Abstract
Background
Postoperative neurocognitive decline occurs frequently. Although predictors of cognitive injury have been well examined, factors that modulate recovery have not. We sought to determine the predictors of cognitive recovery after initial injury following cardiac surgery.
Methods
Two hundred eighty-one patients previously enrolled in cognitive studies who experienced cognitive decline 6 weeks after cardiac surgery were retrospectively evaluated. Eligible patients completed a battery of neurocognitive measures and quality-of-life assessments at baseline, 6 weeks, and 1 year after surgery. Factor analysis was conducted to calculate the cognitive index (CI), a unified, continuous measure of cognitive function. Cognitive recovery was defined as 1-year CI ≥ baseline CI. Potential predictors of cognitive recovery including patient characteristics, quality-of-life factors, comorbidities, medications, and intraoperative variables were assessed with multivariable regression modeling; p<0.05 was considered significant.
Results
Of the 229 patients in our final dataset, 103 (45%) demonstrated cognitive recovery after initial decline in CI at 6 weeks. Multivariable analyses revealed that more education (OR 1. 332 [1. 131-1. 569], p<0. 001), baseline CI (OR 0. 987 [0. 976–0. 998], p=0. 02), less decline in CI at 6 weeks (OR 1. 044 [1. 014–1. 075], p=0. 004), and greater activities of daily living at 6 weeks (OR 0. 891 [0. 810–0. 981], p=0. 02) were significant predictors of cognitive recovery.
Conclusion
Cognitive recovery occurred in approximately one-half of the cardiac surgical patients experiencing early decline. The association between cognitive recovery and Instrumental Activities of Daily Living scores at 6 weeks merits further investigation as it is the only potentially modifiable predictor of recovery.
Introduction
Postoperative cognitive injury is characterized by decline of such mental functions as perception, memory, and information processing after a surgical procedure. 1,2 The occurrence of cognitive dysfunction after coronary artery bypass graft (CABG) surgery is frequent and persistent with 30 to 65% of patients showing cognitive injury at 6 weeks after surgery. 2–5 Despite surgical advancements reducing the once high mortality and morbidity rates associated with CABG, only a few strategies6, 7 have been proposed to moderate postoperative cognitive decline and the associated reduction in quality of life. 8 Preoperative risk factors for cognitive injury after surgical intervention include age, level of education, baseline cognition and genetic predisposition.2, 3, 9, 10 The hypothesized surgical risks for cognitive decline include cerebral embolism, cell salvage, valve surgery, hypoperfusion, systemic inflammatory responses, hemodilution, hyperglycemia, and hyperthermia. 3, 10–13
Preoperative and perioperative factors associated with dysfunction have been well documented; nonetheless, there is much uncertainty and controversy surrounding the underlying pathophysiology, clinical sequelae, and chronicity of the phenomenon. It remains unclear in the literature whether short-term changes in cognitive performance lead to long-term postoperative declines. In a longitudinal study assessing neurocognitive function after CABG, Newman et al. reported that impairment at discharge was associated with a decline from baseline function 5 years after surgery. 2 Other evidence suggests that postoperative cognitive dysfunction (POCD)is transient in nature, with no relationship between immediate and delayed cognitive injury. 5, 14 With the inclusion of a control group and adjustment for the variability of within-subject score changes, Selnes et al. found that while some cognitive dysfunction occurred directly after CABG, there were no significant differences in long-term cognitive decline between patients who had undergone CABG surgery and nonsurgical controls with diagnosed coronary artery disease. 14 Although these observations are of empirical importance to the phenomenon of cognitive decline, they have not identified factors that influence recovery from cognitive injury after cardiac surgery. Understanding the unique factors that contribute to recovery after postoperative cognitive injury may expedite overall recovery, inform risk stratification allowing for improved patient-family education, advance prevention models, and generate implications for treatment, thereby increasing the quality of life for cardiac surgical patients. We therefore sought to identify predictors associated with a return to baseline levels of cognitive performance after initial postoperative injury.
Methods
Study Population and Procedure
After IRB approval, 281 patients undergoing elective cardiac surgery (CABG, Valve, or CABG + Valve) with cardiopulmonary bypass from February 2000 to August 2009 who demonstrated cognitive decline 6 weeks after surgery in prospective cognitive trials4, 10, 12, 15, 16 and had complete data at baseline, 6-weeks, and 1-year assessments were retrospectively evaluated. The requirement for written informed consent was waived by the IRB. Patients were excluded from the parent trials if they presented with characteristics known to have confounding effects on cognition including a history of symptomatic cerebrovascular disease with residual deficit, psychiatric illness (any clinical diagnoses requiring therapy), hepatic insufficiency (liver function tests > 1. 5 times the upper limit of normal), and renal insufficiency (creatinine levels > 2 mg/dL), and who were unable to read and thus unable to complete the cognitive testing or who scored < 24 on a baseline Mini Mental State examination. Patients who experienced an adverse postoperative event, such as a subsequent cardiac event were also excluded in order to create a more homogenous population and to isolate the effect of baseline characteristics on 1-year cognitive functioning. Of note, patients suffering from depression after surgery were not excluded.
Measures
Neurocognitive Assessment
To evaluate cognitive function, trained psychometricians administered a neurocognitive test battery at baseline (1. 61 ± 1. 74 days before surgery), 6 weeks, and 1 year after surgery including the Short story module of the Randt Memory Test, a reliable measurement of discourse memory (immediate and delayed) and oral language comprehension;17 the Modified Visual Reproduction Test from the Wechsler Memory Scale on which patients are required to reproduce from memory several geometric shapes both immediately and after a 30-minute delay, testing for short- and long-term figural memory;18 the Digit Span subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS-R) examination, a measure of short-term auditory memory and attention that calls on subjects to repeat a series of digits that have been orally presented to them both forward and, in an independent test, in reverse order;18 the Digit Symbol subtest of the WAIS-R, an evaluation of psychomotor processing speed, in which number-symbol pairs are transcribed under timed conditions;18 and the Trail Making Test Part B, a timed test in which patients connect alternately letters and numbers in order to assess processing speed, attention, and mental flexibility. 19
Quality of Life Testing
To identify quality-of-life outcomes, the following assessments were also administered at baseline, 6 weeks, and 1 year after surgery:
The Duke Activity Status Index (DASI). 20 A 12-item instrument designed specifically to evaluate functional status and physical capabilities in cardiovascular populations. Limitations experienced by the patients during household tasks, personal care, leisure activities, sexual function, and ambulation were reported on a 4-point Likert scale.
The Duke Older Americans Resources and Services Procedures- Instrumental Activities of Daily Living (OARS-IADL). 21 Six IADL items from the OARS are used to measure patients’ ability to perform important daily self-care activities (e. g. “Could you prepare your own meals?” “Could you do errands, such as shopping for groceries or household necessities?”). Higher scores indicate increasing difficulty in engaging in daily activities.
The Medical Outcomes Study 36-item Short Form Health Survey (SF-36). A survey of health-related limitations and general health conditions used to assess overall health status. 22 Two scales were used: General Health (one item) and Work Activities (four items). Higher Work Activities scores indicate more health-related difficulties.
A social activities measure that indicates degree of social interaction (e. g. “About how often do you visit with friends and relatives?”), with lower scores indicating more social activity.
A symptoms limitations checklist on which patients were asked how often various symptoms (e. g. angina, shortness of breath, arthritis, etc. ) restricted daily activities. 23 Higher scores signified greater limitations.
The Center for Epidemiological Studies Depression Scale. 24 Clinically significant depression is indicated by a score of 16 or higher.
The State-Trait Anxiety Inventory. 25 A 20-item instrument used to measure anxiety on which patients are asked how frequently they experienced a particular symptom (e. g. “I feel worried” “I feel nervous”), on a 4-point Likert scale ranging from “not at all” to “very much so.”
The Perceived Social Support Scale. An assessment of global social support on which patients are asked how often various types of support are available to them. 26
The Cognitive Difficulties Scale. An instrument (based on a 5-point Likert scale) of self-perceived cognitive deficits in memory, concentration, attention, and psychomotor coordination. 27
Statistical Analysis
To account for the correlation among cognitive test scores, baseline raw test scores were subjected to a factor analysis as previously described2. To maintain consistent factor definitions across time, follow-up scores were calculated using weights resulting from this baseline analysis. Analysis yielded independent scores representing 4 cognitive domains: 1) verbal memory and language comprehension; 2) attention, psychomotor processing speed, and concentration; 3) abstraction and visuospatial orientation; and 4) figural memory.
To quantify overall neurocognitive function a composite cognitive index score (CI) was calculated as a mean of the four domain scores. This unified index score has been shown to be a stronger correlate of quality-of-life outcomes than the individual domain scores. 8 Patients who showed any decline in CI score from baseline to 6 weeks were considered “decliners” and comprised the analysis dataset. The outcome variable cognitive recovery was then defined dichotomously as 1-year CI ≥baseline CI. The association between cognitive recovery at 1 year and potential predictors including patient characteristics, quality-of-life factors, comorbidities, medications, and intra operative variables was assessed with chi-square tests for categorical variables and t-tests for continuous variables, followed by multivariable logistic regression modeling. Based on previous findings demonstrating significant associations with neurocognitive outcomes, three variables (age, years of education, and baseline cognition) were prespecified for inclusion in the model. 2, 9 To avoid model overfitting, based on the number of outcomes in our sample we selected the 7 additional variables with the lowest univariate p-values to incorporate into the multivariable modeling. Nonsignificant variables were individually dropped from the multivariable models until only significant variables remained; p<0. 05 was considered significant. Model fit and discrimination were evaluated using the Hosmer-Lemeshow goodness-of-fit test and the area under the receiver operating characteristic curve (c-index).
In secondary analysis, continuous change between 6 weeks and 1 year (rather than the dichotomous variable based on a return to baseline) was assessed using multivariable linear regression. Similarly, we assessed cognitive decline at 6 weeks as a 1 standard deviation or more decline in at least 1 of the 4 cognitive domains with cognitive recovery defined as a return to baseline at 1 year.
Results
Of the 281 patients who had demonstrated a decline in CI from baseline to 6 weeks after surgery, 52 patients were excluded due to 54 major cardiac events in the first year (10 cardiac surgeries and 44 deaths). Of the 229 “decliners” in our final analysis dataset, 178 underwent CABG surgery, 32 had CABG + valve surgery, and 19 had valve surgery alone. Forty-five percent (95% CI: 38. 5 – 51. 5) of the patients (103/229) experienced cognitive recovery while 55%(126/229; 95% CI: 48. 5 – 61. 5) remained below baseline at 1 year assessments. With regard to age, gender, comorbidities, surgical characteristics, statin use, depression, anxiety, marital status, and level of social support, no significant differences were observed between those patients who showed cognitive recovery and those who did not (Table 1).
Table 1.
Patient Characteristics and Cognitive Improvement.
| Not improved (N =126) |
Improved (N =103) |
P value | |
|---|---|---|---|
| Age in years (SD) | 67.6 (10.3) | 65.4 (9.30) | 0.09 |
| Gender (% female) | 31 | 30 | 0.88 |
| Race (% Caucasian) | 79 | 89 | 0.04 |
| Years of education (SD) | 12.6 (3.3) | 13.1 (2.1) | 0.26 |
| Weight in kg (SD) | 81.5 (14.8) | 83.3 (17.1) | 0.40 |
| BMI in kg/m2(SD) | 27.6 (4.5) | 25.6 (5.6) | 0.14 |
| Euroscore (SD) | 6.17 (3.26) | 5.68 (3.03) | 0.31 |
| Hannan comorbidity score (SD) | 0.01 (0.01) | 0.02 (0.03) | 0.47 |
| Diabetes (%) | 29.4 | 27.2 | 0.72 |
| Hypertension (%) | 72.2 | 67.0 | 0.39 |
| Prior smoker (%) | 61.9 | 60.0 | 0.78 |
| COPD (%) | 5.6 | 6.8 | 0.70 |
| LVEF (SD) | 52 (13) | 53 (12) | 0.69 |
| CABG surgery | 77.0 | 78.6 | 0.76 |
| Valve surgery | 14.3 | 13.6 | 0.88 |
| CABG + valve surgery | 8.7 | 7.8 | 0.79 |
| Number of grafts (SD) | 2.8 (1.3) | 2.9 (1.3) | 0.64 |
| CPB time in minutes (SD) | 129.6 (50.5) | 143.7 (76.5) | 0.10 |
| Postopatrial fibrillation (%) | 37.3 | 37.9 | 0.93 |
| Working status (% employed) | 11.1 | 16.5 | 0.24 |
| Marital status (% married) | 77 | 76 | 0.89 |
| Living arrangement (not alone) | 77 | 74 | 0.57 |
| CES-D at baseline (SD) | 11.9 (9.3) | 11.0 (7.0) | 0.56 |
| CES-D at 6 weeks (SD) | 13.3 (10.3) | 13.0 (9.2) | 0.83 |
| Social support score at baseline (SD) | 85.0 (13.1) | 84.6 (13.0) | 0.80 |
| Social support score at 6 weeks (SD) | 81.9 (14.8) | 81.2 (16.8) | 0.86 |
| STAI at baseline (SD) | 37.1 (12.5) | 36.6 (12.4) | 0.86 |
| STAI at 6 weeks (SD) | 34.7 (13.9) | 34.2 (12.2) | 0.82 |
| Optimism at baseline (SD) | 1.4 (0.6) | 1.2 (0.4) | 0.04 |
| Optimism at 6 weeks (SD) | 1.3 (0.6) | 1.4 (0.6) | 0.76 |
| IADL at baseline (SD) | 6.6 (1.6) | 6.9 (1.9) | 0.33 |
| IADL at 6 weeks (SD) | 10.8 (5.3) | 8.7 (3.8) | 0.006 |
| DASI at baseline (SD) | 19.6 (15.4) | 18.1 (15.6) | 0.61 |
| DASI at 6 weeks (SD) | 8.8 (10.6) | 8.9 (8.9) | 0.96 |
| Symptom limitation at baseline (SD) | 14.8 (4.4) | 14.6 (4.8) | 0.79 |
| Symptom limitation at 6 weeks (SD) | 14.8 (5.3) | 14.2 (4.3) | 0.43 |
| Cognitive difficulties score at baseline (SD) | 77.0 (19.5) | 76.9 (20.3) | 0.97 |
| Baseline cognitive score (SD) | 0.11 (0.49) | 0.06 (0.46) | 0.36 |
| Cognitive change at 6 weeks (SD) | −0.23 (0.23) | −0.16 (0.15) | 0.01 |
| Discharge statins (%) | 47 | 42 | 0.44 |
*BMI, body mass index; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; CABG = coronary artery bypass graft surgery; CPB, cardiopulmonary bypass; CES-D, Center for Epidemiological Studies – depression Scale; STAI, Spielberger State Anxiety Inventory; IADL, Instrumental Activities of Daily Living; DASI, Duke Activity Status Index; SD, standard deviation. On the DASI and the Social support scores, a higher score is better, whereas for the IADL, CES-D, STAI, optimism, symptom limitations, and cognitive difficulties, a lower score is better. Univariate p values were calculated with chi-square tests of association for categorical variables and t-tests for continuous variables.
Predictors of Change in Neurocognitive Function
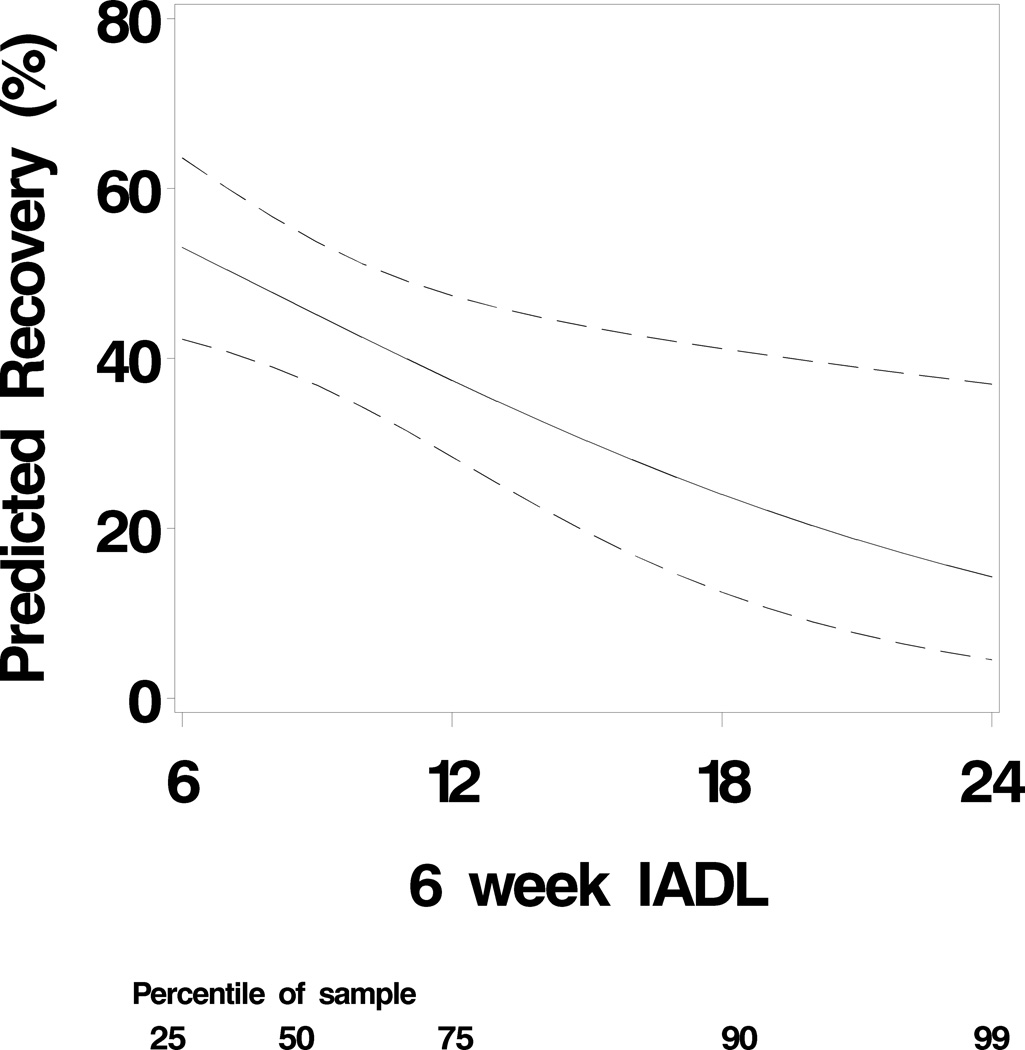
Patients who demonstrated cognitive recovery one year after surgery were more likely to be Caucasian (89% vs. 79%; p = 0. 04) and declined less between baseline and 6 weeks than those who did not recover at one year (−0. 16±0. 15vs. −0. 23±0. 23; p = 0. 01). In addition, cognitive improvement at 1-year post surgery was related to lower IADL scores (higher IADL scores reflect worse daily functioning) 6 weeks after surgery (8. 7±3. 8 vs. 10. 8±5. 3, p = 0. 006) (Table 1). Multivariable logistic regression revealed four independent predictors of cognitive recovery: years of education (p < 0. 001), baseline CI (p = 0. 02), amount of cognitive decline between assessments at baseline and 6 weeks after surgery (p = 0. 004), and greater functional performance (lower IADL scores) at 6 weeks postoperatively (p=0. 02) (Table 2). The c-index for this model was 0. 77, indicating moderately good discrimination of subjects. The Hosmer-Lemeshow test chi-square value was 11.73, p=0. 16, indicating that the observed event rates match the expected event rates. By examining the influence diagnostic plots, we found that there are 3 data points which are not well accounted for by the model, and which may exert a large effect on goodness of fit. To determine if our significant association was affected by these data points, we conducted a follow-up analysis excluding these data points. The model c-index improved to 0. 79 and the p-value for IADL to p=0. 002. The association between IADL and probability of recovery is depicted in Figure 1.
Table 2.
Predictors of cognitive recovery after cardiac surgery. More years of education, lower baseline cognitive index, less decline in cognitive index at 6 weeks, and lower Instrumental Activities of Daily Living (IADL) scores (better performance) at 6 weeks predicts cognitive recovery at 1 year.
| Variable | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Years of education (per year) | 1.332 | 1.131–1.569 | < 0.001 |
| Baseline cognitive index (per 0.01 change) |
0.987 | 0.976–0.998 | 0.018 |
| Decline in cognitive index at 6 weeks (per 0.01 change) |
1.044 | 1.014–1.075 | 0.004 |
| IADL score at 6 weeks (per 1 unit change) |
0.891 | 0.810–0.981 | 0.018 |
*N=229; multivariable logistic regression modeling was developed to predict cognitive recovery. Based on a prior study demonstrating their significance in cognitive outcomes, three variables (age, years of education, and change in cognitive index at 6 weeks) were predetermined for inclusion in the model. To avoid model overfitting, only 7 additional variables with the lowest univariate p-values were incorporated into the multivariable modeling.
Figure 1.
The association between Instrumental Activities of Daily Living at 6 weeks and predicted probability of cognitive recovery. The predicted probability of recovery is derived from the multivariable logistic regression model, which estimates a greater likelihood of cognitive recovery with increasing IADL scores after adjusting for years of education, baseline function, and amount of decline at 6 weeks. That is, a patient with a higher IADL score is more likely to recover cognitive function than a patient with a lower IADL score, even if the two patients have the same baseline score, the same education level, and experienced the same amount of decline. Lower IADL scores indicate enhanced function and the dashed lines represent 95% confidence intervals. The figure footnote indicates the percentage of the sample which had the corresponding IADL score or a lower score; for example, 75% of the sample scored 12 or lower.
This association between IADL scores and cognitive recovery remained significantin the secondary analysis, in which cognitive improvement was assessed as a continuous outcome. IADL also remained a predictor of return to baseline at 1 year when cognitive decline was defined as a 1 standard deviation or more decline in at least 1 of the 4 cognitive domains at 6 weeks. Cognitive recovery at one year (return to baseline) using this 1 standard deviation definition was seen in 40% (95% CI: 31. 1 – 48. 6). Post hoc analysis incorporating the patients who had been excluded for a subsequent cardiac event showed that the adverse event itself was not a significant predictor of cognitive recovery and did not alter the predictive capacity of the other variables in the model.
Discussion
To our knowledge, this is the first study to identify factors associated with cognitive improvement after early cognitive decline in patients undergoing cardiac surgery. We found that almost one-half of the patients who exhibited cognitive decline 6 weeks after cardiac surgery demonstrated cognitive recovery by 1 year. We also identified four predictors that were associated with one-year recovery including higher education level, baseline cognitive performance, less cognitive decline between baseline and 6 weeks, and lower IADL scores at 6 weeks (i. e. better functional performance).
In support of previous studies which showed an association between level of education and cognitive performance, 28–39 we found a relationship between level of education and cognitive recovery after an initial decline. Possible explanations for this effect may lie in education’s influence over important environmental and neurological factors. Less educational attainment may put one at greater risk for cognitive deterioration, decreasing the likelihood of cognitive recovery through its association with other potential risk factors such as nutritional deficiencies, less health care access, psychiatric illness, or exposure to increased occupational hazards. 29,30 Education may also directly influence brain structures early in development, resulting in increased synapses or vascularization and leading to improved lifetime cognitive function and brain plasticity. 28, 30 , 31, 34 Alternatively, education may lead to a reserve of cognitive capacity that does not alter vulnerability to decline, but delays the appearance of clinical symptoms and/or compensates for early cognitive injury.40
Cognitive performance at 6 weeks also was a significant predictor of cognitive recovery at 1 year. Similar to quantity of education, heightened cognitive function can result in increased neuronal count and network capacity, efficiency, or compensation that may decrease risk for future cognitive impairment and increase ability to recover from cognitive damage. 31, 38, 40 Additionally, increases in cognitive function reflect lifestyle patterns that promote stimulation and recovery, thereby preventing or delaying cognitive decline. Epidemiologic studies have found slower rates of decline among those who routinely engage in more cognitively demanding tasks such as reading books and newspapers, playing cards, watching television, and solving puzzles than those who lead less cognitively engaged lifestyles. 31, 40
Our finding that heightened IADL performance 6 weeks after surgery is associated with the likelihood of cognitive recovery at 1year after initial decline supports the previous findings of an association between functional status and cognition. 41–48 IADL are complex, adaptive behaviors that facilitate independent living, reflect on one’s functional capacity, and include the following activities: meal preparation, telephone use, medication management, transportation, financial management, housekeeping duties, and shopping. 42, 46, 48 Functional impairment is first expressed among instrumental activities44, 46, 49 and is an essential feature for the diagnosis of dementia. 44 In other words, functional disability as shown by poor performance on IADL items separates individuals with severe cognitive dysfunction from those with moderate to no cognitive deficit. The functional changes associated with such cognitive decline have repercussions expanding from the individual (e. g. decreased quality of life, increased dysphoria and low self-efficacy) to the communal level (e. g. premature institutionalization, increased caregiver burden, and higher health care costs). 50 The association between increased functional status in the early postoperative period and the likelihood of cognitive recovery in the later postoperative stages suggests that interventions which encourage the performance of instrumental activities immediately after surgery may result in improved cognitive performance.
The DASI, also a measure of functional status, was not found to significantly predict cognitive recovery. Unlike the IADL, which focuses on daily activities that involve planning or increased cognitive control (e. g. can you handle your own money, pay bills, write checks, balance checkbook?), the DASI concentrates more on the completion of more physically demanding or mechanical activities (e. g. can you climb a flight of stairs or walk up a hill?). 51 Both instruments assess functional status and assess ability to perform routine activities; however, the IADL approaches functional capacity from a cognitive framework, and IADL impairment reflects difficulty in organizing, initiating, and performing actions, whereas DASI impairment suggests increased physical limitations, possibly as a result of poor cardiac function. Because the IADL inherently addresses functional capacity with a cognitive component, this distinction may help explain why IADL scores predict future cognitive recovery while DASI scores do not.
Similar to the DASI, the two SF-36 subscales were found to have no significant predictive relationship to cognitive recovery. The SF-36 and the IADL, both measures of quality of life, differ in test objective and agenda. The SF-36 was designed as a health economics tool to assess quality-adjusted life years, a pivotal variable in determining the cost effectiveness of treatment. The SF-36 is foremost a means of monitoring and comparing disease burden and not aptitude, which is the measured intent of the IADL. As with the DASI, poor performance on the SF-36 indicates functional limitation at the level of overall physical condition and not cognitive ability. The inability of both the DASI and the SF-36 to significantly predict cognitive recovery highlights the IADL as a distinct, cognitively focused quality-of-life measure important in assessing the course of postoperative cognitive rehabilitation.
Prior studies have shown depressive symptoms to be independently associated with cognitive decline. 48, 52 However, we were unable to demonstrate an association between depression and social support systems and cognitive recovery at 1 year. Depression and social support systems often occur as byproducts of functional status52 and are therefore less robust predictors of enhanced cognition in comparison to functional status itself.
Our study is limited by the observational and exploratory design; thus our findings should be considered the first step in identifying the factors that may lead to recovered cognitive functioning after an initial postoperative decline. Our study could also be criticized for our definition of cognitive recovery as a return to baseline, which is a dichotomous variable. For this reason, in a secondary supportive analysis, we examined cognitive recovery as a continuous outcome and found that IADL score remains a significant predictor. Furthermore, from the point of view of the patient, a return to baseline functioning is an important landmark with numerous benefits and quality-of-life implications. Another consideration in the interpretation of our study is the inclusion of subjects with any decline from baseline to 6 weeks, rather than a prespecified unit of decline, such as a standard deviation. Although there is little consensus in the literature about how to define POCD and a standard deviation-based definition of decline is often challenged as being arbitrary, we do acknowledge that this decision leaves us unable to fully discount the effects of measurement error or regression to the mean. To further address this concern, we assessed cognitive decline at 6 weeks as a 1 standard deviation or more decline in at least 1 of the 4 cognitive domains and found that 6-week IADL is still a predictor of return to baseline at 1 year. In addition to the inclusion of the IADL in the study battery, future studies should ideally include additional measures of functional status to provide a more comprehensive understanding of the components of functional aptitude and how they relate to cognitive recovery. Future studies should include also include control groups to elucidate the effect of surgery and coronary heart disease on this relationship.
To summarize, in this first study to examine factors predicting recovery from POCD, 45% of patients who experience cognitive decline at 6 weeks return to baseline cognitive function by 1 year. Higher educational levels, baseline cognitive performance, smaller cognitive decline at 6 weeks, and lower IADL scores (better functional performance) at 6 weeks are associated with cognitive recovery 1 year after cardiac surgery. Of note, age, depression, and social support systems do not appear to modulate this recovery. Whether increasing patients' functional capacity through increased occupational activity (e. g. acts of self-care, work, and leisure) before a surgical admission (as opposed to postoperatively) would allow the individual to retain a higher level of functional capacity over their entire perioperative experience with resulting increases in quality of life and cognitive recovery should be further examined.
Acknowledgments
Funding: Supported in part by grants #AG09663 (MFN), #HL54316 (MFN), #HL069081 (MFN) and #M01-RR-30 (Duke Clinical Research Centers Program) from the National Institutes of Health; grants #9951185U (JPM), #9970128N (MFN) from the American Heart Association; and by the Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Department of Anesthesiology, Duke University Medical Center, Durham, NC, USA.
Appendix 1
Neurologic Outcome Research Group of the Duke Heart Center
Director: Joseph P. Mathew, M.D., Co-Director: James A. Blumenthal, Ph.D.
Anesthesiology: Manuel A. Fontes, M.D., Miklos D. Kertai, M.D., Frederick W. Lombard, M.D., Joseph P. Mathew, M.D., David L. McDonagh, M.D., Terri G. Monk, M.D., Mark F. Newman, M.D, Mihai V. Podgoreanu, M.D., Mark Stafford-Smith, M.D., Madhav Swaminathan, M.D., David S. Warner, M.D., Bonita L. Funk, R.N., CCRP, Narai Balajonda, M.D., Roger L. Hall, A.A.S., Tiffany Bisanar, R.N., B.S.N., Karen L. Clemmons, Monique T. Fontes, B.A., Kathleen Lane, R.N., B.S.N., Yi-Ju Li, Ph.D, Jacquelane F. Libed, M.D., Greg Pecora, B.A., Barbara Phillips-Bute, Ph.D., Prometheus T. Solon, M.D., Yanne Toulgoat-Dubois, B.A., Peter Waweru, CCRP, William D. White, M.P.H.
Behavioral Medicine: Michael A. Babyak, Ph.D., James A. Blumenthal, Ph.D., Jeffrey N. Browndyke, Ph.D., Kathleen A. Welsh-Bohmer, Ph.D.
Cardiology:Daniel B. Mark, M.D., M.P.H., Michael H. Sketch, Jr., M.D.
Neurology: Ellen R. Bennett, Ph.D, Carmelo Graffagnino, M.D., Daniel T. Laskowitz, M.D., Warren J. Strittmatter, M.D.
Perfusion Services: Kevin Collins, B.S., C.C.P., Greg Smigla, B.S., C.C.P., Ian Shearer, B.S., C.C.P.
Surgery: Mark F. Berry, M.D., Thomas A. D'Amico, M.D., R. Duane Davis, M.D., Jeffrey G. Gaca, M.D., Donald D. Glower, M.D., R. David Harpole, M.D., G. Chad Hughes, M.D., Robert D.B. Jaquiss, M.D., Shu S. Lin, M.D., Andrew J. Lodge, M.D., Carmelo A. Milano, M.D., Mark W. Onaitis, M.D., Jacob N. Schroeder, M.D., Peter K. Smith, M.D., Betty C. Tong, M.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Investigators who participated from The Neurologic Outcome Research Group of the Duke Heart Center are listed at the end of the article in Appendix 1.
This report was previously presented, in part, at the 2010 Society of Cardiovascular Anesthesiologists
DISCLOSURES:
Name: Monique T. Fontes, BA
Contribution: This author helped analyze the data and write the manuscript
Attestation: Monique T. Fontes reviewed the analysis of the data and approved the final manuscript
Name: R. Cameron Swift, MD
Contribution: This author helped design the study, analyze the data, and write the manuscript
Attestation: R. Cameron Swift has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Barbara Phillips-Bute, PhD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Barbara Phillips-Bute has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Mihai V. Podgoreanu, MD
Contribution: This author helped write the manuscript
Attestation: Mihai V. Podgoreanu approved the final manuscript
Name: Mark Stafford-Smith, MD
Contribution: This author helped design the study and write the manuscript
Attestation: Mark Stafford-Smith reviewed the analysis of the data and approved the final manuscript
Name: Mark F. Newman, MD
Contribution: This author helped write the manuscript
Attestation: Mark F. Newman approved the final manuscript
Name: Joseph P. Mathew, MD, MHSc
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Joseph P. Mathew has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Contributor Information
Monique T. Fontes, Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Duke University Medical Center, Durham, North Carolina.
R. Cameron Swift, Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Duke University Medical Center, Durham, North Carolina.
Barbara Phillips-Bute, Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Duke University Medical Center, Durham, North Carolina.
Mihai V. Podgoreanu, Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Duke University Medical Center, Durham, North Carolina.
Mark Stafford-Smith, Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Duke University Medical Center, Durham, North Carolina.
Mark F. Newman, Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Duke University Medical Center, Durham, North Carolina.
Joseph P. Mathew, Division of Cardiothoracic Anesthesiology and Critical Care Medicine, Duke University Medical Center, Durham, North Carolina.
References
- 1.Newman MF, Grocott HP, Mathew JP, William WD, Landolfo K, Reves JG, Laskowitz DT, Mark DB, Blumenthal JA. Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke. 2001;32:2874–2881. doi: 10.1161/hs1201.099803. [DOI] [PubMed] [Google Scholar]
- 2.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott HP, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 3.Hogue CW, Jr, Palin CA, Arrowsmith JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103:21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 4.Mathew JP, Mackensen GB, Phillips-Bute B, Grocott HP, Glower DD, Laskowitz DT, Blumenthal JA, Newman MF. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40:880–887. doi: 10.1161/STROKEAHA.108.531236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–1185. doi: 10.1213/ANE.0b013e318215217e. [DOI] [PubMed] [Google Scholar]
- 6.Djaiani G, Fedorko L, Borger MA, Green R, Carroll J, Marcon M, Karski J. Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation. 2007;116:1888–1895. doi: 10.1161/CIRCULATIONAHA.107.698001. [DOI] [PubMed] [Google Scholar]
- 7.Hammon JW, Stump DA, Butterworth JF, Moody DM, Rorie K, Deal DD, Kincaid EH, Oaks TE, Kon ND. Coronary artery bypass grafting with single cross-clamp results in fewer persistent neuropsychological deficits than multiple clamp or off-pump coronary artery bypass grafting. Ann Thorac Surg. 2007;84:1174–1178. doi: 10.1016/j.athoracsur.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 8.Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, Mark DB, Newman MF. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med. 2006;68:365–375. doi: 10.1097/01.psy.0000221272.77984.e2. [DOI] [PubMed] [Google Scholar]
- 9.Newman MF, Groughwell ND, Blumenthal JA, Lowry E, White WD, Spillane W, Davis RD, Glower DD, Smith LR, Mahanna EP, Reves JG. Predictors of cognitive decline after cardiac operation. Ann Thorac Surg. 1995;59:1326–1330. doi: 10.1016/0003-4975(95)00076-w. [DOI] [PubMed] [Google Scholar]
- 10.Mathew JP, Podgoreanu MV, Grocott HP, White WD, Morris RW, Stafford-Smith M, Mackensen GB, Rinder CS, Blumenthal JA, Schwinn DA, Newman MF. Genetic variants in P-selectin and C-reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49:1934–1942. doi: 10.1016/j.jacc.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 11.Lombard FW, Mathew JP. Neurocognitive dysfunction following cardiac surgery. Semin Cardiothorac Vasc Anesth. 2010;14:102–110. doi: 10.1177/1089253210371519. [DOI] [PubMed] [Google Scholar]
- 12.Mathew JP, Mackensen GB, Phillips-Bute B, Stafford-Smith M, Podgoreanu MV, Grocott HP, Hill SE, Smith PK, Blumenthal JA, Reves JG, Newman MF. Effects of extreme hemodilution during cardiac surgery on cognitive function in the elderly. Anesthesiology. 2007;107:577–584. doi: 10.1097/01.anes.0000281896.07256.71. [DOI] [PubMed] [Google Scholar]
- 13.Puskas F, Grocott HP, White WD, Mathew JP, Newman MF, Bar-Yosef S. Intraoperative hyperglycemia and cognitive decline after CABG. Ann Thorac Surg. 2007;84:1467–1473. doi: 10.1016/j.athoracsur.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Selnes OA, Grega MA, Borowicz LM, Royall RM, McKhann GM, Baumgartner WA. Cognitive changes with coronary artery bypass graft patients and nonsurgical controls. Ann Thorac Surg. 2003;75:1377–1384. doi: 10.1016/s0003-4975(03)00021-3. [DOI] [PubMed] [Google Scholar]
- 15.Grigore AM, Mathew J, Grocott HP, Reves JG, Blumenthal JA, White WD, Smith PK, Jones RH, Kirchner JL, Mark DB, Newman MF. Prospective randomized trial of normothermic versus hypothermic cardiopulmonary bypass on cognitive function after coronary artery bypass graft surgery. Anesthesiology. 2001;95:1110–1119. doi: 10.1097/00000542-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Mathew JP, Rinder HM, Smith BR, Newman MF, Rinder CS. Transcerebral platelet activation after aortic cross-clamp release is linked to neurocognitive decline. Ann Thorac Surg. 2006;81:1644–1649. doi: 10.1016/j.athoracsur.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 17.Randt C, Brown E. Administration Manual: Randt Memory Test. New York: Life Sciences Associates; 1983. [Google Scholar]
- 18.Wechsler D. The Wechsler Adult Intelligence Scale Revised (Manual) San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- 19.Reitan R. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 20.Hlatky MA, Boineau RE, Higginbotham MB, Lee KL, Mark DB, Califf RM, Cobb FR, Pryor DB. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 21.Fillenbaum GG. Multidimensional functional assessment of older adults (the Duke Older Americans Resources and Services Procedures) Hillsdale, NJ: Erlbaum Associates; 1988. [Google Scholar]
- 22.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric; 2000. [Google Scholar]
- 23.Axelrad K. Locus of Control and Causal Attributions as They Relate to Expectations for Coping with a Heart Attack. Los Angeles, CA: California School of Professional Psychology; 1981. [Google Scholar]
- 24.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Appl Psychol Measures. 1997;1:385–401. [Google Scholar]
- 25.Spielberger CG. State-Trait Anxiety Inventory Manual. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 26.Blumenthal JA, Barefoot J, Williams R, Haney T, Zimet G. Social support, type A behavior, and coronary artery disease. Psychosom Med. 1987;49:331–340. doi: 10.1097/00006842-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 27.McNair DM, Kahn RJ. In: Self-assessment of cognitive deficits, Assessment in geriatric psychopharmacology. Crook T, Ferris S, Bartus R, editors. New Cannan, CT: Mark Powley Associates; 1984. pp. 137–143. [Google Scholar]
- 28.Albert MS. How does education affect cognitive function? Ann Epidemiol. 1995;5:76–78. doi: 10.1016/1047-2797(94)00044-t. [DOI] [PubMed] [Google Scholar]
- 29.Farmer RO. Education and change in cognitive function. Ann Epidemiol. 1995;5:76–78. doi: 10.1016/1047-2797(94)00047-w. [DOI] [PubMed] [Google Scholar]
- 30.Friedland RO. Epidemiology, education, and the ecology of Alzheimer's disease. Neurology. 1993;43:246–249. doi: 10.1212/wnl.43.2.246. [DOI] [PubMed] [Google Scholar]
- 31.McDowell I, Xi G, Lindsay J, Tierney M. Mapping the connections between education and dementia. J Clin Exp Neuropsychol. 207(29):127–141. doi: 10.1080/13803390600582420. [DOI] [PubMed] [Google Scholar]
- 32.Mortimer J. Do Psychosocial Risk Factors Contribute to Alzheimer's disease? In: Henderson AS, Henderson JH, editors. Etiology of dementia of Alzheimer's type. New York, NY: Wile & Sons; 1988. [Google Scholar]
- 33.Qui C, Backman L, Wingbald B, Aguero-Torres H, Fratiglioni L. The influence of education on clinically diagnosed dementia incidence and mortality data from the Kungsholmen Project. Arch Neurol. 2001;58:2034–2039. doi: 10.1001/archneur.58.12.2034. [DOI] [PubMed] [Google Scholar]
- 34.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 35.Richards M, Sacker A. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol. 2003;25:614–624. doi: 10.1076/jcen.25.5.614.14581. [DOI] [PubMed] [Google Scholar]
- 36.Richards M, Sacker A. Is education causal? Yes. Int J Epidemiol. 2011;40:516–518. doi: 10.1093/ije/dyq166. [DOI] [PubMed] [Google Scholar]
- 37.Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, Brown TR, DeCarli C, Stern Y. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging. 2011;32:1588–1598. doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, Flynn J, Steffener J, Brown T. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex. 2008;18:959–967. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall CB, Lipton RB, Silwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell-McGinty S, Podell K, Franzen M, Baird AD, Williams MJ. Standard measures of executive function in predicting instrumental activities of daily living in older adults. Int J Geriatr Psychiatry. 2002;17:828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- 42.Cahn-Winer DA, Malloy PF, Bole PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly indviduals. Clin Neuropsychol. 2000;24:609–614. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- 43.Grisby J, Kaye K, Baxter J, Shetterly SM, Hamman RF. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. J Am Geriatr Soc. 1998;46:590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 44.Jefferson AL, Cahn-Winer DA, Boyle PA, RH P, Moser DJ, Gordon N, Cohen RA. Cognitive predictors of functional decline in vascular dementia. Int J Geriatr Psychiatry. 2006;21:752–754. doi: 10.1002/gps.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katz S. Assessing self maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–726. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 46.Kempen GI, Summerijer TP. The development of hierachial polychtomous ADL-IADL scale for noninstitutionalized elders. Gerontolgist. 1990;30:497–502. doi: 10.1093/geront/30.4.497. [DOI] [PubMed] [Google Scholar]
- 47.Lawton MP. Scales to measure competence in everyday activities. Psychopharmacol Bull. 1988;24:609–614. [PubMed] [Google Scholar]
- 48.Mehta KM, Yaffe K, Covinsky KE. Cognitive impairment, depressive symptoms, and functional decline in older people. J Am Geriatr Soc. 2002;50:1045–1050. doi: 10.1046/j.1532-5415.2002.50259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spector WD, Katz S, Murphy JB, Fulton JP. The hierarchical relationship between activities of daily living and instrumental activities of daily living. J Chronic Dis. 1987;40:481–489. doi: 10.1016/0021-9681(87)90004-x. [DOI] [PubMed] [Google Scholar]
- 50.Petersen RC, Smith GE, SC W, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 51.Parissis JT, Nikolaou M, Birmpa D, Farmakis D, Paraskevaidis I, Bistola V, Katsolulas T, Filippatos G, Kermastinos D. Clinical and prognostic value of Duke's activity status index along with plasma B-type natriuretic peptide levels in chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2009;103:73–75. doi: 10.1016/j.amjcard.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 52.Gallo JJ, Rabins PV. Depression without sadness: Alternative presentations of depression in late life. Am Fam Physician. 1999;60:820–826. [PubMed] [Google Scholar]