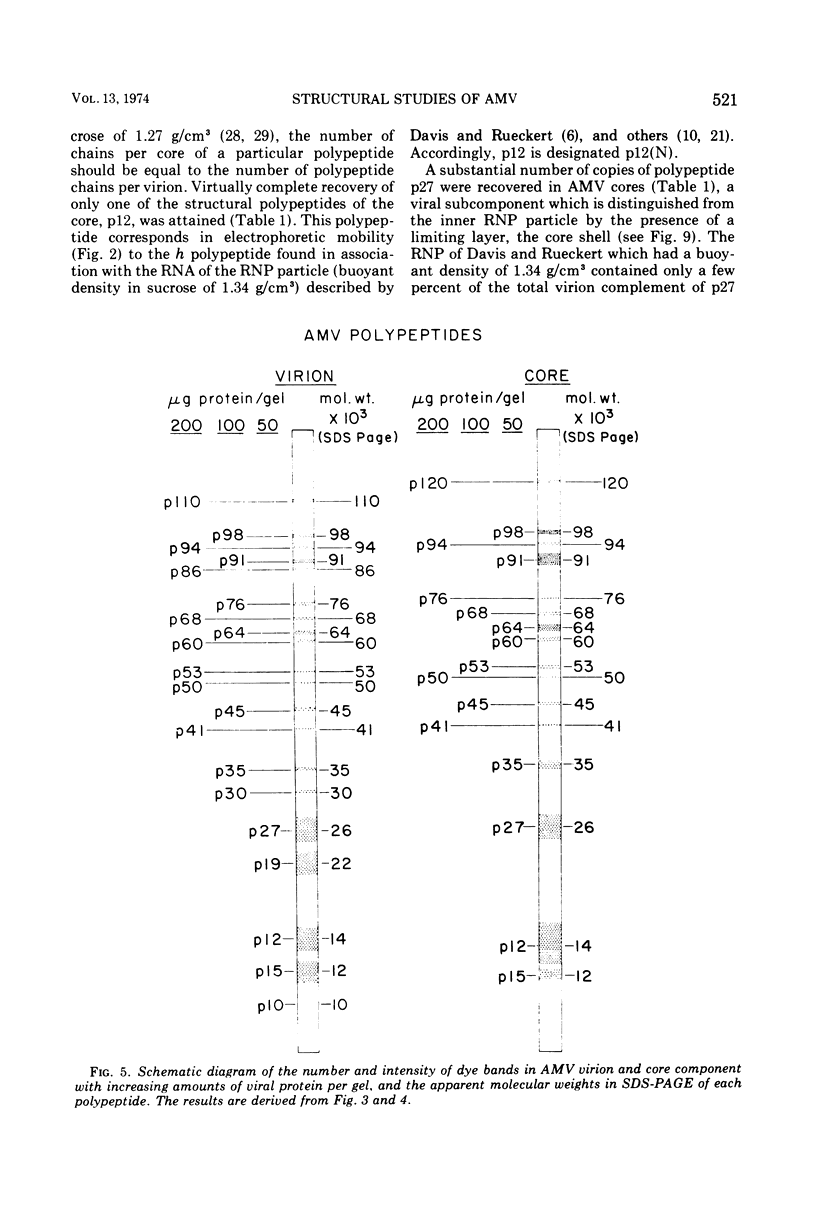
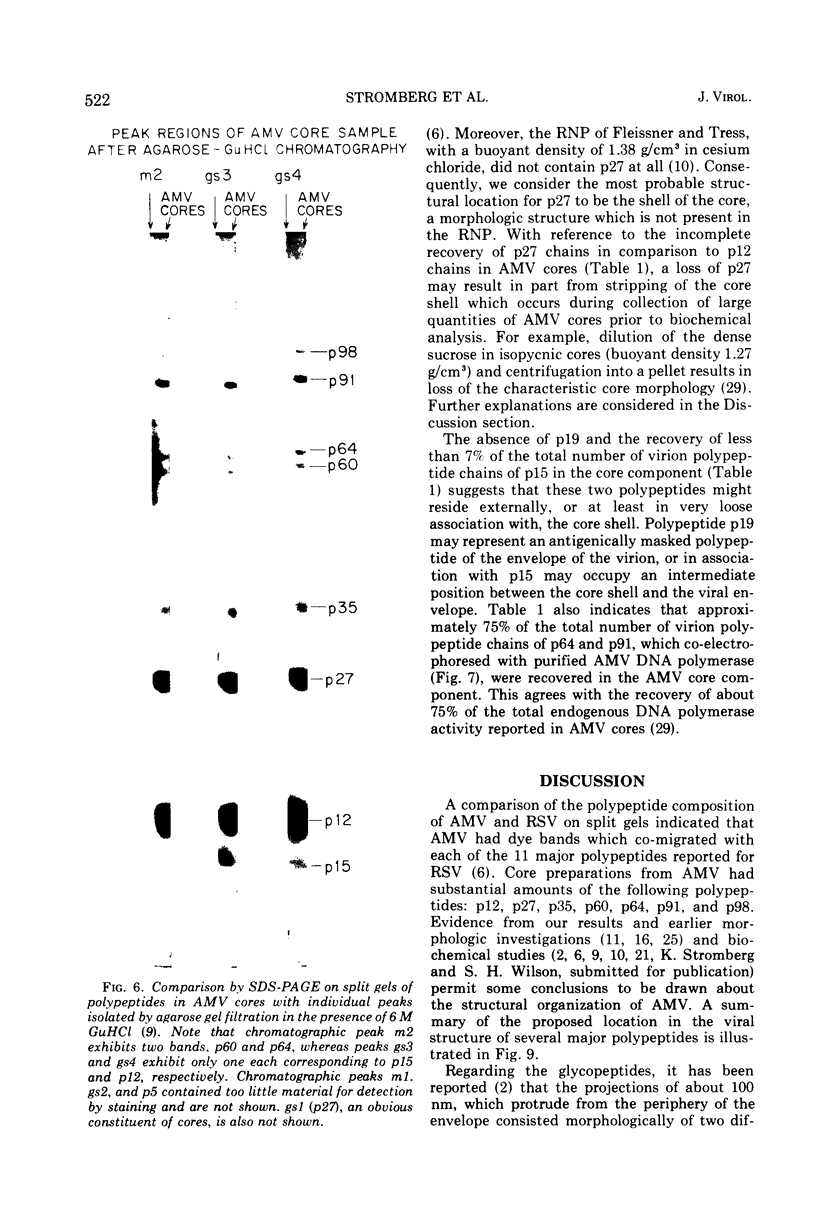
Abstract
Two different systems of dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in separate laboratories detected analogous patterns of dye bands in virions of avian myeloblastosis virus (AMV). At least 11 of the dye bands co-migrated with the major polypeptides reported in Rous sarcoma virus. Particles with the morphology of the AMV core component, obtained after exposure of AMV to the nonionic surfactant Sterox SL, contained major polypeptides p12, p27, p60, p64, p91, and p98. The polypeptide p12 has been previously shown to be the major constituent of the inner ribonucleoprotein (RNP) of the AMV core, and has been designated p12(N). Two RNP polypeptides, p64 and p91, co-electrophoresed with purified AMV DNA polymerase and have now been designated p64(P) and p91(P). The polypeptide p27 has been identified as a probable constituent of the core shell, and has accordingly now been designated p27(C). In comparison to virions of AMV, the AMV core component contained a greatly reduced amount of polypeptide p15 and appeared to lack a major polypeptide, p19. Consequently, these polypeptides may be associated either with the exterior of the core shell or the interior of the viral envelope. Glycopeptides were not detected in AMV cores, in agreement with earlier reports that they reside in external projections from the viral envelope.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolognesi D. P., Bauer H., Gelderblom H., Hüper G. Polypeptides of avian RNA tumor viruses. IV. Components of the viral envelope. Virology. 1972 Mar;47(3):551–566. doi: 10.1016/0042-6822(72)90545-4. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H. Polypeptides of avian RNA tumor viruses. 1. Isolation and physical and chemical analysis. Virology. 1970 Dec;42(4):1097–1112. doi: 10.1016/0042-6822(70)90357-0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Gelderblom H., Bauer H., Mölling K., Hüper G. Polypeptides of avian RNA tumor viruses. V. Analysis of the virus core. Virology. 1972 Mar;47(3):567–578. doi: 10.1016/0042-6822(72)90546-6. [DOI] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Comparison of Rous sarcoma virus-specific deoxyribonucleic acid polymerases in virions of Rous sarcoma virus and in Rous sarcoma virus-infected chicken cells. J Virol. 1971 May;7(5):625–634. doi: 10.1128/jvi.7.5.625-634.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Martin G. S., Vogt P. K. Glycoprotein components of avian and murine RNA tumor viruses. Virology. 1970 Aug;41(4):631–646. doi: 10.1016/0042-6822(70)90428-9. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner E., Tress E. Isolation of a ribonucleoprotein structure from oncornaviruses. J Virol. 1973 Dec;12(6):1612–1615. doi: 10.1128/jvi.12.6.1612-1615.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H., Bauer H., Bolognesi D. P., Frank H. Morphogenese und Aufbau von RNS-Tumorviren. Elektronenoptische Untersuchungen an Virus-Partikeln vom C-Typ. Zentralbl Bakteriol Orig A. 1972 May;220(1):79–90. [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacour F., Fourcade A., Verger C., Delain E. Coiled structure of the nucleocapsid of avian myeloblastosis virus. J Gen Virol. 1970 Oct;9(1):89–92. doi: 10.1099/0022-1317-9-1-89. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S. An electron microscope study of Rauscher leukemia virus. Virology. 1971 Nov;46(2):277–297. doi: 10.1016/0042-6822(71)90030-4. [DOI] [PubMed] [Google Scholar]
- Medappa K. C., McLean C., Rueckert R. R. On the structure of rhinovirus 1A. Virology. 1971 May;44(2):259–270. doi: 10.1016/0042-6822(71)90258-3. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Rifkin D. B., Compans R. W. Isolation and characterization of ribonucleoprotein substructures from Rous sarcoma virus. Virology. 1972 Oct;50(1):65–75. doi: 10.1016/0042-6822(72)90346-7. [DOI] [PubMed] [Google Scholar]
- Rifkin D., Compans R. W. Identification of the spike proteins of Rous sarcoma virus. Virology. 1971 Nov;46(2):485–489. doi: 10.1016/0042-6822(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Hung P., Robinson H. L., Ralph D. D. Proteins of avian tumor viruses with different coat antigens. J Virol. 1970 Nov;6(5):695–698. doi: 10.1128/jvi.6.5.695-698.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Robinson H. L. DNA polymerase in defective Rous sarcoma virus. Virology. 1971 May;44(2):457–462. doi: 10.1016/0042-6822(71)90278-9. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Nowinski R. C., Moore D. H. Helical nucleocapsid structure of the oncogenic ribonucleic acid viruses (oncornaviruses). J Virol. 1971 Oct;8(4):564–572. doi: 10.1128/jvi.8.4.564-572.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Litwack M. D., Desmukes B. Structural studies on avian myeloblastosis virus: rapid purification and quantitation. Proc Soc Exp Biol Med. 1972 Oct;141(1):215–221. doi: 10.3181/00379727-141-36745. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Litwack M. D. Structural studies of avian myeloblastosis virus: presence of transfer RNA in core component. Biochim Biophys Acta. 1973 Aug 24;319(2):140–152. doi: 10.1016/0005-2787(73)90005-1. [DOI] [PubMed] [Google Scholar]
- Stromberg K. Surface-active agents for isolation of the core component of avian myeloblastosis virus. J Virol. 1972 Apr;9(4):684–697. doi: 10.1128/jvi.9.4.684-697.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]










