Abstract
Hersh (Mem Cogn 2:771–774, 1974) investigated the role of irrelevant relations in college students’ pattern learning and performance for letter series completion problems. He created irrelevant relations in sequences by inserting items to make pattern structure ambiguous such that it was open to multiple interpretations during initial pattern processing. He reported irrelevant relations impaired humans’ performance more when placed at the beginning of patterns than at the end. However, once pattern structure was induced, irrelevant relations were not impairing. Here, we examined the impact on rat serial pattern learning of irrelevant relations positioned at the beginning or end of a serial pattern. Rats pressed levers in a circular array according to the same structured serial pattern, 123 234 345 456 567, where digits indicated the clockwise position of the correct lever. This structured serial pattern was interleaved with repeating responses on lever 2 to produce irrelevant relations at the beginning of the pattern (Beginning: 122232 223242 324252 425262 526272), on lever 6 to produce irrelevant relations at the end of the pattern (End: 162636 263646 364656 465666 566676), or on lever 8 to produce no irrelevant relations (No Irrelevant Relations: 182838 283848 384858 485868 586878. Irrelevant relations significantly retarded learning regardless of their placement within the pattern. However, irrelevant relations retarded learning significantly more when placed at the pattern beginning versus end. The results indicate that rats, like humans, process patterns from beginning to end.
Keywords: Rat, Serial pattern learning, Sequence learning, Pattern learning, Irrelevant relations, Pattern structure
Introduction
Are animals constrained to learning sequences based on pairwise associations between consecutive items, as in Skinner’s (1934) chaining theory? Or is their sequence learning more flexible and akin to that of humans? Humans are able to learn and reproduce complex sequences readily. For example, if presented with the sequence AMBNCODPE_, a human typically demonstrates sensitivity to the structural organization of the sequence by cognitively sorting it into two separate subpatterns—ABCDE and MNOP. This ability to cognitively sort a sequence into component subpatterns has been taken as evidence that an active cognitive search for pattern structure plays an important role in rule abstraction. According to this idea, once rule abstraction by cognitive sorting (Fountain and Annau 1984) is accomplished, the subject can readily complete the missing final element of such a sequence, “Q” in this case, by extrapolating the appropriate subpattern. Humans’ ability to reorganize pattern elements from nonadjacent serial positions into chunks that were never presented by the experimenter indicates humans are not merely linking one element of an interleaved pattern with the next. Instead, humans appear to represent the abstract rules describing the sequence. This notion is supported by Hersh’s (1974) investigation into whether irrelevant relations in a pattern of stimuli would affect college students’ processing of sequential patterns.
In Hersh’s (1974) study, college students were presented with interleaved letter series such as MMMNMO (subpatterns: MMM and MNO) or AMANAO (subpatterns AAA and MNO). Inducing the pattern in the former series is harder than in the latter. This is because as one encounters the items sequentially, it is difficult to tell whether the pattern is obeying a “repeat” rule implied by successive pattern elements or “next” rules found in two interleaved subpatterns that coincidentally contain identical successive elements. In Hersh’s studies, students encountered sequences varying with respect to the location of irrelevant relations between letters (i.e., beginning, middle, or end). Results indicated that students induced the structure of the interleaved letter patterns. However, irrelevant relations at the beginning of a series increased pattern learning difficulty. Further, the difficulty of processing varied with irrelevant relations placement only during pattern induction, but not subsequent item generation. That is, Hersh found that irrelevant relations impaired the students’ ability to abstract the rule describing the letter series they were presented. However, once the students abstracted the rule describing the series, they were able to produce subsequent elements consistent with that rule. This suggested that after the structure of an interleaved sequence was induced, irrelevant relations were no longer problematic. All of the foregoing bear on judging the efficacy of theories that describe the nature of the processes people use to abstract structure from sequential patterns (see Hersh 1974).
Today, much evidence suggests that animals, like people, do not merely chain successive items together in a variety of tasks including sequential choice tasks, transitive inference tasks, and spatial navigation tasks (e.g., Menzel 1973; Fountain and Annau 1984; Roitblat et al. 1987; Capaldi and Miller 1988; Phelps and Roberts 1989; Dallal and Meck 1990; Phelps and Roberts 1991; Roitblat et al. 1991; Fountain and Rowan 1995a, b; Macuda and Roberts 1995; Rapp et al. 1996; Fountain et al. 1999; Fountain and Benson 2006; Brown and Giumetti 2006). Instead, animals appear to represent sequential events by coding hierarchical representations characterized by relations between nonadjacent events (e.g., Roitblat et al. 1987; Roitblat et al. 1991; Fountain et al. 1999; Fountain and Benson 2006). That is, animals detect relationships between pattern elements even when they are not adjacent to one another.
For example, Fountain et al. (1999) investigated whether rats would learn interleaved subpatterns at different rates as a function of subpattern complexity. In two experiments, rats learned a structured or unstructured sequence interleaved with elements of a repeating sequence or an alternating sequence. The goal of the first experiment was to determine whether rats would show signs of sensitivity to the organization of nonadjacent items from interleaved subpatterns when one subpattern was composed of a single repeating element and the second subpattern was either highly structured or unstructured. For rats in the hierarchically-structured subpattern condition, a 123 234 345 456 567 subpattern was interleaved with the repeating subpattern 888 888 888 888 888. This resulted in the structured-repeating pattern 182838 283848 384858 485868 586878. For rats in the unstructured subpattern condition, a 153 236 345 426 547 subpattern was interleaved with the same repeating element subpattern used in the structured-repeating pattern: 888 888 888 888 888. This resulted in the unstructured-repeating pattern: 185838 283868 384858 482868 584878. For both groups, the integers in the patterns reflect the clockwise position of correct levers, which were rewarded by BSR pulses, in an octagonal chamber on successive trials and spaces represent pauses that served as phrasing cues (see Fountain et al. 2006). The results indicated acquisition of the interleaved structured pattern was significantly faster than the interleaved unstructured pattern. Notice that the unstructured subpattern, 153 236 345 426 547, was generated by exchanging only two pairs of elements in the structured pattern, as indicated by underlining. Exchanging the pairs did not disrupt pairwise associations in the interleaved patterns because all the relocated items continued to be preceded by “8” trials. However, the effects of disrupting the pattern structure were apparent throughout the pattern in that the performance of the unstructured group was even significantly poorer in the third chunk (384858), which was identical in both the structured and unstructured patterns. This suggests rats found this chunk more difficult to learn in the context of the unstructured pattern than the context of the structured pattern.
In a second experiment, rats learned two interleaved sequences created from sets composed of more than one element. As in the first experiment, interleaved patterns were composed of two subpatterns. A structured or unstructured subpattern was interleaved with a subpattern of two alternating elements, 787878. For one group, the structured subpattern, 123456, was interleaved with the alternating subpattern to create the structured-alternating pattern 172837485768. For another group of rats, the unstructured subpattern, 153426, was interleaved with the same alternating subpattern to produce the unstructured-alternating pattern 175837482768. The unstructured subpattern was formed by exchanging only two items of the structured subpattern, as indicated by underlining. The results indicated rats learned the subpatterns of their interleaved patterns at different rates both within and between pattern groups.
Based on subpattern structure, we would expect the formally simpler pattern to be learned most quickly (e.g., Jones 1974; Hulse and Dorsky 1977; Hulse 1978). In the case of the unstructured-alternating pattern, the alternating pattern was acquired faster than the unstructured pattern, as expected based on subpattern structure. Similarly, the structured subpattern was learned faster than the unstructured subpattern. In the case of the structuredalternating pattern, at first glance it is unclear which pattern should be learned most quickly, as both are structured. However, if structural complexity is equated, then we might expect rats to show the same predisposition that humans do to detect repeating items before other structural features (Kotovsky and Simon 1973). Evidence supporting this theory was obtained in that rats in the structured-alternating group showed facilitated acquisition for the alternating pattern over the structured subpattern of the interleaved pattern despite similar structural complexity. The differential acquisition of the structured and unstructured subpatterns supports the view that accurate interleaved subpattern performance depended on a mnemonic representation characterized by relations for nonadjacent events (e.g., Roitblat et al. 1987, 1991). Further, these results indicate that rats are sensitive to the organization of nonadjacent elements in serial patterns, as well as able to detect, sort, and learn about the structural relationships present in such patterns. Moreover, the results indicate pattern and subpattern structure drive the ways in which animals sort, chunk, and represent nonadjacent pattern elements related by common rules or features.
Such research suggests that rats, like humans, do not simply link together elements of an interleaved pattern as they encounter them. Instead, rats appear to encode a representation of the abstract rules describing the sequence. To date, researchers have not explored how irrelevant information might affect rats’ encoding of pattern structure. However, this issue has been explored in studies with people. Hersh (1974) presented college students with letter series completion problems varying with respect to the location of irrelevant relations between letters (i.e., at the beginning, middle, or end of the series). The results indicated students induced the structure of the interleaved letter patterns. However, irrelevant relations between letters at the beginning of a series increased pattern learning difficulty. In particular, irrelevant relations at the beginning of the series produced longer latencies and more errors than irrelevant relations at the end of the series. Whether irrelevant relations influence animals’ pattern learning similarly has not been determined. The current experiment investigated this issue.
Rats learned a hierarchically-structured subpattern (123 234 345 456 567) interleaved with one of three repeating subpatterns (888 888 888 888 888, 222 222 222 222 222, or 666 666 666 666 666) in our serial multiple-choice paradigm (e.g., Fountain et al. 2006). We chose this paradigm which employed a circular array of levers over a linear array of stimuli constructed from, for example, food quantities or a row of spatial locations (e.g., Fountain 1990; Fountain and Hulse 1981) because it allowed us the flexibility to present rats with long, complex patterned sequences and to reduce cueing effects of salient elements at the ends of linear arrays. Each group learned a different repeating subpattern in order to vary the number and placement of irrelevant relations between groups. This resulted in the following three patterns:
| No Irrelevant Relations | 182838 283848 384858 |
| (Group NoIR): | 485868 586878 |
| Beginning Irrelevant Relations | 122232 223242 324252 |
| (Group Beginning): | 425262 526272 |
| End Irrelevant Relations | 162636 263646 364656 |
| (Group End): | 465666 566676 |
Each digit represents an octagonal chamber lever, moving in the clockwise direction. Underlining signifies irrelevant relations, and spaces indicate temporal pauses.
The current study is based on Hersh’s (1974) work with college students showing that students actively search for pattern structure and on the current hypothesis that rats likewise actively search sequences of events for pattern structure. If rats actively search sequences for pattern structure, then irrelevant relations that necessarily produce structural ambiguity should retard acquisition relative to a control group experiencing no irrelevant relations. Additionally, irrelevant relations located at the beginning of an interleaved pattern should make the pattern more difficult to learn relative to a pattern with irrelevant relations located at the end. Thus, significantly faster acquisition and better performance was predicted for Group NoIR relative to Groups Beginning and End. Secondly, Group End was predicted to show significantly faster acquisition and fewer errors than Group Beginning. Such results would suggest irrelevant relations affected rats’ pattern processing in a manner similar to humans in that rats represented pattern structure over nonadjacent elements, were impaired by irrelevant relations during initial pattern induction, and processed the interleaved patterns from beginning to end.
Method
Subjects
The subjects were 20 naïve male hooded rats (Rattus norvegicus). Subjects were at least 90 days old at the time of surgery. All rats were implanted with bipolar electrodes (MS301, Plastic Products, Roanoke, VA) for hypothalamic BSR (coordinates, skull level: 4.5 mm posterior, 1.5 mm lateral, 8.5 mm below the surface of the skull). Before surgery, rats were deeply anesthetized by .04 mg/kg atropine sulfate and 7.0 mg/kg Xylazine by intraperitoneal injection followed by 1.0–3.0% oxygen/isoflurane. Rats were monitored carefully for infection following surgery and were allowed at least 1 week for recovery from surgery. They were housed in individual cages with food and water available ad libitum. Rats were maintained on a 15:9- h light:dark cycle. All experimental activities occurred during the light portion of the cycle.
A total of 18 subjects, 6 in each group, completed the study. Two subjects were excluded from the study during the acquisition phase due to electrode failure. Their data are not included in the results presented below.
Apparatus
One shaping chamber (30 × 30 × 30 cm) equipped with a single retractable response lever mounted 5.0 cm above the floor and a commutating device located in the ceiling was utilized for shaping the leverpress response for BSR. Each chamber was constructed of clear Plexiglas with a floor of stainless steel rods. The shaping chamber was enclosed in a sound-attenuating shell constructed from particleboard (20 × 60 × 65 cm). The shaping chamber was housed in a separate room from the test chambers.
Two testing chambers were used. Each was octagonal in shape with clear Plexiglas walls (15 cm wide × 30 cm tall; 40 cm between parallel walls; see Fountain et al. 2006). Each chamber rested on a floor of hardware cloth. A retractable response lever was centered on each wall 5.0 cm above the floor. Each lever required approximately 0.15 N of force for activation. In the testing chambers, rats were connected to a stimulator by a flexible cord (Plastic Products MS304) and a commutating device centrally located in the ceiling of the chamber. Each chamber was located in a separate testing room (approximately 2.0 × 2.6 m). Each room contained a platform suspended from the ceiling, which was centrally located above the octagonal testing chamber. A speaker, through which continuous soft white noise (31 dB–house noise) or loud white noise (79 dB–cue noise) could be presented (Med Associates), two light bulbs (15 W–house light, 100 W–cue light), and a closed-circuit television camera were mounted on the platform. At the beginning of each session, the house noise and light were turned on and remained on except when cue noise and lights were on and during intervals between patterns. The cue noise and light were turned on during temporal pauses within each pattern, as described below. All lights and sounds were turned off between patterns. The camera allowed monitoring of rats’ activity throughout testing from an adjacent room. Major distal cues in the rooms included wall-mounted electrical outlet panels on two walls, a third smooth painted wall, and a fourth wall containing a door. The experiment was controlled from an adjoining room via a microcomputer and interface (interface and Med-State Software, Med Associates).
Procedure
Throughout all phases of the experiment, rats received reinforcement consisting of 1–3 200-ms BSR “pulses” of a 60-Hz sinusoidal pulse train from a constant current source of 20–100 µA. In all procedures, rats received a set number (1–3) of pulses for each correct response. We determined how many pulses to administer to each rat for correct responses during the shaping phase. Each rat received the lowest number of pulses that was sufficiently rewarding to maintain leverpressing.
Following at least 1 week of recovery from surgery, rats were trained to press a lever for BSR in a shaping chamber. At the session beginning, the lever was inserted and remained inserted throughout the 30-min session. Rats were required to make at least 1,000 leverpress responses within a 30-min session. Rats that failed to meet this criterion were excluded.
After rats were trained to leverpress for BSR pulses, they were divided into 3 groups and trained 5 days per week in the octagonal chamber to press levers in a particular order, as described below. A discrete-trial 8-choice procedure with correction was utilized (Fountain et al. 2006). At the start of each session, the house noise and light were turned on to indicate the beginning of the experimental session and remained on except when cue noise and lights were on and during intervals between patterns. At the beginning of each trial, all 8 levers were inserted into the chamber. If a correct choice was made, all 8 levers were retracted and BSR was administered. If an incorrect choice was made, all levers except the correct lever were withdrawn from the chamber and the rat was required to produce the correct response to obtain BSR before continuing to the next trial. As such, rats always received feedback regarding the correct response on each trial. For each trial, the first lever chosen by the subject was recorded for later analysis.
Acquisition
During acquisition, rats learned to press levers in a particular order (with correction) in an octagonal chamber for BSR according to one of three interleaved patterns:
| Group NoIR: | 182838 283848 384858 485868 586878 |
| Group Beginning: | 122232 223242 324252 425262 526272 |
| Group End: | 162636 263646 364656 465666 566676 |
The patterns consisted of five 6-element chunks. In all patterns, integers represent the clockwise position of levers in the octagonal chamber. Spaces represent 1 s temporal pauses. To facilitate rats’ phrasing of the pattern, the cue noise and light were turned on during these temporal pauses. At the end of each 30-element pattern, all noise and lights were extinguished for 2 s. Underlining indicates the placement of irrelevant relations in each pattern. Importantly, both Group Beginning and Group End encountered portions of their pattern characterized by repeating responses and adjacent-lever responses (viz., 122232 223242 and 465666 566676 located at the beginning versus the end of their patterns, respectively).
In total, rats completed 32 blocks of 20 patterns. During the first 2 days of training, rats received 5 patterns per day. For the next 7 days, rats received 10 patterns per day. For the remainder of the experiment, rats received 1 block or 20 patterns per day. Thus, block 1 was composed of the patterns rats completed on days 1–3, block 2 was composed of the patterns rats completed on days 4–5, block 3 was composed of the patterns completed on days 6–7, and block 4 was composed of the patterns completed on days 8–9. Beginning on day 10, rats completed 1 block of patterns per day. Rats’ responses were recorded on each trial, and percent correct rates were calculated on the hierarchically-structured and repeating subpatterns by subpattern type and on an element-by-element basis. Second, we recorded the percent correct responses on within chunk elements and chunk boundary elements. Within chunk elements refer to the second and third elements of each chunk of the hierarchically- structured subpattern. Chunk boundary elements consisted of the first element of each structural chunk of the hierarchically-structured subpattern.
Data analysis
In all reported analyses, main effects and interactions were considered significant if α 0.05. Appropriate repeated measures analysis of variance (ANOVA) tests were conducted to evaluate the groups’ performance on the interleaved pattern throughout acquisition and transfer phases. We then conducted subsequent planned comparisons employing a critical t based on the appropriate error term from the ANOVA and α = 0.05 to identify significant effects.
Results
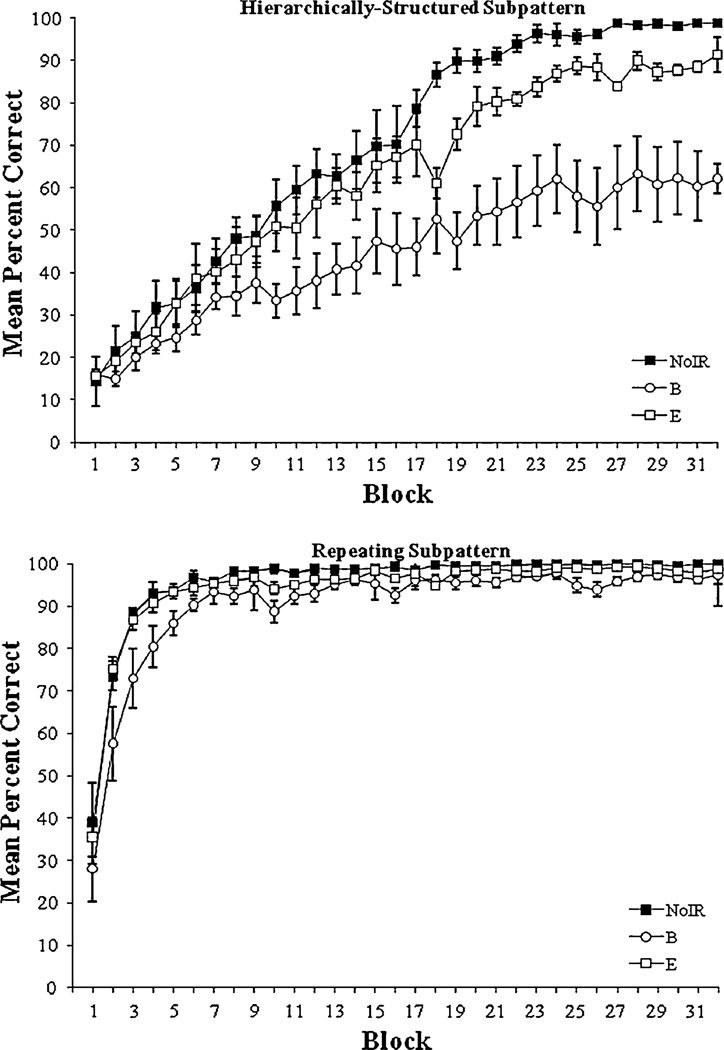
Acquisition curves depicting block-by-block group mean correct response rates for all groups are shown in Fig. 1 for the hierarchically-structured (top panel) and repeating (bottom panel) subpatterns of Experiment 1. As seen in the figure, the performance of all groups improved throughout acquisition. All groups learned their repeating subpattern more quickly than the hierarchically-structured subpattern. However, over the course of acquisition the groups performed significantly differently in terms of their mean overall percentage correct. Groups End and NoIR performed significantly better than Group Beginning throughout acquisition. This indicated irrelevant relations in the beginning of the pattern significantly affected subjects’ ability to detect pattern structure. Group NoIR did not significantly differ from Group End in terms of total percentage correct. However, significant differences emerged between all groups when performance was divided by subpattern. These differences indicated any irrelevant relations significantly retarded learning. However, the effects of irrelevant relations were most detrimental when they occurred at the beginning of the pattern. These conclusions are supported by the analyses reported below.
Fig. 1.
Acquisition curves depicting block-by-block group mean correct response rates for Group NoIR (No Irrelevant Relations), Group B (Beginning Irrelevant Relations), and Group End (End Irrelevant Relations) for the hierarchically-structured (top panel) and repeating (bottom panel) subpatterns of Experiment 1. Error bars: ±SEM
To explore differences between groups over acquisition, we conducted a group × block × subpattern repeated measures ANOVA on rats’ mean percentage correct per block. Main effects for group (F2,15= 14.32, P < 0.001), block (F31,465= 134.96, P < 0.001), and subpattern (F1,15= 220.40, P < 0.001) were significant. Block × group (F62,465= 1.66, P = 0.002), subpattern × group (F2,15 = 6.94, P = 0.007), block × subpattern (F31,465 = 47.64, P < 0.001), and block × subpattern × group (F62,465 = 4.11, P < 0.001) interactions were also significant. We next conducted subsequent planned comparisons based on the appropriate error term from the ANOVA as described in the Method. In particular, we were interested in how rats’ learning differed as a function of block during acquisition, subpattern, and group membership.
The significant block × subpattern × group interaction indicated the groups acquired their interleaved patterns at different rates and that their acquisition depended on subpattern type. These differences are depicted in Fig. 1 (top panel: hierarchically-structured subpattern; bottom panel: repeating subpattern). On the hierarchically-structured subpattern, Group NoIR performed significantly better than Group Beginning on blocks 4 and 7–32. Group NoIR also performed significantly better than Group End on blocks 11, 14, 17–24, and 27–31 of the hierarchically-structured subpattern. Additionally, Group End performed significantly better than Group Beginning on blocks 6 and 8–32 on the hierarchically-structured subpattern. These results suggested Group NoIR acquired the hierarchically-structured subpattern significantly faster and more completely than either Groups End or Beginning. Additionally, Group End acquired the hierarchically-structured subpattern significantly faster and to a higher level of accuracy than Group Beginning.
On the repeating subpattern, Group NoIR performed significantly better than Group Beginning on blocks 1–4 and 10. Group NoIR did not perform significantly better than Group End on any repeating subpattern blocks. Group End performed significantly better than Group Beginning on repeating subpattern blocks 2–4. These results indicated Groups NoIR and End were not significantly different in their acquisition of the repeating subpattern and acquired the repeating subpattern faster than Group Beginning. Despite initial significant differences, all groups eventually acquired the repeating subpattern to the same high performance level (>90% correct) by the conclusion of acquisition.
This pattern of differences was also seen when rats’ errors were sorted by two element types. Chunk boundary elements are defined as the first element of each structural chunk of the hierarchically-structured subpattern. Within chunk elements are defined as the second and third elements of each structural chunk of the hierarchically-structured subpattern. To explore the differences between groups with respect to element type over the course of acquisition, we conducted a group × block × element type repeated measures ANOVA on rats’ block-by-block mean percentage correct for each element type (chunk boundary or within chunk). Main effects for group (F2,15 = 8.62, P = 0.003), block (F31,465 = 127.84, P < 0.001), and element type (F1,15 = 228.58, P < 0.001) were observed. Significant block × group (F62,465 = 3.78, P < 0.001), element type × group (F2,15 = 7.76, P = 0.005), block × element type (F31,465 = 27.06, P < 0.001), and block × element type × group (F62,465 = 4.31, P < 0.001) interactions were also found. We next conducted subsequent planned comparisons based on the appropriate error term from the ANOVA as described in the Method. In particular, we were interested in how rats’ performance differed over acquisition as a function of group membership and element type.
The significant block × element type × group interaction indicated the distribution of correct responses with respect to element type differed over acquisition and depended on group membership and block. Group NoIR performed significantly worse at chunk boundaries than on within chunk elements during blocks 1–18 of acquisition, and Group End performed significantly worse at chunk boundaries than on within chunk elements during blocks 1–23 of acquisition. Group Beginning performed significantly worse at chunk boundaries than on within chunk elements throughout acquisition.
We also compared the performance over element type between groups. Group NoIR performed significantly better on within chunk elements than Group Beginning during blocks 2–32 of acquisition. Additionally, Group NoIR performed significantly better on within chunk elements than Group End during block 18. Group End performed significantly better on within chunk elements than Group Beginning during blocks 2–17 and 19–32. Group Beginning performed significantly better at chunk boundaries than Group NoIR during blocks 1, 6–7, and 9. However, Group NoIR performed significantly better at chunk boundaries than Group Beginning during blocks 14–32. Group NoIR performed significantly better at chunk boundaries than Group End during blocks 4–5, 7–8, 10–23, and 27–32. Group Beginning performed significantly better at chunk boundaries than Group End during blocks 1–9 and 11–13. Lastly, Group End performed significantly better at chunk boundaries than Group Beginning during blocks 20–32.
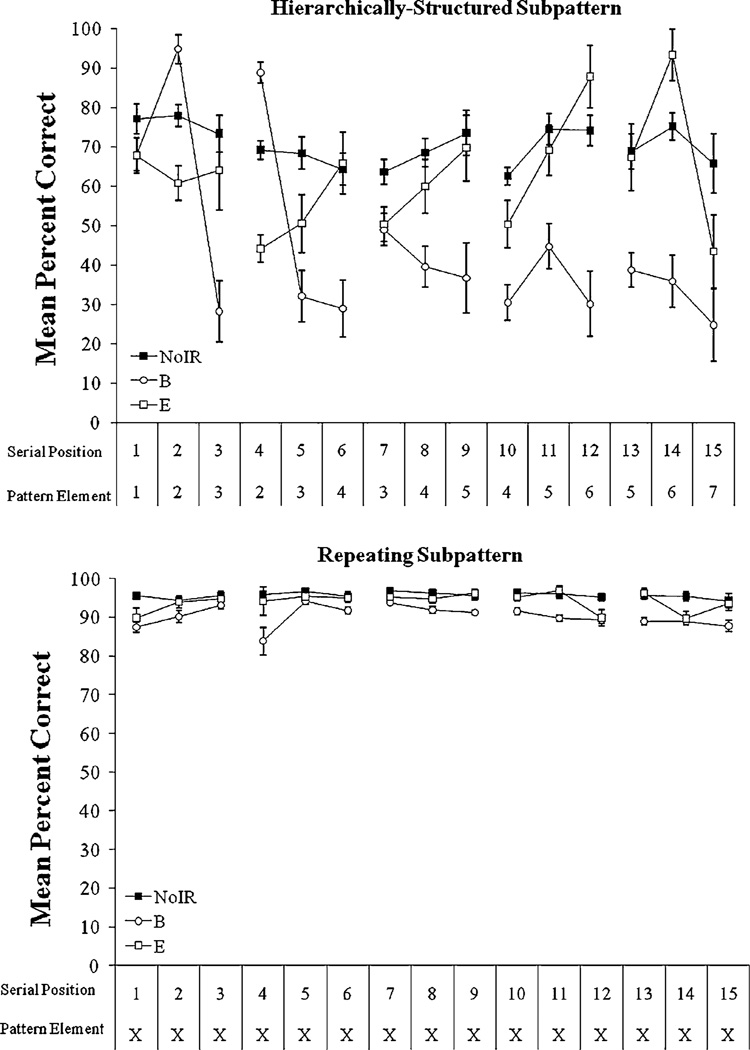
Figure 2 depicts rats’ performance in their respective groups on an element-by-element basis for the hierarchically-structured (top panel) and repeating (bottom panel) subpatterns. To characterize these differences across pattern elements, we calculated mean percentage correct for each rat for each pattern element. Then, we conducted a group × subpattern × chunk × element repeated measures ANOVA. This analysis showed significant main effects for group (F2,15 = 14.32, P < 0.001), subpattern (F1,15 = 220.40, P < 0.001), chunk (F4,60 = 12.64, P < 0.001), and element (F2,30 = 10.08, P < 0.001). Subpattern × group (F2,15 = 6.94, P = 0.007), chunk × group (F8,60 = 14.19, P < 0.001), element × group (F4,30 = 16.21, P < 0.001), subpattern × chunk (F4,60 = 18.08, P < 0.001), subpattern × element (F2,30 = 9.86, P < 0.001), and chunk × element (F8,120 = 13.61, P < 0.001) interactions were also significant. Further significant interactions were indicated for subpattern × chunk × group (F8,60 = 14.70, P < 0.001), Subpattern × element × group (F4,30 = 23.75, P < 0.001), chunk × element × group (F16,120 = 9.9, P < 0.001), and subpattern × chunk × element (F8,120 = 25.83, P < 0.001). The subpattern × chunk × element × group interaction was also significant (F16,120 = 16.45, P < 0.001). We next conducted subsequent planned comparisons based on the appropriate error term from the ANOVA as described in the “Method”. In particular, we were interested in how rats’ performance differed as a function of group membership, pattern type, chunk within the pattern, and element within the chunk.
Fig. 2.
Rats’ element-by-element group mean correct response rates for Group NoIR (No Irrelevant Relations), Group B (Beginning Irrelevant Relations), and Group End (End Irrelevant Relations) for the hierarchically-structured (top panel) and repeating (bottom panel) subpatterns of Experiment 1 pooled over the 32 blocks of acquisition. X’s represent the repeating element of the repeating subpattern (namely, Group NoIR: the repeating “8” element; Group B: the repeating “2” element; Group End: the repeating “6” element). Error bars: ±SEM
The subpattern × chunk × element × group interaction indicated the groups performed differentially on the interleaved pattern and that this performance differed by subpattern, chunk, and element. As shown in the top panel of Fig. 2, within the hierarchically-structured subpattern, planned comparisons indicated that Group NoIR performed significantly better at chunk boundary 1 than chunk boundaries 2–5. Group Beginning performed significantly better at chunk boundary 2 than chunk boundaries 1 and 3–5; additionally, Group Beginning performed significantly better at chunk boundary 1 than chunk boundaries 3–5. Group End performed significantly better at chunk boundaries 1 and 5 than at chunk boundaries 2–4, where chunk boundaries 1 and 5 were not significantly different. Additionally, while Group NoIR overall maintained a similar level of performance across the hierarchically-structured subpattern elements, Group Beginning had significant difficulty with element 3 within each chunk relative to other chunk elements, while Group End had significant difficulty with element 1 within each chunk relative to other chunk elements.
On an element-by-element basis, Group NoIR performed significantly better than Group Beginning at all serial positions of the hierarchically-structured subpattern except for serial positions 2 and 4. On these elements, Group Beginning significantly outperformed Group NoIR. Group Beginning also significantly outperformed Group End at serial positions 2 and 4 of the hierarchically-structured subpattern. Group NoIR performed significantly better than Group End at serial positions 1–5, 7–8, 10, and 15 of the hierarchically-structured subpattern. Group End significantly outperformed Group NoIR at serial positions 12 and 14. Group End performed significantly better than Group Beginning at serial positions 3, 5–6, and 8–15 of the hierarchically-structured subpattern.
As shown in the bottom panel of Fig. 2, Group NoIR performed significantly better than Group Beginning at serial positions 1, 4, 11, and 13–15 on the repeating subpattern. Group NoIR did not perform significantly better than Group End on any repeating subpattern elements. Group End performed significantly better than Group Beginning at repeating subpattern serial positions 4, 11, and 13.
Discussion
The results of the current experiment indicated that rats were sensitive to pattern structure relating nonadjacent elements, as the performance of all groups on their respective interleaved patterns improved significantly over acquisition. However, the groups did not learn the repeating and hierarchically-structured subpatterns at the same rate. All groups learned the repeating subpattern significantly quicker than the hierarchically-structured subpattern, in accordance with the findings of Kotovsky and Simon (1973) with humans and Fountain et al. (1999) with rats that subjects were predisposed to detect the relationship between repeating elements before other structural pattern features. It is true that the simplicity of the repeating subpattern is necessarily confounded in this experiment with amount of reinforcement for a particular lever. However, a number of researchers have noted that as pattern structure becomes increasingly difficult, learning also becomes more difficult (e.g., Fountain and Rowan 1995a). These results add to the growing body of literature suggesting animals, like humans, do not just chain successive items together in sequential tasks (e.g., Roitblat et al. 1991; Fountain and Rowan 1995a, b; Fountain and Benson 2006). Instead, the evidence increasingly indicates animals represent sequential events by coding hierarchical representations characterized by relations between nonadjacent events.
While evidence showed that all groups were sensitive to the structure of interleaved patterns, any irrelevant relations significantly impaired subjects’ pattern induction. This was particularly apparent with respect to learning the hierarchically-structured subpattern. The group that encountered irrelevant relations at the beginning of its interleaved pattern performed significantly poorer on the hierarchically-structured subpattern than the group that did not encounter any irrelevant relations in its interleaved pattern and the group that encountered irrelevant relations only at the end of its interleaved pattern. These differences became apparent early in acquisition. This indicated irrelevant relations located in the beginning of the interleaved pattern significantly affected subjects’ ability to induce pattern structure. Additionally, the results indicated that the group that encountered irrelevant relations at the end of its interleaved pattern performed significantly poorer on the hierarchically-structured subpattern than the group that encountered no irrelevant relations. This difference became apparent during the latter half of acquisition. Taken together, these results indicate that the presence of any irrelevant relations interfered with the induction of pattern structure.
The element-by-element analysis revealed more information about the way rats represented pattern structure. Within the hierarchically-structured subpattern, the group that encountered no irrelevant relations performed significantly better at chunk boundary 1 than chunk boundaries 2–5. The group that encountered irrelevant relations at the beginning of its interleaved pattern performed significantly better at chunk boundary 2 than chunk boundaries 1 and 3–5. Additionally, the group that encountered irrelevant relations at the beginning of its interleaved pattern performed significantly better at chunk boundary 1 than chunk boundaries 3–5. The group that encountered irrelevant relations at the end of its interleaved pattern performed significantly better at chunk boundaries 1 and 5 than at chunk boundaries 2–4, where chunk boundaries 1 and 5 were not significantly different. These results suggested that rats in all groups used the extinguishing of light and sound for 2 s as a good predictor of the first chunk boundary, relative to other chunk boundaries that did not contain a common repeating subpattern element.
Additionally, the group that encountered no irrelevant relations maintained a similar level of performance across the hierarchically-structured subpattern elements. However, the group that encountered irrelevant relations in the beginning of the interleaved pattern had significant overall difficulty with element 3 within each chunk, while the group that encountered irrelevant relations at the end had significant overall difficulty with element 1 within each chunk. This indicated the group that encountered irrelevant relations at the end of the interleaved pattern had difficulty with chunk boundaries (the first chunk element for the hierarchically-structured subpattern), while the group that encountered irrelevant relations at the beginning of the interleaved pattern had difficulty with the final element within each chunk. In contrast, the group that encountered no irrelevant relations found the elements of similar difficulty.
With respect to performance between groups, the analyses indicated the group that encountered no irrelevant relations performed significantly better than the group that encountered irrelevant relations at the beginning of its interleaved pattern on all elements of the hierarchically-structured subpattern except at serial positions 2 and 4. At these serial positions, a response on lever 2 was required. On these elements, the group that encountered irrelevant relations at the beginning of its interleaved pattern performed significantly better. This suggested rats in this group detected the overlap of elements between the two subpatterns, perhaps learning to repetitively press lever 2 as it was a commonly rewarded response in the first two chunks of the pattern. This led to facilitated performance at chunk boundary 2 for this group. The group that encountered no irrelevant relations performed significantly better than the group that encountered irrelevant relations at the end of its interleaved pattern at serial positions 1–5, 7–8, 10, and 15 of the hierarchically-structured subpattern. However, the group that encountered irrelevant relations at the end of its interleaved pattern performed significantly better than the group that encountered no irrelevant relations at serial positions 12 and 14, where a response on lever 6 was required. This suggested that rats in the group that encountered irrelevant relations at the end of its pattern detected the overlap of elements between the two subpatterns, perhaps learning to repetitively press lever 6 as it was a commonly rewarded response in the last two chunks of the pattern. This led to facilitated performance by this group at chunk boundary 5 relative to chunk boundaries 2–4.
While the results indicated the impact of irrelevant relations on pattern induction was largely confined to the hierarchically-structured subpattern, there was some evidence that irrelevant relations impacted learning of the repeating subpattern. More specifically, the group that encountered no irrelevant relations performed significantly better than the group that encountered irrelevant relations in the beginning of its interleaved pattern at serial positions 1, 4, 11, and 13–15 on the repeating subpattern. The group that encountered irrelevant relations at the end of its interleaved pattern performed significantly better than the group that encountered irrelevant relations at the beginning of its interleaved pattern at repeating subpattern serial positions 4, 11, and 13. However, the group that encountered irrelevant relations at the end of its interleaved pattern performed similarly to the group that encountered no irrelevant relations. These results suggested that irrelevant relations only affected the induction of the repeating subpattern structure when they occurred in the beginning of the interleaved pattern. That is, the presence of irrelevant relations in the beginning of the interleaved pattern led to an overall disruption in interleaved pattern learning in both the hierarchical and repeating subpatterns.
Overall, these results indicated any irrelevant relations significantly impaired pattern structure induction. However, this impairment was most profound for subjects that encountered irrelevant relations in the beginning of their interleaved pattern. This result provides evidence regarding how rats built structural representations of the interleaved patterns. Subjects that encountered irrelevant relations at the beginning of their pattern committed more errors overall than subjects that encountered irrelevant relations at the end and subjects that encountered no irrelevant relations. This indicates that when abstracting pattern structure, subjects processed their interleaved pattern from beginning to end. In summary, the current experiment provided more evidence that rats, like humans, can induce pattern structure over nonadjacent elements. However, irrelevant relations interfered with pattern structure induction, especially when irrelevant relations between elements were located at the pattern beginning. This suggested that rats tend to build pattern structure representations from the beginning to end of a series.
Despite such parallels, debate has persisted for decades as to whether organisms learn rules describing hierarchically-structured sequences (Hulse and Dorsky 1977; Lashley 1951; Restle and Brown 1970; Simon and Kotovsky 1963) or rely on associative mechanisms (e.g., Capaldi and Molina 1979; Skinner 1934). Unfortunately, researchers have not reached a consensus. For example, recent investigations point to important roles for both associative learning (e.g., Stempowski et al. 1999; Fountain et al. 2000; Wallace et al. 2008; Kundey and Fountain 2010; Muller and Fountain, in press) and rule learning (e.g., Fountain and Rowan 1995b; Fountain et al. 1999; Fountain and Benson 2006; Fountain et al. 2007; Fountain 2008; Fountain et al. in press). Perhaps the strongest evidence for rule learning in rats arises from exploring learning of interleaved patterns (e.g., Fountain et al. 1999; Fountain and Benson 2006), such as in the current study. These results suggest rats are not limited to learning sequences of information based on pairwise associations between items. Instead, their learning of patterned sequences appears more flexible and bears increasing similarity to that of humans.
The pattern of results obtained in the current experiment mirrors those obtained by Hersh (1974) in a study with college students. In particular, Hersh (1974) found that irrelevant relations disrupted humans’ pattern encoding— especially when irrelevant relations were located at the beginning of a sequence. Our results, like those of Hersh (1974), also suggest that the presence of irrelevant relations disrupts rats’ pattern encoding, particularly when they are encountered at the beginning of a sequence. This implies that rats, like humans, process patterns from beginning to end when attempting to abstract structure and that rule induction processes in rats, like those of humans, are easily misled by structural ambiguity created by very basic rules, for example, identity (i.e., repeat the last item or response) or a +1 rule (i.e., increment 1 unit in the alphabet or, in the case of rats in the current study, move one lever in the clockwise direction). These parallels highlight further similarities between pattern processing in rats and humans.
The current experiment investigated rats’ ability to abstract pattern structure when the pattern contained no irrelevant relations, irrelevant relations at the beginning of the pattern, or irrelevant relations at the end of the pattern. Rats pressed levers in a circular array according to the same structured serial pattern interleaved with repeating responses on lever 2, 6, or 8: 122232 223242 324252 425262 526272 (Beginning), 162636 263646 364656 465666 566676 (End), or 182838 283848 384858 485868 586878 (No Irrelevant Relations). The results of an analogous study with college students (Hersh 1974) indicated that subjects induced pattern structure relating nonadjacent elements. However, any irrelevant relations impaired performance, especially when they were located at the beginning of a series. This suggested that humans tend to build representations of pattern structure as they encountered them from the beginning to the end of a series. The current experiment demonstrated similar results in rats in a serial multiple-choice paradigm. The claim is that the serial multiple-choice paradigm is sufficiently conceptually similar to Hersh’s (1974) letter series completion task that the two tasks likely recruit analogous cognitive processes in rats and college students. Specifically, the evidence from the current experiment indicates that both rats and humans actively search sequences of events for pattern structure and that the cognitive processes involved are sensitive to structural ambiguity created by irrelevant relations in patterns.
Acknowledgments
Preparation of this article was supported in part by Award Number R15DA023349 from the National Institute on Drug Abuse to S. B. Fountain. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. We thank Courtney Taylor and Cody Pollack for assistance in collecting data, as well as the Kent State Graduate Student Senate for funding. This work was reported in another form by Shannon M. A. Kundey as a dissertation in partial fulfillment of the degree of Doctor of Philosophy in Psychology at Kent State University. The experiments reported comply with all current laws in the United States.
Footnotes
We have no conflicts of interest to report.
References
- Brown M, Giumetti G. Spatial pattern learning in the radial arm maze. Learn Behav. 2006;34:102–108. doi: 10.3758/bf03192875. [DOI] [PubMed] [Google Scholar]
- Capaldi EJ, Miller DJ. The rat’s simultaneous anticipation of remote events and current events can be sustained by event memories alone. Anim Learn Behav. 1988;16:1–7. [Google Scholar]
- Capaldi EJ, Molina P. Element discriminability as a determinant of serial-pattern learning. Anim Learn Behav. 1979;7:318–322. [Google Scholar]
- Dallal NL, Meck WH. Hierarchical structures: chunking by food type facilitates spatial memory. J Exp Psychol Anim Behav Process. 1990;16:69–84. [PubMed] [Google Scholar]
- Fountain SB. Rule abstraction, item memory, and chunking in rat serial-pattern tracking. J Exp Psychol Anim Behav Process. 1990;16:96–105. [PubMed] [Google Scholar]
- Fountain SB. Pattern structure and rule induction in sequential learning. Comp Cogn Behav Rev. 2008;3:66–85. [Google Scholar]
- Fountain SB, Annau Z. Chunking, sorting, and rule-learning from serial patterns of brain-stimulation reward by rats. Anim Learn Behav. 1984;12:265–274. [Google Scholar]
- Fountain SB, Benson DM., Jr Chunking, rule learning, and multiple item memory in rat interleaved serial pattern learning. Learn Motiv. 2006;37:95–112. [Google Scholar]
- Fountain SB, Hulse SH. Extrapolation of serial stimulus patterns by rats. Anim Learn Behav. 1981;9:381–384. [Google Scholar]
- Fountain SB, Rowan JD. Coding of hierarchical versus linear pattern structure in rats and humans. J Exp Psychol Anim Behav Process. 1995a;21:187–202. doi: 10.1037//0097-7403.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Sensitivity to violations of “run” and “trill” structures in rat serial-pattern learning. J Exp Psychol Anim Behav Process. 1995b;21:78–81. [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Benson DM. Rule learning in rats: serial tracking in interleaved patterns. Anim Cogn. 1999;2:41–54. [Google Scholar]
- Fountain SB, Benson AM, Wallace DG. Number, but not rhythmicity, of temporal cues determines phrasing effects in rat serial-pattern learning. Learn Motiv. 2000;31:301–322. [Google Scholar]
- Fountain SB, Rowan JD, Muller MD, Smith DPA, Chenoweth AM, Wallace DG. Sequence production paradigms for exploring the organization of sequential behavior. In: Anderson MJ, editor. Tasks and techniques: a sampling of methodologies for the investigation of animal learning, behavior, and cognition. Hauppauge: Nova Science; 2006. pp. 245–260. [Google Scholar]
- Fountain SB, Rowan JD, Carman HM. Encoding structural ambiguity in rat serial pattern learning: the role of phrasing. Int J Comp Psychol. 2007;20:25–34. [Google Scholar]
- Fountain SB, Rowan JD, Muller MD, Kundey SMA, Pickens LRG, Doyle KE. Wasserman EA, Zentall TR. Handbook of comparative cognition. Oxford: Oxford University Press; The organization of sequential behavior: conditioning, memory, and abstraction. (in press) [Google Scholar]
- Hersh HM. The effects of irrelevant relations on the processing of sequential patterns. Mem Cogn. 1974;2:771–774. doi: 10.3758/BF03198153. [DOI] [PubMed] [Google Scholar]
- Hulse SH. Cognitive structure and serial pattern learning by animals. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale: Erlbaum; 1978. pp. 311–340. [Google Scholar]
- Hulse SH, Dorsky NP. Structural complexity as a determinant of serial pattern learning. Learn Motiv. 1977;8:488–506. [Google Scholar]
- Jones MR. Cognitive representations of serial patterns. In: Kantowitz B, editor. Human information processing: tutorials in performance and cognition. 1st edn. Hillsdale: Erlbaum; 1974. pp. 187–230. [Google Scholar]
- Kotovsky K, Simon HA. Empirical tests of a theory of human acquisition of concepts for sequential patterns. Cogn Psychol. 1973;4:399–424. doi: 10.1037/h0043901. [DOI] [PubMed] [Google Scholar]
- Kundey SMA, Fountain SB. Blocking in rat serial pattern learning. J Exp Psychol Anim Behav Process. 2010;36:307–312. doi: 10.1037/a0016523. [DOI] [PubMed] [Google Scholar]
- Lashley KS. The problem of serial order in behavior. In: Jeffress LA, editor. Cerebral mechanisms in behavior. 1st edn. New York: Wiley; 1951. pp. 112–146. [Google Scholar]
- Macuda T, Roberts WA. Further evidence for hierarchical chunking in rat spatial memory. J Exp Psychol Anim Behav Process. 1995;21:20–32. [PubMed] [Google Scholar]
- Menzel EW. Chimpanzee spatial memory organization. Sci. 1973;182:943–945. doi: 10.1126/science.182.4115.943. [DOI] [PubMed] [Google Scholar]
- Muller MD, Fountain SB. Concurrent cognitive processes in rat serial pattern learning: item memory, serial position, and pattern structure. Learn Motiv. doi: 10.1016/j.lmot.2010.08.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps MT, Roberts WA. Central-place foraging by Rattus norvegicus on the radial maze. J Comp Psychol. 1989;103:326–338. doi: 10.1037/0735-7036.103.4.326. [DOI] [PubMed] [Google Scholar]
- Phelps MT, Roberts WA. Pattern tracking on a radial maze: tracking multiple patterns at different spatial locations. J Exp Psychol Anim Behav Process. 1991;17:411–422. [Google Scholar]
- Rapp P, Kansky M, Eichenbaum H. Learning and memory for hierarchical relationships in the monkey: effects of aging. Behav Neuro. 1996;110:887–897. doi: 10.1037//0735-7044.110.5.887. [DOI] [PubMed] [Google Scholar]
- Restle F, Brown ER. Serial pattern learning. J Exp Psychol. 1970;83:120–125. [Google Scholar]
- Roitblat H, Scopatz R, Bever T. The hierarchical representation of three-item sequences. Anim Learn Behav. 1987;15:179–192. [Google Scholar]
- Roitblat H, Bever TG, Helweg DA, Harley HE. On-line choice and the representation of serially structured stimuli. J Exp Psychol Anim Behav Process. 1991;17:55–67. doi: 10.1037//0097-7403.17.1.55. [DOI] [PubMed] [Google Scholar]
- Simon HA, Kotovsky K. Human acquisition of concepts for sequential patterns. Psychol Rev. 1963;70:534–546. doi: 10.1037/h0043901. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The extinction of chained reflexes. P Natl Acad Sci USA. 1934;20:234–237. doi: 10.1073/pnas.20.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempowski NK, Carman HM, Fountain SB. Temporal phrasing and overshadowing in rat serial-pattern learning. Learn Motiv. 1999;30:74–100. [Google Scholar]
- Wallace DG, Rowan JD, Fountain SB. Determinants of phrasing effects in rat serial pattern learning. Anim Cogn. 2008;11:199–214. doi: 10.1007/s10071-007-0110-7. [DOI] [PubMed] [Google Scholar]