail ended in a small disk (15 nm wide) which is presumably for attachment to the host. The hexagonal head measures 68 by 57 nm and is composed of donut-shaped units 9 nm in diameter.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE N. A., COWLES P. B. Studies on the host-virus relationship in a lysogenic strain of Bacillus megaterium. II. The relationship between growth and bacteriophage production in cultures of Bacillus megaterium. J Bacteriol. 1952 Feb;63(2):177–186. doi: 10.1128/jb.63.2.177-186.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D. The physical properties of the deoxyribonucleic acid from T7 bacteriophage. J Mol Biol. 1962 Dec;5:643–649. doi: 10.1016/s0022-2836(62)80092-8. [DOI] [PubMed] [Google Scholar]
- DELAPORTE B., SIMINOVITCH L. Recherches cytologiques sur un Bacillus megatherium lysogène au cours du développement du bactériophage. Ann Inst Pasteur (Paris) 1952 Jan;82(1):90–97. [PubMed] [Google Scholar]
- FRIEDMAN M., COWLES P. B. The bacteriophages of Bacillus megaterium. I. Serological, physical, and biological properties. J Bacteriol. 1953 Oct;66(4):379–385. doi: 10.1128/jb.66.4.379-385.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D. An upper limit to the protein content of the germinal substance of bacteriophage T2. Virology. 1955 May;1(1):108–127. doi: 10.1016/0042-6822(55)90009-x. [DOI] [PubMed] [Google Scholar]
- Hill L. R. An index to deoxyribonucleic acid base compositions of bacterial species. J Gen Microbiol. 1966 Sep;44(3):419–437. doi: 10.1099/00221287-44-3-419. [DOI] [PubMed] [Google Scholar]
- MAISCH W. F., WACHSMAN J. T. THYMINELESS INDUCTION OF BACTERIOPHAGE IN BACILLUS MEGATERIUM. J Bacteriol. 1964 Nov;88:1388–1393. doi: 10.1128/jb.88.5.1388-1393.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTHROP J. H. Growth and phage production of lysogenic B. megatherium. J Gen Physiol. 1951 May;34(5):715–735. doi: 10.1085/jgp.34.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTHROP J. H., MURPHY J. S. Appearance of new phage types and new lysogenic strains after adaptation of lysogenic B. megatherium to ammonium sulfate culture medium. J Gen Physiol. 1956 Mar 20;39(4):607–624. doi: 10.1085/jgp.39.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. INCORPORATION OF BACTERIOPHAGE GENOME BY SPORES OF BACILLUS SUBTILIS. J Bacteriol. 1964 Jun;87:1499–1502. doi: 10.1128/jb.87.6.1499-1502.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIGLE J. Densities of transducing lambda bacteriophages. J Mol Biol. 1961 Aug;3:393–398. doi: 10.1016/s0022-2836(61)80052-1. [DOI] [PubMed] [Google Scholar]
- YOUNG I. E., FITZ-JAMES P. C. Chemical and morphological studies of bacterial spore formation. II. Spore and parasporal protein formation in Bacillus cereus var. alesti. J Biophys Biochem Cytol. 1959 Dec;6:483–498. doi: 10.1083/jcb.6.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehle C. O., Doi R. H. Stabilization of Bacillus subtilis phage with dimethylsulfoxide. Can J Microbiol. 1965 Aug;11(4):745–746. doi: 10.1139/m65-099. [DOI] [PubMed] [Google Scholar]