Abstract
Polycystic ovary syndrome (PCOS) is a complex disease with heterogeneous clinical and anatomical features that were first described in 1721 by Antonio Vallisneri. There is still a lack of consensus regarding the criteria to be used for diagnosis of PCOS. Transvaginal ultrasonography with Doppler studies of the ovarian and pelvic vasculature plays an important role in its diagnosis, but findings must be interpreted in light of the patient's symptoms and laboratory findings.
Keywords: PCOS, Hyperandrogenism, Metabolic syndrome, Ultrasonography
Sommario
La complessità e l'eterogeneità anatomica e clinica dell'espressione della sindrome dell'ovaio policistico (PCOS) costituisce a tutt'oggi una problematica nella quale la valutazione ecografica rappresenta una componente importante nella diagnosi, che si deve integrare con i sintomi clinici e le alterazioni biochimiche proprie della sindrome descritta per la prima volta da Antonio Vallisneri nel 1721. I criteri per la diagnosi sono eterogenei come la stessa patologia.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in reproductive-age women. Nonetheless, it is one of the most highly debated and controversial issues in reproductive medicine and gynecological endocrinology. There is no internationally accepted definition of PCOS, and criteria for its diagnosis have yet to be standardized. These difficulties are a reflection of certain intrinsic characteristics of the syndrome. The symptoms, in fact, are heterogeneous and highly variable. Furthermore, laboratory and imaging findings often fall within normal limits, making it difficult to arrive at widely accepted cut-offs for use in clinical settings [1,2].
In its classic form, PCOS is characterized by chronic anovulation (80%), irregular menses (80%), and hyperandrogenism that may be associated with hirsutism (60%), acne (30%), seborrhea and obesity (40%). The clinical and pathological features of the “polycystic” or “micropolycystic” ovary were first described in 1721 by Antonio Vallisneri. The syndrome itself, however, was defined much later by Stein and Leventhal, based on their observation in 1935 [3] of a constellation of symptoms consisting in amenorrhea, hirsutism, and obesity in women whose ovaries were enlarged with multiple follicular cysts and fibrotic thickening of the tunica albuginea and cortical stroma. The criteria established by these two authors for the diagnosis of PCOS were quite rigid, and they excluded numerous clinical pictures that resembled one another. It is important to note that the ovarian dysfunction leading to hyperandrogenism is not the main cause of the ovarian features described above: they are the result of conditions related to the hyperandrogenism, such as obesity, diabetes, hyperprolactinemia, and adrenal or thyroid disease.
To further complicate matters, polycystic ovaries have also been described in a high percentage of women who are absolutely normal with no alterations of the ovarian and/or endocrine phenotype [4].
This finding confirms the relative importance of ovarian morphology in diagnosis of PCOS. The presence of endocrine dysfunction or true endocrine disease should be suspected only when there are clinical disturbances and specific endocrine changes. In short, it is easy to see why there is a lack of consensus regarding the criteria to be used for reliable diagnosis of PCOS.
Pathophysiology of PCOS
Despite the enormous quantity of clinical, laboratory, and experimental data published on PCOS, its pathogenesis is still a subject of speculation, and the syndrome has often been referred to as a pathogenetic enigma. In 1993, Crowley et al. [5] attempted to draw up a clear, accurate summary of the four pathogenetic hypotheses that are considered the most credible.
-
(1)
The so-called “top–down school” attributes the syndrome to primary central dysregulation of the production of luteinizing hormone (LH) that translates into inadequate follicular maturation. Increases in serum LH levels lead to hyperplasia of the ovarian thecal cells, which promotes hypersecretion of ovarian androgens.
-
(2)
According to the “bottom–up school,” the problem begins in the periphery, with the conversion of adrenal androgens (D4 androstenedione, D4 A) into estrone (E1) at the level of peripheral adipose tissues. E1 is capable of sensitizing the pituitary gland and causing it to hypersecrete LH, which stimulates the theca and increases ovarian androgen production.
-
(3)
The “androgen theory” holds that ovarian or adrenal hyperandrogenism is the prime mover in PCOS. It is interesting to note that a single gene encodes cytochrome P450c17a, the enzyme that mediates both 17α-hydroxylase and 17-20-desmolase activities at the ovarian and adrenal levels.
-
(4)
The “insulin school” focuses on the association between high androgen levels and hyperinsulinemia, which was recognized in PCOS patients over a decade ago. It is often accompanied by insulin resistance, especially in women who are obese [6]. As a result of these findings, attention has shifted toward the metabolic component as a major factor in the pathophysiology of PCOS. Hyperinsulinemia with insulin resistance is considered one of the most important factors that predispose women to PCOS because it promotes hyperandrogenism and chronic anovularity by multiple mechanisms. In fact, it increases LH incretion [7] and decreases serum levels of the sex hormone-binding globulins (SHBGs), which in turn increases the fraction of unbound circulating androgens that can act on target organs [8]. It also decreases androgen clearance, diminishes aromatase activity, and enhances steroidogenesis [9–14] in the adrenal glands and ovarian theca.
Puberty, in those cases with the characteristics of “hyperpuberty,” is also a risk factor for the development of PCOS. We know that the physiological course of puberty typically includes certain fundamental events, including increases in the number and amplitude of LH pulses and in mean serum levels of insulin, and activation of adrenal androgen production in the reticular zone leading to increased blood levels of dehydroepiandrosterone sulfate (DHEA-S), D4 A, and testosterone (T) [15].
All of this reflects peripubertal evidence of PCOS-type endocrine/metabolic characteristics in adolescents with hyperandrogenism [16]. The results of other studies indicate that the pathogenesis of the disease has a genetic base. Battaglia et al.'s analysis of the prepubertal daughters of women with PCOS revealed sonographic evidence of ovarian polycystosis, but the hormone profiles were not significantly different from those of controls [17]. Ovaries that appear polycystic in the daughter of a PCOS patient can thus be considered evidence of a genetic predisposition to development of the syndrome. The presence of environmental factors, “additional metabolic disturbances” like hyperinsulinemia, insulin resistance, hypertriglyceridemia, and low blood levels of high-density lipoprotein (HDL) cholesterol [18], may lead to full clinical and hormonal expression of the syndrome.
Diagnosis
The criteria used to diagnose PCOS are as variegated as the disease itself. The definition of PCOS used in North America emerged from an NIH (National Institutes of Health) conference held in 1990. It stresses the importance of biochemical and clinical signs of hyperandrogenism and ovarian dysfunction. Ovarian morphology is not an essential component of the diagnosis [18,4]. In contrast, the approach used in Europe assigns central importance to the appearance of the ovaries on ultrasound [19].
Both definitions have their limitations: the one used in the United States is restrictive and fails to consider the fact that US findings of polycystic ovaries in the absence of other manifestations of PCOS might still reflect an early or latent stage of the syndrome. As a result, it is likely to underestimate the incidence of the syndrome and to be less effective in preventing its metabolic sequelae.
On the other hand, if diagnosis of PCOS is based on the isolated sonographic finding of an ovary with polycystic morphology in asymptomatic patients (5–23%) [20] or teenagers with no evidence of hyperandrogenism [21], the incidence of the syndrome is likely to be overestimated. In fact, these findings may represent physiological variants of normal ovarian morphology instead of cryptic or unexpressed forms of PCOS [21–25].
This explains the wide variability of the prevalence figures reported for PCOS. When the American criteria are used, the prevalence falls between 4% and 9% [26,27]. If instead the European criteria are used (in which sonographic findings are the sine qua non), the prevalence rises to 15–20% [21].
As an expert like Homburg [19] has noted, “It has become painfully apparent that it is not only the Atlantic Ocean that divides North America from Europe, but also the definition and diagnosis of polycystic ovarian syndrome (PCOS)”.
After the Rotterdam PCOS Workshop that was held in May 2003, a consensus statement was drawn up that establishes criteria that can be universally adopted to ensure simple, practical, standardized diagnosis of this complex syndrome [28].
The diagnosis is reached after exclusion of other diseases with similar clinical features. PCOS is considered to be present when at least two of the following are present:
-
-
oligo-/amenorrhea and/or anovulation;
-
-
clinical and/or biochemical signs of hyperandrogenism, in particular an LH/FSH ratio of >2.5, increased levels of testosterone, or an elevated free androgen index (FAI);
-
-
sonographic evidence of polycystic ovaries.
This proposal would therefore result in the correct diagnosis of patients with symptoms and biochemical alterations but no evidence of ovarian polycystosis on ultrasound, as well as those with symptoms and polycystic ovarian morphology but no biochemical alterations. The result would be an increase in the prevalence of PCOS compared with that based on either the European or American criteria.
The roles and limitations of ultrasonography in the diagnosis of PCOS
Pelvic US can make an enormous contribution to the diagnosis of PCOS, but it must always be supplemented with a careful history and laboratory work-up.
In 1985, Adams et al. [29] defined the sonographic features of a polycystic ovary: multiple (≥10), small (mean diameter: 2–8 mm) follicles within the ovarian cortex, increased stromal density in the central cortex, increased ovarian volume (≥8 mL).
Abnormal ultrasound findings are generally present in both ovaries [30], although some cases of unilateral, partial PCOS involving only one of the ovaries have been reported. According to some authors [31], these cases show that the peripheral expression of LH receptors and environmental factors (surgery, infections, vascularization characteristics) can limit PCOS involvement (including both morphological and functional alterations) to a single ovary or even to a specific portion of an ovary.
Recent reports suggest that the vaginal approach is preferable to a transabdominal (TA), when possible [32,33]. The results of the TA examination can vary considerably in terms of follicle number, ovarian volume, and stromal density. The transvaginal US examination shows more precise correlation with laparoscopic and histologic findings [32], and this allows the definition of more precise criteria for the identification of polycystic ovaries on US. Moreover, use of a high-frequency transducer eliminates the need for bladder filling, which patients often find uncomfortable, as well as the risk of interpretation errors caused by abdominal adipose tissue (particularly common in women who are overweight or obese).
Constant US findings in PCOS patients include increases in the number of follicles, the density of the stroma, and the volume of the ovary itself.
The number of follicles that reportedly characterizes the polycystic ovary varies widely, from >5 to >15. A minimum of six follicles is currently recommended [34].
Another important variable in the diagnosis of PCOS is ovarian volume, which is calculated according to the following formula: 0.5233 (π6) × A × B × C, where A, B, and C represent the longitudinal, anteroposterior, and transverse diameters of the ovary, respectively (Fig. 1). Volume increases alone, however, are not sufficient for a reliable diagnosis of PCOS [21].
Fig. 1.
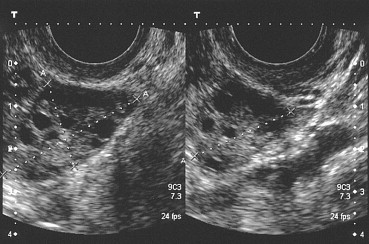
Measurement of the three diameters of the ovary to estimate its volume. The stroma surrounding the microfollicles shows clear signs of hypertrophy.
The same applies to stromal echogenicity. Investigation of this aspect of the ovary was originally regarded as an important part of sonographic studies for suspected PCOS. Its assessment is highly subjective and prone to wide operator-dependent variability. This makes it difficult to define a reliable cut-off that distinguishes between normal and polycystic ovaries, and consequently, the diagnostic specificity of this parameter is not high [35] (Fig. 2).
Fig. 2.
Echogenicity of the ovarian stroma is subjectively rated with a three-point score, where 3 indicates maximum intensity.
The criteria established by Adams et al. for follicle number, ovarian size, and in part also for stromal echogenicity are not universally accepted, and the question of US diagnosis of PCOS is therefore still open. Evaluation of these US parameters alone (in the absence of clinical and laboratory data) is often the cause of erroneous diagnosis of PCOS in women with multifollicular ovaries (MFO), which is associated with a clinical picture completely different from that of PCOS [36].
The multifollicular pattern is in fact a physiological expression of functional ovarian immaturity. It is often observed during normal puberty, during body-weight regain, or in association with nutrition-related amenorrhea (typical in adolescents suffering from high stress, who are often bulimic). However, it is not associated with hyperandrogenism, and the sonographic picture often normalizes with gonadotropin-releasing hormone (GnRH) substitution therapy alone.
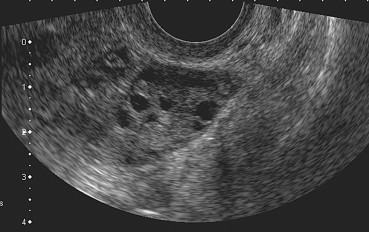
The differential diagnosis of PCOS includes MFO (Fig. 3), which is associated with the presence of normal or mildly enlarged ovaries containing multiple follicles. The follicles are distributed throughout the entire ovarian section and are often somewhat larger than those of PCOS (4–10 mm). Unlike PCOS, MFO is not associated with accentuation of the stromal component [29].
Fig. 3.
Multifollicular ovary (MFO): numerous follicles Ø 6–10 mm are distributed throughout the ovarian section.
In recent years, a number of attempts have been made to improve the specificity of US in the diagnosis of PCOS with the evaluation of additional parameters, with particular emphasis on stromal hypertrophy (Fig. 4).
Fig. 4.
PCOS: the stromal area is increased by more than 25%. This finding displays a specificity of 96% for diagnosis of this syndrome.
With advanced sonographic software, pixel intensity can be measured at the level of the stroma to provide an objective description of stromal echogenicity. This approach has shown that stromal hypertrophy is a frequent and specific feature of PCOS that is correlated with androgen levels [37]. Unfortunately, these techniques are too complex and difficult to apply in clinical practice [35].
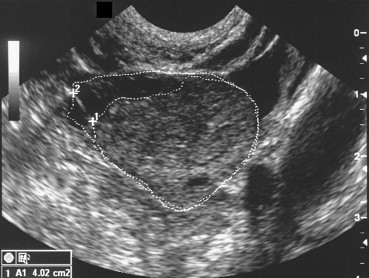
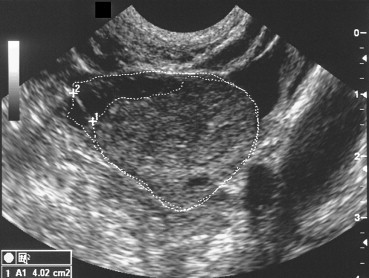
An alternative solution proposed by Fulghesu et al. [38] involves calculation of the ratio of the stromal area of the ovary (i.e., the area that appears hyperechoic) to the total area of the ovary (S/A ratio). The areas are measured on longitudinal scans of the ovary performed with the transvaginal probe (Fig. 5a and b). The S/A ratio is easy to evaluate with standard sonographic equipment and has a low coefficient of inter-examiner variation (less than 5%). An S/A ratio cut-off of 0.34 has been shown to diagnose PCOS with 100% sensitivity and specificity, and it effectively reduces the risk of false PCOS diagnosis in patients with MFO. Unlike classic US parameters, the S/A ratio displays strong correlation with androgen levels and with body mass indices.
Fig. 5.
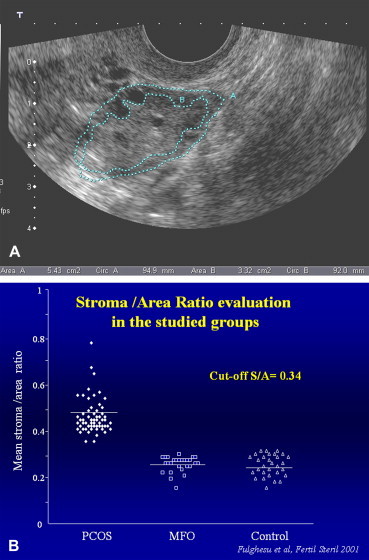
(a) PCOS: calculation of the S/A ratio. The stromal area corresponds to the hyperechoic zone located mainly in the center of the ovary. The total ovarian area is measured by outlining with the caliper the external limits of the ovary in the maximum plane section. (b) In patients with PCO, the S/A ratio is always >0.34. Those of patients with MFO and healthy controls always fall below this cut-off. Fulghesu et al. [38].
The addition of color Doppler greatly improves the diagnostic efficacy of the transvaginal US examination. It provides additional morphologic and pathophysiologic data related to the flow dynamics in ovarian and pelvic vessels [39].
Initially, attention was focused on large vessels such as the uterine and ovarian arteries [40].
Doppler studies [41] of women with PCOS revealed an overall increase in the pulsatility index (PI) of the uterine arteries. The diminished uterine perfusion reflected by this finding is caused by the effects of high androgen levels on the uterine arteries (Fig. 6), and it may explain the increased incidence of abortion among women with PCOS.
Fig. 6.
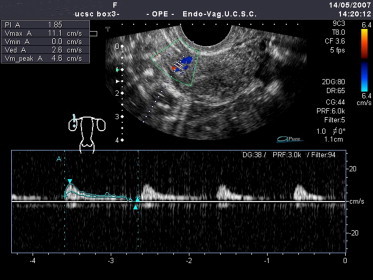
In patients with PCOS, the pulsatility index (PI: 1,85) of the intrauterine artery is higher than normal and vascularization is thus reduced.
More recently, the small vessels of the ovarian stroma have also been investigated [42]. These studies have revealed important changes involving the intraovarian arteries, which show close correlation with the LH/FSH ratio.
High levels of LH are thought to be responsible for the increased stromal vascularization and the diminished resistance of intraovarian vessels it causes: higher LH leading to stromal hyperplasia are associated with lower resistivity indices (RI) in the intraovarian arteries (Fig. 7).
Fig. 7.
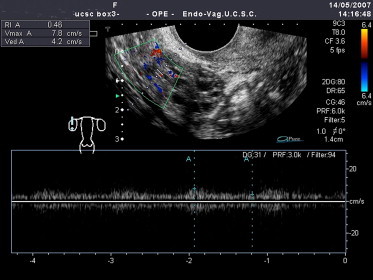
In patients with PCOS, the intraovarian arterioles display lower than normal resistivity indices (RI: 0.46) indicative of enhanced stromal vascularization.
These findings shed light on the patterns observed during US examinations of polycystic ovaries [43]. The peripheral cystic pattern (PCP), in which the follicles are located predominantly in the periphery of the ovary (80% of all cases), and the general cystic pattern (GCP), which is characterized by uniform distribution of the follicles throughout the ovarian parenchyma (15% of all cases), are now regarded [44] as two different phases of the same functional disorder.
Doppler studies have demonstrated that resistivity indices in the intraovarian arteries are generally lower in the subcortical pattern than in the intraparenchymal one. This finding suggests that the latter pattern is an evolution of the former and that the transformation is due to the specific effects of LH on the ovarian parenchyma, which increase vascularization, leading to stromal hyperplasia and a consequent overproduction of androgens.
As the number of microcysts and the volume of the ovary increase and Doppler indices become increasingly abnormal, the clinical and hormonal manifestations of PCOS become more evident, and menstrual disturbances, more severe [45].
Obese PCOS patients have also been found to have higher uterine artery PIs, higher serum levels of insulin and triglycerides, higher hematocrits, and lower levels of HDL cholesterol than their non-overweight counterparts: therefore, in patients who are overweight, hyperinsulinemia might represent the link between the increased resistance in the uterine vasculature, obesity, altered lipid profiles, and the cardiovascular risk [46].
In addition to providing temporary relief of symptoms, prompt treatment might also be capable of slowing the disease progression and reducing the reproductive, metabolic, and cardiovascular risks [47].
In women with hyperandrogenism and/or true PCOS, evaluation of the ovarian morphology and vasculature with transvaginal US and Doppler studies provides more detailed information on the stage of the ovarian dysfunction that is an important index of the patient's cardiovascular risk.
Encouraging results have been obtained with three-dimensional transvaginal US. Compared with conventional US, the three-dimensional approach provides more detailed information on all types of ovarian and uterine pathology, and its use in reproductive medicine is becoming more and more common. It also seems to provide more reliable estimates of organ volumes and blood flow, and most importantly, it facilitates standardization of sonographic examination procedures [48,49].
The introduction of this advanced technique has improved the precision and reproducibility of ovarian measurements. The stromal volume can be calculated as the difference between the total ovarian volume and the total follicular volume (Fig. 8). With this technique, Kyei-Mensah et al. [50] documented a positive correlation between stromal volumes and androstenedione levels, whereas Nardo et al. [51] found no correlation between stromal volumes and biochemical indices of the menstrual cycle.
Fig. 8.
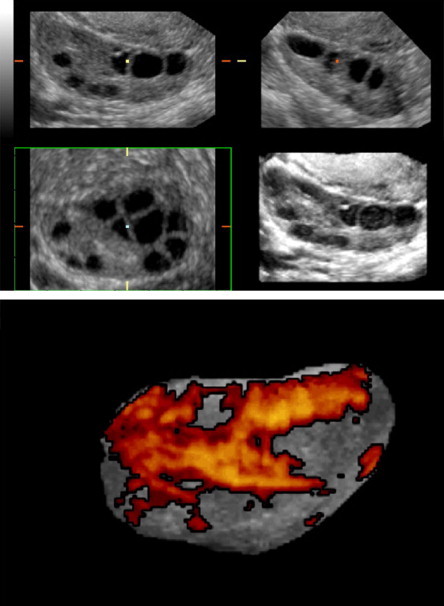
Three-dimensional ultrasound facilitates assessment of the ovarian stroma based on its mean grey signal intensity, volume, and vascularity. The stromal volume can be calculated by subtracting total follicular volume from total ovarian volume. Raine-Fenning and Fleischer [49].
The three-dimensional approach also allows quantitative assessment of the ovarian vasculature by quantification of power Doppler signals. Three indices can be calculated [52]: the vascularization index, which represents the ratio of power Doppler information within the total dataset relative to both color and grey information; the flow index, which represents the mean intensity of the power Doppler signal; and the vascularization flow index, which is a combination of the former two indices.
It remains to be determined how closely these indices correlate with in vivo blood flow and vascularization. However, their inter- and intrasubject variability has already been demonstrated, and this suggests that they can play a valuable role in the identification and classification of substantial differences between groups of patients [53].
The role of magnetic resonance imaging (MRI) in the diagnosis of PCOS is still controversial. The study of small ovarian cysts involves the use of T2-weighted, turbo spin echo (TSE) sequences (which can be acquired more rapidly than spin echo sequences) and coronal and axial scansion planes, which provide the best visualization of the adnexae [54]. The images reveal multiple subcortical cysts, of uniform size and with very thin walls that may be contrast-enhanced. They generally appear hypointense in T1-weighted images and hyperintense in T2-weighted images. The size of the ovaries varies, and the central stroma appears hypointense in T2-weighted images as a result of the increased medullary component. In any case, the signal is more intense than that of the ovarian cortex.
As demonstrated by the work of Marrian [55], MRI is fairly sensitive in the detection of follicular cysts, but it is not specific enough to allow diagnosis of PCOS without supporting history and laboratory data. In fact, ovaries with a polycystic appearance have been observed in patients without PCOS, in patients with oligomenorrhea who have not been diagnosed with PCOS, and in patients being treated with estrogens or clomiphene. Compared with US, MRI plays a supplementary role in the diagnosis of PCOS. It merely provides additional evidence of the polycystic morphology of the ovaries and at this point it has no real use in clinical practice.
Conclusions
PCOS and the hyperandrogenism that accompanies it is a complex disease associated with significant metabolic alterations, such as obesity and insulin resistance, which have been and continue to be the subject of intense research.
Collectively speaking, the data generated by these studies depict PCOS as an entirely new disease entity with repercussions that extend far beyond the boundaries of the reproductive system. PCOS has important clinical and systemic implications in terms of morbidity related to type II diabetes, dyslipidemia (high levels of total and LDL cholesterol and triglycerides), hypertension, and cardiovascular disease [56], and for this reason, it requires prompt diagnosis and appropriate treatment.
In spite of its complex pathophysiology and the heterogeneous clinical and anatomical manifestations of this syndrome, transvaginal US studies of the ovaries with Doppler analysis of the intraovarian and uterine arteries plays a pivotal role in diagnosing and staging PCOS. In light of the close correlation that has been observed with laparoscopic findings, the Doppler US examination is probably the single most important tool for diagnosing this syndrome [41].
References
- 1.Franks S. Polycystic ovary syndrome: a changing perspective. Clin Endocrinol. 1989;31:87–120. doi: 10.1111/j.1365-2265.1989.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 2.Balen A.H., Conway G.S., Kaltsas G. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1985;10:2107–2111. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- 3.Stein I.F., Leventhal M.L. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. [Google Scholar]
- 4.Polson D.W., Wadsworth J., Adams J. Polycystic ovaries: a common finding in normal women. Lancet. 1988;1:870–872. doi: 10.1016/s0140-6736(88)91612-1. [DOI] [PubMed] [Google Scholar]
- 5.Crowley W.F., Jr., Hall J.E., Martin K.A., Adams J., Taylor A.E. An overview of the diagnostic considerations in polycystic ovarian syndrome. Ann N Y Acad Sci. 1993;687:235–241. doi: 10.1111/j.1749-6632.1993.tb43871.x. [DOI] [PubMed] [Google Scholar]
- 6.Lanzone A., Fulghesu A.M., Guido M., Fortini A., Caruso A., Mancuso S. Differential androgen response to adrenocorticotropic hormone stimulation in polycystic ovarian syndrome: relationship with insulin secretion. Fertil Steril. 1992;58:296–301. doi: 10.1016/s0015-0282(16)55220-0. [DOI] [PubMed] [Google Scholar]
- 7.Nestler J.E., Usiskin K.S., Barlascini C.O., Welty D.F., Clore J.N., Blackard W.G. Suppression of serum insulin by diazoxide reduces serum testosterone levels in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1989;68:1027–1032. doi: 10.1210/jcem-68-6-1027. [DOI] [PubMed] [Google Scholar]
- 8.Nestler J.E., Powers L.P., Matt D.W. A direct effect of hyperinsulinemia serum sex-hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–89. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 9.Ciampelli M., Lanzone A. Insulin and polycystic ovary syndrome. Gynecol Endocrinol. 1998;12:277–292. doi: 10.3109/09513599809015601. [DOI] [PubMed] [Google Scholar]
- 10.Burghen G.A., Givens J.R., Kitabchi A.E. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113–116. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 11.Stuart C.A., Peters E.J., Prince M.J., Richards G., Cavallo A., Meyer W.J., 3rd Insulin resistance with acanthosis nigricans; the role of obesity and androgen excess. Metabolism. 1986;35:197–205. doi: 10.1016/0026-0495(86)90201-5. [DOI] [PubMed] [Google Scholar]
- 12.Diamanti Kandarakis E., Mitrakou A., Hennes M.M. Insulin sensitivity and antiandrogenic therapy in women with polycystic ovary syndrome. Metabolism. 1995;44:525–531. doi: 10.1016/0026-0495(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 13.Toprak S., Yonem A., Cakir B. Insulin resistance in nonobese patients with polycystic ovary syndrome. Horm Res. 2001;55:65–70. doi: 10.1159/000049972. [DOI] [PubMed] [Google Scholar]
- 14.Moghetti P., Castello R., Negri C. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab. 1996;81:881–886. doi: 10.1210/jcem.81.3.8772544. [DOI] [PubMed] [Google Scholar]
- 15.Potau N., Ibanez L., Riquè S. Pubertal changes in insulin secretion and peripheral insulin sensitivity. Horm Res. 1997;48:219–226. doi: 10.1159/000185519. [DOI] [PubMed] [Google Scholar]
- 16.Apter D., Butzow T., Laughlin G.A. Metabolic features of polycystic ovary syndrome are found in adolescent girls with hyperandrogenism. J Clin Endocrinol Metab. 1995;80:2966–2973. doi: 10.1210/jcem.80.10.7559882. [DOI] [PubMed] [Google Scholar]
- 17.Battaglia C., Regnani G., Mancini F., Iughetti L., Flamigni C., Venturoli S. Polycystic ovaries in childhood: a common finding in daughters of PCOS patients. A pilot study. Hum Reprod. 2002;17:771–776. doi: 10.1093/humrep/17.3.771. [DOI] [PubMed] [Google Scholar]
- 18.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 19.Homburg R. What is polycystic ovarian syndrome? A proposal for a consensus on the definition and diagnosis of polycystic ovarian syndrome. Hum Reprod. 2002;17:2495–2499. doi: 10.1093/humrep/17.10.2495. [DOI] [PubMed] [Google Scholar]
- 20.Loucks T.L., Talbott E.O., McHugh K.P., Keelan M., Berga S.L., Guzick D.S. Do polycystic-appearing ovaries affect the risk of cardiovascular disease among women with polycystic ovary syndrome? Fertil Steril. 2000;74:547–552. doi: 10.1016/s0015-0282(00)00695-6. [DOI] [PubMed] [Google Scholar]
- 21.Michelmore K.F., Balen A.H., Dunger D.B., Vessey M.P. Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol (Oxf) 1999;51:779–786. doi: 10.1046/j.1365-2265.1999.00886.x. [DOI] [PubMed] [Google Scholar]
- 22.Speca S., Summaria V. Ultrasonography in gynecology: normal anatomy. Eur J Ultrasound. 1996;4:77–89. [Google Scholar]
- 23.Carmina E., Wong L., Chang L. Endocrine abnormalities in ovulatory women with polycystic ovaries on ultrasound. Hum Reprod. 1997;12:905–909. doi: 10.1093/humrep/12.5.905. [DOI] [PubMed] [Google Scholar]
- 24.Norman R.J., Hague W.M., Masters S.C. Subjects with polycystic ovaries without hyperandrogenaemia exhibit similar disturbances in insulin and lipid profiles as those with polycystic ovary syndrome. Hum Reprod. 1995;10:2258–2261. doi: 10.1093/oxfordjournals.humrep.a136280. [DOI] [PubMed] [Google Scholar]
- 25.Homburg R. Polycystic ovary syndrome – from gynaecological curiosity to multisystem endocrinopathy. Hum Reprod. 1996;11:29–39. doi: 10.1093/oxfordjournals.humrep.a019031. [DOI] [PubMed] [Google Scholar]
- 26.Diamanti Kandarakis E., Kouli C., Bergiele A.T. A survey of the polycystic ovary syndrome in the Greek Island of Lesbos: ormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 27.Asuncion M., Calvo R.M., San Millan J.L., Sancho J., Avila S., Escobar-Morreale H.F. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women in Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 28.Fauser B. Revised consensus on diagnostic criteria and long-term risk related to polycystic ovary syndrome. Rotterdam ESHRE/ASRM, in press.
- 29.Adams J., Franks S., Polson D.W. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2:1375–1378. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 30.Parisi L., Tramonti M., Derchi L.E. Polycistic ovarian desease: ultrasonic evaluation and correlation with clinical and hormonal data. J Clin Ultrasound. 1984;12:21–26. doi: 10.1002/jcu.1870120106. [DOI] [PubMed] [Google Scholar]
- 31.Battaglia C., Regnani G., Artini P.G. Polycistic ovary syndrome: a new ultrasonographic and color Doppler pattern. Gynecol Endocrin. 2000;14:417–424. doi: 10.3109/09513590009167713. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K., Ozaki T., Okada M. Relationship between ultrasonography and histopathological changes in PCOS. Hum Reprod. 1994;9:2225–2258. doi: 10.1093/oxfordjournals.humrep.a138432. [DOI] [PubMed] [Google Scholar]
- 33.Yee B., Barnes R.B., Vargyas J.M., Marrs R.P. Correlation of transabdominal and transvaginal ultrasound measurements of follicle size and number with laparoscopic findings for in vitro fertilization. Fertil Steril. 1987;47:828–832. doi: 10.1016/s0015-0282(16)59173-0. [DOI] [PubMed] [Google Scholar]
- 34.Battaglia C., Gennazzani A.D., Salvatori M. Doppler, ultrasonographic and endocrinological environment with regard to the number of small subcapsular follicles in PCOS. Gynaecol Endocrinol. 1999;13:123–129. doi: 10.3109/09513599909167544. [DOI] [PubMed] [Google Scholar]
- 35.Buckett W.M., Bouzayen R., Watkin K.L., Tulandi T., Tan S.L. Ovarian stromal echogenicity in women with normal and polycystic ovaries. Hum Reprod. 1999;14:618–621. doi: 10.1093/humrep/14.3.618. [DOI] [PubMed] [Google Scholar]
- 36.Ardaens Y., Robert Y., Lemaitre L., Fossati P., Dewailly D. Polycystic ovarian disease: contribution of vaginal endosonography and reassessment of ultrasonic diagnosis. Fertil Steril. 1991;55:1062–1068. doi: 10.1016/s0015-0282(16)54353-2. [DOI] [PubMed] [Google Scholar]
- 37.Robert Y., Dubrulle F., Gaillandre L. Ultrasound assessment of ovarian stroma hypertrophy in hyperandrogenism and ovulation disorders: visual analysis versus computerized quantification. Fertil Steril. 1995;64:307–312. [PubMed] [Google Scholar]
- 38.Fulghesu A.M., Ciampelli M., Belosi C., Apa R., Pavone V., Lanzone A. A new ultrasound criterion for the diagnosis of polycystic ovary syndrome: the ovarian stroma/total area ratio. Fertil Steril. 2001;76:326–331. doi: 10.1016/s0015-0282(01)01919-7. [DOI] [PubMed] [Google Scholar]
- 39.Kuriak A., Kupesic-Urek S., Schulman H. Transvaginal color flow Doppler in the assessment of ovarian and uterine flow in infertile women. Fertil Steril. 1991;56:870–873. doi: 10.1016/s0015-0282(16)54657-3. [DOI] [PubMed] [Google Scholar]
- 40.Mercè L., Garces D., Barco M.J. Intraovarian Doppler velocimetry in ovulatory, dysovulatory and anovulatory cycles. Ultrasound Obstet Gynecol. 1992;2:197–202. doi: 10.1046/j.1469-0705.1992.02030197.x. [DOI] [PubMed] [Google Scholar]
- 41.Ajossa S., Guerriero S., Paoletti A.M., Orru M., Melis G.B. The antiandrogenic effect of flutamide improves uterin perfusion in women with polycystic ovary syndrome. Fertil Steril. 2002;77:1136–1140. doi: 10.1016/s0015-0282(02)03101-1. [DOI] [PubMed] [Google Scholar]
- 42.Battaglia C. The role of ultrasound and Doppler analysis in the diagnosis of polycystic ovary syndrome. Ultrasound Obstet Gynecol. 2003;22:225–232. doi: 10.1002/uog.228. [DOI] [PubMed] [Google Scholar]
- 43.Matsunaga I., Hata T., Kitao M. Ultrasonographic identification of polycystic ovaries. Asia Oceania J Obstet Gynaecol. 1985;11:227–232. doi: 10.1111/j.1447-0756.1985.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 44.Battaglia C., Artini P.G., Salvatori M. Ultrasonographic patterns of polycystic ovaries: color Doppler and hormonal correlations. Ultrasound Obstet Gynecol. 1998;11:332–336. doi: 10.1046/j.1469-0705.1998.11050332.x. [DOI] [PubMed] [Google Scholar]
- 45.Battaglia C., Artini P.G., Gennazzani A.D. Color Doppler analysis in oligo and amenorrheic women with polycystic ovary syndrome. Gynecol Endocrinol. 1997;11:105–110. doi: 10.3109/09513599709152520. [DOI] [PubMed] [Google Scholar]
- 46.Battaglia C., Artini P.G., Gennazzani A.D. Color Doppler analysis in lean and obese women with polycystic ovary syndrome. Ultrasound Obstet Gynecol. 1996;7:342–346. doi: 10.1046/j.1469-0705.1996.07050342.x. [DOI] [PubMed] [Google Scholar]
- 47.Lobo R.A., Carmina E. The importance of diagnosing the woman with polycystic ovary syndrome. Ann Intern Med. 2000;132:989–993. doi: 10.7326/0003-4819-132-12-200006200-00010. [DOI] [PubMed] [Google Scholar]
- 48.Pretorius D.H., Nelson T.R. Three-dimensional ultrasound. Ultrasound Obstet Gynecol. 1995;5:219–221. doi: 10.1046/j.1469-0705.1995.05040219.x. [DOI] [PubMed] [Google Scholar]
- 49.Raine-Fenning N., Fleischer A.C. Clarifying the role of three-dimensional transvaginal sonography in reproductive medicine: an evidenced-based appraisal. J Exp Clin Assist Reprod. 2005;2:10. doi: 10.1186/1743-1050-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kyei-Mensah A., Maconochie N., Zaidi J. Transvaginal three-dimensional ultrasound: reproducibility of ovarian and endometrial volume measurements. Fertil Steril. 1998;66:718–722. doi: 10.1016/s0015-0282(16)58624-5. [DOI] [PubMed] [Google Scholar]
- 51.Nardo L.G., Buckett W.M., White D., Digesu A.G., Franks S., Khullar V. Three-dimensional assessment of ultrasound features in women with clomiphene citrate-resistant ovarian syndrome (PCOS): ovarian stromal volume does not correlate with biochemical indices. Hum Reprod. 2002;17:1052–1055. doi: 10.1093/humrep/17.4.1052. [DOI] [PubMed] [Google Scholar]
- 52.Pairleitner H., Steiner H., Hasenoehrl G., Staudach A. Three-dimensional power Doppler sonography: imaging and quantifying blood flow and vascularization. Ultrasound Obstet Gynecol. 1999;14:139–143. doi: 10.1046/j.1469-0705.1999.14020139.x. [DOI] [PubMed] [Google Scholar]
- 53.Raine-Fenning N.J., Campbell B.K., Clewes J.S., Kendall N.R., Johnson I.R. The reliability of virtual organ computer-aided analysis (VOCAL) for the semiquantification of ovarian, endometrial and subendometrial perfusion. Ultrasound Obstet Gynecol. 2003;22:633–639. doi: 10.1002/uog.923. [DOI] [PubMed] [Google Scholar]
- 54.McLeary M.S., Kjellin I.B., Kirk S.R. Magnetic resonance imaging of the pediatric female pelvis: a pictorial essay. Journal of Women's Imaging. 2001;3:38–44. [Google Scholar]
- 55.Marrian G., Stein M. Polycystic ovarian disease (Stein–Leventhal syndrome) Obstetrics/gynecology. 2005:24. section 4. [Google Scholar]
- 56.Fulghesu A.M., Apa R., Ciampelli M. Iperinsulinemia e Iperandrogenismi. Endocrinologia. 2000;4(1) [Google Scholar]


