Abstract
Aims
To investigate the relationships between tobacco dependence, biomarkers of nicotine and carcinogen exposure, and biomarkers of nicotine and carcinogen exposure per cigarette in Black and White smokers.
Design and participants
204 healthy Black (n=69) and White (n=135) smokers were enrolled in two clinical studies.
Measurement
Nicotine equivalents (nicotine and its metabolites), 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol (NNAL), and polycyclic aromatic hydrocarbon (PAH) metabolites were measured in urine. The Fagerström Test for Nicotine Dependence (FTND) and time to first cigarette (TFC) measured tobacco dependence.
Findings
Average TFC and FTND for Blacks and Whites were not significantly different. Urine NNAL and nicotine equivalents increased with increasing FTND in Whites but did not increase in Blacks (race x FTND interaction, both p<0.031). The interaction term was not significant for PAHs. An inverse relationship was seen between FTND and nicotine equivalents, NNAL, and PAH metabolites per cigarette in Blacks but remained flat in Whites (race x FTND interaction, all p≤0.039). Regardless of dependence (low dependence, TFC>15 minutes; high dependence, TFC≤15 minutes), FTND and TFC were not significantly correlated with urine nicotine equivalents and carcinogen exposure in Blacks. We found moderate correlations between FTND and TFC and nicotine equivalents and carcinogen exposure among Whites of low dependence and non-significant correlations among Whites of high dependence.
Conclusion
In the US, tobacco dependence measures were linearly related to nicotine intake and carcinogen exposure in White but not in Black smokers. The relationship between dependence measures and tobacco biomarkers in Black smokers regardless of level of dependence resembled highly dependent White smokers.
Keywords: Tobacco dependence, nicotine addiction, carcinogen exposure, FTND, time to first cigarette (TFC), racial differences, NNAL, PAH
INTRODUCTION
Cigarette smoking is a major preventable cause of cancer and various other diseases. Among racial/ethnic groups in the United States, non-Hispanic Blacks experience a disproportionate burden of smoking-related diseases despite smoking fewer days per month, fewer cigarettes per day (CPD), and having lower adult prevalence of heavy smoking than non-Hispanic Whites (1, 2). Blacks are more susceptible to lung cancer than Whites, especially among lower consumption smokers (3).
Racial differences in cancer prevalence among smokers are likely to involve the interplay of genetics, smoking behavior, and tobacco dependence factors. Variations in CYP2A6 genes, which encode cytochrome P450 2A6 (CYP2A6) nicotine metabolizing enzymes, and CHRNA5-CHRNA3-CHRNA4 nicotinic receptor gene cluster have been associated with differential lung cancer risks among racial/ethnic groups (4, 5). We have previously shown that Blacks smoke cigarettes differently compared to Whites (6) and take on average 30% more nicotine per individual cigarette smoked (7). Further, evidence suggests that Black smokers have higher level of tobacco dependence, are more likely to attempt to quit smoking but have lower success rates, and have longer lifetime smoking duration than White smokers (1, 8, 9).
Polycyclic aromatic hydrocarbons (PAH) and tobacco-specific nitrosamines (TSNA) are two important classes of cancer-causing compounds in cigarette smoke (10). Several PAH metabolites can be simultaneously measured in urine (11), and can serve as proxies for exposure to carcinogenic PAHs such as benzo[a]pyrene. The TSNA, 4-(methylnitrosamino)-1-(3)pyridyl-1-butanone (NNK), is a pulmonary carcinogen associated with increased risk of lung cancer among active and passive smokers (4, 12). The NNK metabolite, 4-(methylnitrosamino)-1-(3)pyridyl-1-butanonol (NNAL), also a pulmonary carcinogen, can be measured in urine and reflects NNK exposure (10).
We have previously published data indicating that CPD predicts concentrations of tobacco carcinogens much more poorly in Black compared to White smokers (6). CPD is one of two objective measures in the Fagerström Test for Nicotine Dependence (FTND), the most commonly used behavioral scale of tobacco dependence (13). The other measure is the time to first cigarette after waking (TFC), which is used independently as a measure of tobacco dependence and has been shown to be associated with lung cancer risk (14). Muscat and colleagues showed that CPD has a positive linear relationship with cotinine among smokers who reported long TFC (low dependence) but not among smokers with short TFC (high dependence) (15). A linear relationship between CPD and smoke intake indicates smoke intake is determined more by the cigarette (without smoker compensation at lower levels of smoking) and suggests lower dependence. A flat relationship suggests a high degree of compensation by the smoker to optimize daily nicotine intake, which might be expected in highly dependent smokers. The authors did not report on racial differences in that paper.
Given the racial differences in tobacco dependence, racial differences in the relationship between CPD and carcinogen intake, as well as differences in the relationship between CPD and cotinine at long and short TFC (or low and high tobacco dependence), we hypothesized that the relationship between measures of tobacco dependence (FTND and TFC) and nicotine and carcinogen exposure differ in Black and White smokers. Therefore, the aim of our study was to analyze the relationships between tobacco dependence measures (FTND and TFC) and (a) biomarkers of nicotine and carcinogen exposure and (b) biomarkers of nicotine and carcinogen exposure per cigarette in Black and White smokers. The examination of these racial differences can provide valuable information to further understand disparities in smoking-related cancer outcomes.
METHODS
Studies
Previously unpublished data analyses from two clinical studies are presented in this article. Study 1 was a clinical trial of reduced-nicotine content cigarettes in which smokers were randomly assigned to a control or research arm after a 2-week baseline period in which they smoked their usual brand of cigarettes. Details of this study have been described elsewhere (16). In the present analysis, we focused on data collected during the baseline period. Data from Study 2 that focused on racial differences in the relationship between CPD and biomarkers of nicotine and carcinogen exposure as well as urine menthol in relation to biomarkers of nicotine and carcinogen exposure have been published previously (6, 17). The relationships between dependence measures and biomarkers were not reported in any of the published manuscripts.
Subjects
Study 1 subjects included 77 self-identified Black and White cigarette smokers. Study 2 subjects were 127 cigarette smokers who met the inclusion criteria of being self-identified Black or White, with four grandparents of the same race. Other inclusion criteria for both studies included being between the ages of 18–65; being healthy based on medical history and screening blood tests; and smoking 10 or more cigarettes per day for the past year or longer as ascertained by telephone screening. Exclusion criteria included pregnancy or breast feeding; current alcohol or drug abuse; current use of smokeless tobacco, pipes, cigars, and nicotine medications; and regular use of medications other than vitamins, oral contraceptives, hormone replacements, or aspirin. No subject participated in both studies.
Experimental protocols
Subjects from both studies were screened for eligibility by telephone. Eligible subjects were asked to come to a community-based clinic (Study 1) or the Clinical Research Center at San Francisco General Medical Center (Study 2) where the respective study was explained and written informed consent was obtained from each participant. Questionnaires were administered regarding tobacco dependence (FTND) as well as health history, drug use history, and smoking. Cigarette consumption was taken as the average of CPD in the 3 days prior to the study visit.
Following completion of questionnaires, a blood sample was taken and urine collected. Subjects were compensated financially for participation. The study was approved by the Institutional Review Board at the University of California, San Francisco.
Analytical chemistry
Plasma concentrations of cotinine and 3-HC and urine concentrations of nicotine and its metabolites cotinine, 3-HC, and their respective glucuronides were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (18). Urine total NNAL (free plus glucuronide) and PAH metabolites, 2-naphthol, 1-, 2-, 3-, 4-hydroxyphenanthrenes, 1-, 2-, 3-hydroxyfluorenes, and 1-hydroxypyrene were measured by LC-MS/MS (11, 19). The methods papers cited above contain details on quality control measures for the assays listed. Urine creatinine was measured in the San Francisco General Hospital clinical laboratory using a standard colorimetric assay.
Total PAH was expressed as the molar sum of all PAH metabolites per mg creatinine. Urine nicotine equivalents (NEq) was determined as the molar sum of nicotine, cotinine, 3-HC, and their respective glucuronides per mg creatinine. Urine NEq, when measured at steady state, accounts for 80%–90% of the daily dose of nicotine (20).
Statistical analysis
We used log-transformed biomarker data in all regression analyses. Demographic data were normally distributed and were analyzed on a raw-scale. We present biomarker data as geometric means and 95% confidence intervals (CI) and demographic data as means and interquartile ranges and/or sample sizes and percentages. We used Wilcoxon’s rank-sum test to compare demographic characteristics, smoking consumption, and exposure biomarkers of tobacco smoke constituents in Black versus White smokers.
We used mixed effects regression models to test the hypotheses relating biomarkers of exposure to race and measures of tobacco dependence (i.e., FTND and TFC), with subjects as a random effect. In one model we included log-transformed biomarkers as the dependent variable, and FTND as the independent variable, adjusting for the effects of BMI, age, sex, and race, and years of education and employment status, as indicators of socioeconomic status (SES). FTND was analyzed as a continuous variable in one set of models and as ordinal (quartiles of FTND) in another set. We introduced an FTND x race interaction to test the hypothesis that race modifies the relationship between FTND score and exposure biomarkers.
Regression models were tested to examine the effects of the independent variables described earlier on log-transformed NEq/CPD (an indicator of intensity of smoking), NNAL/CPD, and PAH/CPD as the dependent variables.
In other models, we introduced TFC as the independent variable of interest. TFC was analyzed as a continuous variable. A race x TFC interaction term was introduced and models were adjusted for the effects of age, BMI, CPD, years of education (all continuous variables), and sex, race, and employment status.
To evaluate whether the relationship between FTND and TFC and tobacco biomarkers varied among race as well as dependence level, we grouped our sample according to median TFC (TFC>15 min, low dependence; and TFC≤15 min, high dependence) and repeated the analyses for FTND and TFC, respectively, for each dependence group as described earlier.
We computed Pearson correlation coefficients between various smoker characteristics such as FTND, TFC, CPD, and biomarkers of tobacco exposure by race as well as by race and dependence level based on TFC level. All analyses were carried out using SAS v. 9.3 (SAS Institute, Inc., Cary, NC, USA) and statistical tests were considered significant at α = 0.05.
RESULTS
Demographic, smoking history and dependence
Of 204 subjects enrolled, 66% were White and 42% were females (Table 1). The average age of the participants was 38.3 years. On average, Black smokers were significantly older and had higher BMI. Blacks smoked significantly fewer CPD on average compared to Whites (17.5 vs. 19.8) and a significantly higher proportion of Blacks used menthol cigarettes (65% vs. 16%). Although subjects reported smoking on average 10 or more CPD over the past year during telephone screening, 25% of Blacks and 10% of Whites smoked 10 or fewer CPD over the three days preceding blood and urine sampling. FTND scores and TFC were not significantly different between the two racial groups.
TABLE 1.
Demographic and biomarkers of cigarette smoke exposure by race
| Characteristic | Blacks | Whites | All subjects | p Value |
|---|---|---|---|---|
| Demographic | ||||
| N | 69 | 135 | 204 | |
| Sex, n (%) | ||||
| Female | 27 (39.1) | 59 (43.7) | 86 (42.2) | 0.552 |
| Male | 42 (60.9) | 76 (56.3) | 118 (57.8) | |
| Age (years) | 42.0 (37.0, 48.0) | 36.4 (27.0, 45.0) | 38.3 (29, 46.0) | <0.001† |
| Education (years) | 13.7 (12, 14) | 15.1 (14, 16) | 14.7 (13, 16) | <0.001† |
| Employed, n (%) | ||||
| Yes | 28 (40.6) | 81 (60.0) | 109 (53.4) | 0.012† |
| No | 41 (59.4) | 54 (40.0) | 95 (46.6) | |
| BMI | 29.2 (24.3, 32.3) | 25.5 (22.4, 28.0) | 26.8 (22.7, 30.0) | <0.001† |
| CPD | 17.5 (10.7, 20.0) | 19.8 (15.0, 22.7) | 19.0 (14.7 (21.5) | 0.005† |
| CPD category | ||||
| 1–10 | 17 (24.6) | 13 (9.6) | 30 (14.7) | 0.018 |
| 11–20 | 37 (53.6) | 84 (62.2) | 121 (59.3) | |
| > 20 | 15 (21.7) | 38 (28.2) | 53 (26.0) | |
| TFC (min) | 23.1 (5.0, 30.0) | 27.3 (5.0, 30.0) | 25.9 (5.0, 30.0) | 0.392 |
| TFC category | ||||
| > 15 min | 28 (40.6) | 57 (42.2) | 85 (41.7) | 0.881 |
| ≤ 15 min | 41 (59.4) | 78 (57.8) | 119 (58.3) | |
| FTND | 5.1 (3.0, 7.0) | 5.1 (4.0, 7.0) | 5.1 (4.0, 7.0) | 0.936 |
| Menthol, n (%) | 45 (65.2) | 22 (16.3) | 67 (32.8) | <0.001† |
| Exposure biomarkers | ||||
| Plasma (ng/mL) | ||||
| Cotinine | 184.2 (151.1, 224.6) | 178.8 (157.8, 202.5) | 180.0 (162, 201.6) | 0.576 |
| 3-HC | 52.3 (41.2, 66.5) | 67.6 (58.8, 77.8) | 61.0 (53.6, 69.3) | 0.079 |
| 3-HC/cotinine | 0.28 (0.24, 0.33) | 0.38 (0.34, 0.42) | 0.34 (0.31, 0.37) | 0.004† |
| Cotinine/CPD | 12.8 (10.0, 16.2) | 10.0 (8.9, 11.2) | 11.0 (9.8, 12.4) | 0.018† |
| Urine | ||||
| Cotininea | 6.3 (5.0, 7.8) | 7.1 (6.3, 8.1) | 6.8 (6.1, 7.7) | 0.741 |
| 3-HCa | 15.6 (12.4, 19.5) | 21.6 (19.2, 24.3) | 19.3 (17.3, 21.5) | 0.049† |
| NEqa | 45.2 (39.5, 51.7) | 53.2 (48.5, 58.4) | 50.4 (46.7, 54.4) | 0.074 |
| NNALb | 1.0 (0.8, 1.2) | 1.2 (1.0, 1.4) | 1.1 (1.0, 1.3) | 0.120 |
| 3-HC/cotinine | 2.5 (2.1, 3.0) | 3.0 (2.7, 3.4) | 2.8 (2.6, 3.1) | 0.089 |
| Sum of fluorb | 14.9 (12.8, 17.2) | 16.6 (15.0, 18.3) | 15.9 (14.7, 17.3) | 0.220 |
| 1-HPb | 1.0 (0.8, 1.3) | 1.2 (1.0–1.3) | 1.1 (1.0–1.2) | 0.255 |
| 2-Naphtholb | 62.6 (54.1, 72.3) | 112.9 (102.2, 124.7) | 91.7 (83.8, 100.4) | <0.001† |
| Sum of phenb | 3.2 (2.7, 3.8) | 3.6 (3.2, 4.0) | 3.4 (3.2, 3.8) | 0.175 |
| Total PAHb | 84.4 (73.9, 96.5) | 137.0 (124.7, 150.5) | 115.5 (106.3, 125.5) | <0.001† |
| Cotinine/CPD | 0.43 (0.33, 0.56) | 0.39 (0.34, 0.44) | 0.40 (0.35, 0.45) | 0.359 |
| NEq/CPD | 3.1 (2.5, 3.8) | 2.8 (2.6, 3.1) | 2.9 (2.7, 3.2) | 0.911 |
| NNAL/CPD | 0.069 (0.055, 0.088) | 0.065 (0.057, 0.074) | 0.066 (0.059, 0.075) | 0.467 |
| Total PAH/CPD | 5.8 (4.7, 7.0) | 7.4 (6.7, 8.1) | 6.8 (6.2, 7.4) | 0.016† |
NOTES: Demographic data as means and interquartile ranges or n (%); biomarker data as geometric means and 95% CI; BMI = body mass index; CPD = cigarettes per day; TFC = time to first cigarette (median = 15 min); FTND = Fagerström Test for Nicotine Dependence; NEq = nicotine equivalent; NNAL = 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol; PAH = polycyclic aromatic hydrocarbons; fluor = fluorene; 1-HP = 1-hydroxypyrene; phen = phenanthrene;
units in nmol/mg creatinine;
units in pmol/mg creatinine;
significant difference between racial groups by Wilcoxon’s rank-sum test
Tobacco exposure biomarkers
Plasma and urine concentrations of nicotine and carcinogen biomarkers by race are presented in Table 1. Plasma cotinine levels were not significantly different between Black and White smokers but plasma cotinine/CPD was significantly higher in Blacks. Urine 2-naphthol and total PAH metabolites were significantly lower in Black compared to White smokers. Total PAH metabolites per CPD was significantly lower in Black smokers.
FTND versus nicotine intake and carcinogen exposure
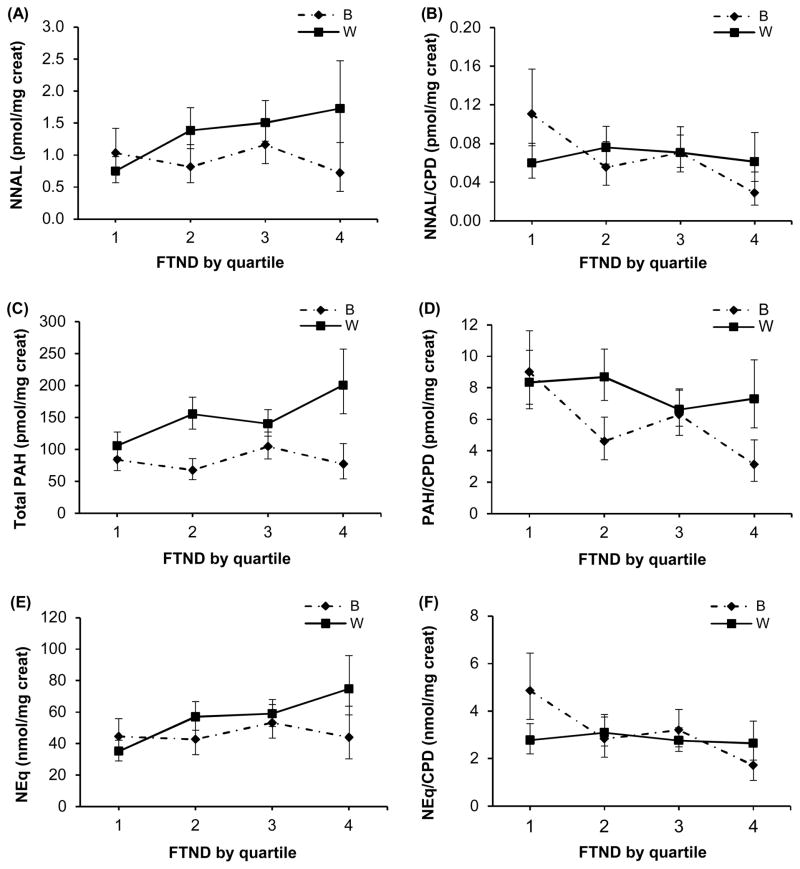
In regression analyses presented in Table 2A (model 1), the race x FTND interaction was significant for NNAL (p=0.029) and nicotine equivalents (p=0.031). In White smokers, concentrations of urine NNAL, total PAHs, and nicotine equivalents had significant positive relationships with FTND score (all p<0.001) while these biomarkers had no significant association with FTND in Blacks (Table 2A). When FTND was assessed categorically, the race x FTND interaction was significant for all three biomarkers (all p<0.05) (Figure 1). On average, Black and White smokers in the first quartile of FTND scores (lowest scores) had comparable levels of urine NNAL, total PAHs, and nicotine equivalents while these biomarkers were higher in Whites at the higher quartiles of FTND.
TABLE 2A.
Multiple linear regression models of predictors of urine total NNAL, urine total PAH metabolites, and urine nicotine equivalents
| Dependent Variable | Modela | Parameter | Estimate | 95% CI | p Value |
|---|---|---|---|---|---|
| Log NNAL (pmol/mg creat) | 1 | Race (White) | −0.145 | −0.389, 0.099 | 0.241 |
| R2 = 0.20 | FTND for Blacks | 0.008 | −0.025, 0.042 | 0.606 | |
| FTND for Whites | 0.056 | 0.029, 0.084 | <0.001 | ||
| White x FTND interaction | 0.048 | 0.005, 0.090 | 0.029 | ||
|
| |||||
| 2 | Race (White) | 0.123 | −0.005, 0.252 | 0.059 | |
| R2 = 0.19 | TFC for Blacks | −0.001 | −0.004, 0.002 | 0.357 | |
| TFC for Whites | −0.002 | −0.003, −0.001 | <0.001 | ||
| White x TFC interaction | −0.001 | −0.004, 0.002 | 0.681 | ||
|
| |||||
| Log total PAH (pmol/mg creat) | 1 | Race (White) | 0.095 | −0.071, 0.260 | 0.260 |
| R2 = 0.35 | FTND for Blacks | 0.018 | −0.004, 0.041 | 0.114 | |
| FTND for Whites | 0.035 | 0.016, 0.053 | <0.001 | ||
| White x FTND interaction | 0.016 | −0.012, 0.045 | 0.266 | ||
|
| |||||
| 2 | Race (White) | 0.163 | 0.078, 0.249 | <0.001 | |
| R2 = 0.37 | TFC for Blacks | −0.002 | −0.004, 0.0001 | 0.061 | |
| TFC for Whites | −0.001 | −0.002, −0.0004 | 0.004 | ||
| White x TFC interaction | 0.0007 | −0.001, 0.003 | 0.507 | ||
|
| |||||
| Log NEq (nmol/mg creat) | 1 | Race (White) | −0.129 | −0.299, 0.042 | 0.138 |
| R2 = 0.22 | FTND for Blacks | 0.016 | −0.008, 0.040 | 0.192 | |
| FTND for Whites | 0.049 | 0.030, 0.067 | <0.001 | ||
| White x FTND interaction | 0.033 | 0.003, 0.062 | 0.031 | ||
|
| |||||
| 2 | Race (White) | 0.038 | −0.051, 0.126 | 0.400 | |
| R2 = 0.21 | TFC for Blacks | −0.002 | −0.004, −0.0002 | 0.031 | |
| TFC for Whites | −0.002 | −0.002, −0.0008 | <0.001 | ||
| White x TFC interaction | 0.0007 | −0.001, 0.003 | 0.548 | ||
NOTE: FTND = Fagerström Test for Nicotine Dependence; TFC = time to first cigarette (min); NNAL = 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol; PAH = polycyclic aromatic hydrocarbon metabolites; NEq = nicotine equivalent;
Model 1 was adjusted for sex, age, body mass index, years of education and employment status; Model 2 was adjusted for sex, age, body mass index, years of education, employment status and cigarettes per day
FIGURE 1.
Relationship between FTND scores and urine total NNAL (plot A), FTND and urine total NNAL/CPD (plot B), FTND and urine total PAH (plot C), FTND and urine total PAH/CPD (plot D), FTND and urine nicotine equivalents (NEq) (plot E), and FTND and urine NEq/CPD (plot F), comparing Black (B) and White (W) smokers. The race x FTND interaction was significant for all biomarkers and biomarkers/CPD (p<0.05); creat = creatinine; values are geometric means and 95% CIs and FTND quartiles are in increasing order of FTND scores
TFC versus nicotine intake and carcinogen exposure
In regression analyses, the race x TFC interaction was not significant for urine NNAL, total PAHs and nicotine equivalents (Table 2A, model 2). However, we found significant negative associations between TFC and NNAL (p<0.001) and total PAH (p=0.004) in Whites but no significant associations for Blacks. TFC was significantly associated to nicotine equivalents in both Whites (p<0.001) and Blacks (p=0.031).
FTND versus nicotine and carcinogen intake per individual cigarette
Significant race x FTND interactions were observed for all three biomarkers per CPD (all p≤0.039) (Table 2B, Model 1). Urine NNAL, PAH, and nicotine equivalents per CPD decreased significantly with increasing FTND in Black smokers (all p≤0.002) while the concentration of these three biomarkers per cigarette smoked remained flat with increasing FTND among White smokers. Significant race x FTND interactions were also observed when FTND was analyzed as quartiles (all p<0.05) (Figure 1).
TABLE 2B.
Multiple linear regression models of predictors of urine total NNAL, urine total PAH metabolites, and urine nicotine equivalents per cigarette smoked
| Dependent Variable | Modela | Parameter | Estimate | 95% CI | p Value |
|---|---|---|---|---|---|
| Log NNAL/CPD (pmol/mg creat) | 1 | Race (White) | −0.347 | −0.619, −0.074 | 0.013 |
| R2 = 0.13 | FTND for Blacks | −0.060 | −0.098, −0.023 | 0.002 | |
| FTND for Whites | 0.005 | −0.026, 0.035 | 0.759 | ||
| White x FTND interaction | 0.065 | 0.018, 0.113 | 0.008 | ||
|
| |||||
| 2 | Race (White) | 0.083 | −0.047, 0.212 | 0.209 | |
| R2 = 0.28 | TFC for Blacks | −0.0006 | −0.004, 0.002 | 0.692 | |
| TFC for Whites | −0.002 | −0.003, −0.001 | 0.002 | ||
| White x TFC interaction | −0.001 | −0.004, 0.002 | 0.399 | ||
|
| |||||
| Log PAH/CPD (pmol/mg creat) | 1 | Race (White) | −0.109 | −0.304, 0.085 | 0.270 |
| R2 = 0.25 | FTND for Blacks | −0.051 | −0.078, −0.024 | <0.001 | |
| FTND for Whites | −0.016 | −0.037, 0.006 | 0.156 | ||
| White x FTND interaction | 0.035 | 0.002, 0.069 | 0.039 | ||
|
| |||||
| 2 | Race (White) | 0.124 | 0.035, 0.213 | 0.007 | |
| R2 = 0.43 | TFC for Blacks | −0.001 | −0.003, 0.0009 | 0.298 | |
| TFC for Whites | −0.001 | −0.002, −0.0003 | 0.009 | ||
| White x TFC interaction | 0.000 | −0.002, 0.002 | 0.990 | ||
|
| |||||
| Log Neq/CPD (nmol/mg creat) | 1 | Race (White) | −0.343 | −0.553, −0.134 | 0.001 |
| R2 = 0.15 | FTND for Blacks | −0.056 | −0.085, −0.026 | <0.001 | |
| FTND for Whites | −0.003 | −0.026, 0.020 | 0.802 | ||
| White x FTND interaction | 0.053 | 0.016, 0.090 | 0.005 | ||
|
| |||||
| 2 | Race (White) | −0.003 | −0.093, 0.086 | 0.939 | |
| R2 = 0.42 | TFC for Blacks | −0.001 | −0.003, 0.001 | 0.170 | |
| TFC for Whites | −0.001 | −0.002, −0.0007 | <0.001 | ||
| White x TFC interaction | 0.000 | −0.002, 0.002 | 0.959 | ||
NOTE: FTND = Fagerström Test for Nicotine Dependence; TFC = time to first cigarette (min); NNAL = 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol; PAH = polycyclic aromatic hydrocarbon metabolites; NEq = nicotine equivalent;
Models were adjusted for sex, age, body mass index, years of education and employment status.
TFC versus nicotine and carcinogen intake per individual cigarette
The race x TFC interactions were not significant for NNAL/CPD, PAH/CPD, and nicotine equivalents per CPD. However, among Whites, exposure to carcinogens and nicotine per CPD decreased with increasing TFC (all p≤0.009) while the slopes for Blacks were not significantly different from zero (Table 2B, model 2).
Cross-correlations among FTND, TFC, and biomarkers by race
Pearson correlations between FTND, TFC, and log-transformed biomarkers of tobacco smoke exposure were moderate and statistically significant for White smokers while they were non-significant for Black smokers. Correlations with FTND were as follows: versus urine NNAL, Black r = 0.07 (ns), White r = 0.44 (p<0.05); versus urine total PAH, Black r = 0.13 (ns), White r = 0.37 (p<0.05); versus urine nicotine equivalents, Black r = 0.10 (ns), White r = 0.46 (p<0.05); and versus plasma cotinine, Black r = 0.22 (ns), White r = 0.46 (p<0.05). Correlations with TFC were as follows: versus urine NNAL, Black r = −0.11 (ns), White r = −0.34 (p<0.05); versus urine total PAH, Black −0.16 (ns), White r = −0.29 (p<0.05); versus urine nicotine equivalents, Black r = −0.23 (ns), White r = −0.35 (p<0.05); and versus plasma cotinine, Black r = −.24 (ns), White r = −0.48 (p<0.05) [ns = non-significant].
Dependence, CPD, and exposure biomarkers at long and short TFC
FTND, TFC, and CPD were not significantly correlated with tobacco exposure biomarkers and carcinogen biomarkers among Blacks regardless of reporting long or short TFC [short TFC is ≤ 15 min (high dependence); long TFC is >15 min(low dependence)] (Table 3). Among Whites, FTND, TFC, and CPD were significantly correlated with nicotine intake and carcinogens in smokers who reported long TFC (low dependence) while the correlations were not significant at short TFC (high dependence).
TABLE 3.
Pearson correlation coefficients between tobacco dependence, cigarette consumption, and tobacco biomarkers in Black and White smokers by tobacco dependence
| Variable | Group | NNAL | Total PAH | NEq | Urine COT | Plasma COT |
|---|---|---|---|---|---|---|
| FTND | Black smokers | |||||
| low dependence | −0.15 | −0.21 | −0.20 | 0.04 | 0.08 | |
| high dependence | −0.07 | 0.16 | −0.01 | −0.20 | −0.05 | |
| White smokers | ||||||
| low dependence | 0.52 | 0.41 | 0.46 | 0.20 | 0.16 | |
| high dependence | 0.16 | 0.25 | 0.30 | 0.26 | 0.27 | |
|
| ||||||
| TFC | Black smokers | |||||
| low dependence | 0.09 | 0.00 | −0.10 | −0.10 | −0.04 | |
| high dependence | −0.02 | −0.21 | −0.02 | −0.01 | −0.15 | |
| White smokers | ||||||
| low dependence | −0.34 | −0.31 | −0.35 | −0.30 | −0.25 | |
| high dependence | −0.11 | −0.14 | −0.13 | −0.05 | −0.25 | |
|
| ||||||
| CPD | Black smokers | |||||
| low dependence | −0.03 | 0.03 | −0.25 | −0.19 | −0.09 | |
| high dependence | −0.12 | 0.1 | 0.02 | 0.16 | 0.15 | |
| White smokers | ||||||
| low dependence | 0.33 | 0.38 | 0.36 | 0.21 | 0.38 | |
| high dependence | 0.14 | 0.12 | 0.18 | −0.05 | 0.22 | |
|
| ||||||
| NEq | Black smokers | |||||
| low dependence | 0.77 | 0.61 | 1 | 0.69 | 0.76 | |
| high dependence | 0.39 | 0.73 | 1 | 0.68 | 0.72 | |
| White smokers | ||||||
| low dependence | 0.63 | 0.64 | 1 | 0.78 | 0.74 | |
| high dependence | 0.61 | 0.59 | 1 | 0.72 | 0.57 | |
NOTGES: Tobacco dependence was measured by time to first cigarette (TFC) (median = 15 min); low tobacco dependence = TFC >15 min; high tobacco dependence = TFC ≤ 15 min; Black, low dependence, n = 28; Black, high dependence, n = 41; White, low dependence, n = 57; White, high dependence, n = 78; NNAL = 4-(methylnitrosamino)-1-(3)pyridyl-1-butanol; total PAH = polycyclic aromatic hydrocarbon metabolites; NEq = nicotine equivalent; CPD = cigarettes per day; Fluor is molar sum of 1-, 2-, and 3-fluorenols; COT is cotinine; all biomarker data were log10-transformed; significant correlations are in bold.
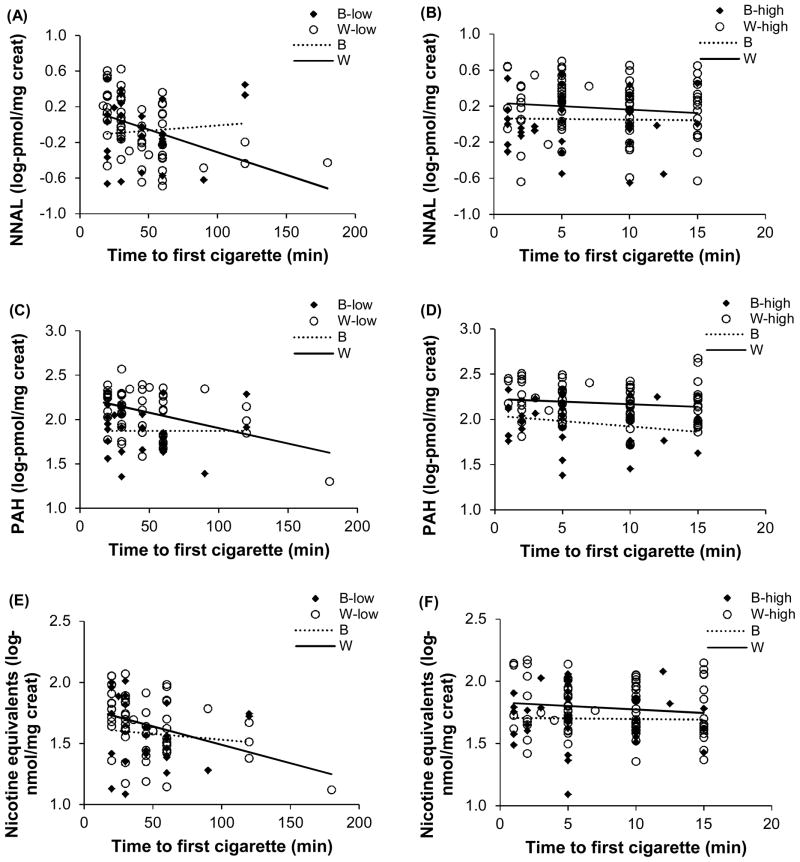
Figure 2 shows the relationship between TFC and carcinogen biomarkers and urine nicotine equivalents in Blacks and Whites by low and high dependence based on TFC. Regression analyses revealed significant negative associations between TFC and NNAL (p=0.028) and nicotine equivalents (p=0.016) and non-significant association with total PAHs (p=0.059) among White smokers with low dependence. The slopes for TFC versus biomarkers among Blacks regardless of low or high dependence and among Whites with high dependence were not significantly different from zero.
FIGURE 2.
Relationship between time to first cigarette (TFC) and urine NNAL (plots A and B), TFC and urine total PAHs (plots C and D), and TFC and urine nicotine equivalents (plots E and F), comparing Black (B) and White (W) smokers by low dependence (low) and high dependence (high). W-low slopes for plots A and E are significantly different from zero (p<0.05). Slopes of all other plots are non-significant.
DISCUSSION
Main observations
We present several results which may help enhance our understanding of disparities in smoking-related cancer and disease outcomes in Black compared to White smokers. First, we observed that tobacco dependence measures were linearly related to nicotine intake and carcinogen exposure in White but not in Black smokers. The relationship between FTND versus urine nicotine equivalents, a measure of daily nicotine intake, and NNAL and PAH metabolites, measures of carcinogen exposure, was relatively flat for Black smokers, whereas a moderate positive relationship was observed for Whites. Also, while nicotine equivalents, NNAL, and PAH metabolites generally decreased with longer TFC in both Black and White smokers, this inverse relationship was stronger for Whites than for Blacks. Second, we found that the intake of nicotine and carcinogens per CPD, measures of intensity of smoking each cigarette, had an inverse relationship with FTND for Blacks while it was relatively flat for Whites. These observations persisted after the statistical adjustment for years of education and employment status as well as age, BMI, sex, and race. Finally, FTND, TFC, and CPD had poor associations with nicotine intake and carcinogen exposure in Blacks regardless of tobacco dependence and in Whites of high tobacco dependence while the associations in White smokers of low tobacco dependence were moderate and significant.
Tobacco dependence measures versus nicotine intake and carcinogen exposure
Smoking and nicotine addiction are complex behaviors which are influenced by multiple factors. Although most of the toxicity of smoking is directly related to other constituents of tobacco smoke, it is primarily the pharmacologic effects of nicotine that causes and sustains the powerful addicting effects of tobacco (21). One would then expect that nicotine intake and exposure to other constituents present in tobacco smoke would be directly related with the degree of dependence.
The expected relationship was observed in White smokers. However, the observed flat relationship between FTND and nicotine equivalents and carcinogen exposure in Blacks suggests that the smoking behavior of Blacks and Whites may be influence differently by nicotine and/or they may be smoking for different motives. We previously reported findings of a flat relationship between CPD and nicotine and carcinogen exposure in Blacks compared to Whites (6). Since CPD is a major contributor to the FTND score it is plausible that the racial differences observed in the relationship between FTND and nicotine and carcinogen exposure is a reflection of the previously reported racial differences in cigarette consumption. However, we also found that TFC, the other core item of the FTND, had weaker linear relationships with nicotine intake and carcinogen exposure among Blacks compared to Whites. Further, we found that FTND, TFC, and CPD were significantly associated with nicotine equivalents and carcinogens in White smokers of low dependence (long TFC) while the associations in White smokers of high dependence (short TFC) and all Black smokers regardless of dependence were not significant. Our findings suggest that important differences exist in how tobacco dependence determines smoking behavior and carcinogen exposure in Black and White smokers. Our findings also suggest that dependence and smoking behavior of most Black smokers seem to resemble that of high dependent White smokers.
Variations in the CYP2A6 gene have been associated with different rates of nicotine metabolism (22). The effect of rate of nicotine metabolism on dependence and smoking behavior is not fully understood, but there is some evidence that slow metabolism is associated with a lower level of nicotine dependence. Blacks in general metabolize nicotine more slowly than Whites, reflected in a lower nicotine metabolite ratio (NMR or 3-hydroxycotinine/cotinine). However, the NMR was not associated to FTND and TFC in a previous publication of Study 1 data (23).
Tobacco dependence versus nicotine intake and carcinogen exposure per cigarette
We report differences in the relationship between FTND and nicotine equivalents and carcinogen intake per cigarette among Black and White smokers. These measures reflect the intensity of smoking each cigarette. Biomarkers per CPD decreased with FTND in Blacks but remained flat among Whites. Blacks appear to smoke cigarettes more intensely than Whites, particularly at lower FTND scores and CPD, suggesting a greater degree of nicotine compensation when smoking fewer CPD. This suggests that Blacks at the lower level of FTND have a greater level of nicotine dependence than the FTND scale reveals.
The use of primarily mentholated cigarettes by Blacks compared to Whites (65% vs. 16%) may contribute to higher intake of nicotine and carcinogens per cigarette among Blacks but we found no evidence that menthol predicts nicotine and carcinogen intake in a previous publication using Study 2 data (17). Also, differences in SES might be a possible explanation for differences in smoking intensity between Blacks and Whites. However, statistical adjustment for years of education and employment status led to marginal or no changes in the regression parameter estimates in Tables 2A and 2B. Years of education was associated with only total PAH and PAH/CPD (both p≤0.005), and not to NNAL, nicotine equivalents, and their concentrations per CPD (in models with FTND or TFC as predictor). PAH delivery of cigarettes has been shown to vary with tobacco varieties (24) and the relationship between education and PAH and PAH/CPD may be reflective of differences in brands of cigarettes smoked. Employment status was not significantly associated with any biomarker or biomarker per CPD.
Study limitations
Some limitations of the study should be noted. First, Black subjects in our study smoked on average more cigarettes than the national average while White subjects’ consumption was closer to the national average, hence limiting the generalizability of our findings. Also, some subjects smoked fewer CPD in the past 3 days prior to screening sessions than they reported smoking on average in the prior year. Changes in cigarette consumption a few days before blood and urine sampling may impact biomarker concentrations since we assume our biomarker assessment represents steady-state exposure in relation to cigarettes smoked in the preceding 3 days. Nicotine and PAH metabolites are likely to represent recent exposures because they have relatively short half-lives. However, NNAL has a half-life of 10–18 days so that levels will not achieve steady state in someone who has recently changed their cigarette consumption (25). Nonetheless, we think our biomarker assessments are not seriously biased because we found similar correlations between NNAL and PAH metabolites and nicotine equivalents.
CONCLUSION
We present novel data that can help further our understanding of why disparities in smoking-related cancer risk exists between Blacks and Whites in America. We found tobacco dependence measures were linearly related to nicotine intake and carcinogen exposure in White but not in Black smokers. Our findings indicate that among smokers of lower tobacco dependence, Blacks seem to take in more nicotine and carcinogens per individual cigarette than White smokers. Finally, we found that the relationship between tobacco dependence measures and tobacco biomarkers in Black smokers regardless of level of dependency resembled highly dependent White smokers. The ultimate measure of tobacco dependence is the ability to stop smoking when a person attempts to. There is much evidence that Blacks have as much or more difficulty quitting than do Whites. Among White smokers, the linear relationship between FTND and TFC and nicotine intake and other smoke constituents is consistent with the idea that the level of dependence is pharmacologically related to daily nicotine intake. However, the lack of relationship in Blacks suggests that daily nicotine intake is not the determinant of dependence or that FTND and TFC are not good measures of dependence in Blacks. More research on other measures of tobacco dependence in relation to nicotine within Black smokers is encouraged.
Acknowledgments
The authors thank Dr Faith Allen for data management, Dr Katherine Dains for project management, Sandra Tinetti for clinical coordination; Christopher Havel, Fredysha McDaniel, Lita Ramos, Minjiang Duan and Janice Chen for performing analytical chemistry; and Marc Olmsted for editorial assistance.
Funding
Supported by US Public Health Service grants DA02277 and DA12393 from the National Institute on Drug Abuse and CA78603 and R25CA113710 from the National Cancer Institute, National Institutes of Health. Carried out in part at the General Clinical Research Center at San Francisco General Hospital Medical Center (NIH/NCRR UCSF-CTSI UL1 RR024131)
Footnotes
Declarations of interest:
NLB serves as a consultant to several pharmaceutical companies that market smoking cessation medications and has served as a paid expert witness in litigation against tobacco companies. Other authors have no conflicts to declare.
References
- 1.USDHHS. Tobacco Use Among US Racial/ethnic Minority Groups: African Americans, American Indians and Alaska Natives, Asian Americans, and Pacific Islanders, Hispanics: Executive Summary: a Report of the Surgeon General. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 1998. [Google Scholar]
- 2.Trinidad DR, Pérez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res. 2009;11(2):203. doi: 10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 4.Le Marchand L, Derby KS, Murphy SE, Hecht SS, Hatsukami D, Carmella SG, et al. Smokers with the CHRNA lung cancer–associated variants are exposed to higher levels of nicotine equivalents and a carcinogenic tobacco-specific nitrosamine. Cancer Res. 2008;68(22):9137. doi: 10.1158/0008-5472.CAN-08-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship Between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 Variation and Smoking Behaviors and Lung Cancer Risk. J Natl Cancer Inst. 2011;103(17):1342–6. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;13(9):772–83. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Stable EJ, Herrera B, Jacob P, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 8.Ahijevych K, Gillespie J. Nicotine dependence and smoking topography among black and white women. Res Nurs Health. 1997;20(6):505–14. doi: 10.1002/(sici)1098-240x(199712)20:6<505::aid-nur5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Siahpush M, Singh G, Jones P, Timsina L. Racial/ethnic and socioeconomic variations in duration of smoking: results from 2003, 2006 and 2007 Tobacco Use Supplement of the Current Population Survey. J Public Health (Oxf) 2010;32(2):210–8. doi: 10.1093/pubmed/fdp104. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 11.Jacob P, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79(2):587–98. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 12.USDHHS. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Centers for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2006. [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 14.Muscat JE, Ahn K, Richie JP, Jr, Stellman SD. Nicotine dependence phenotype and lung cancer risk. Cancer. 2011;117(23):5370–6. doi: 10.1002/cncr.26236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muscat JE, Stellman SD, Caraballo RS, Richie JP. Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3415. doi: 10.1158/1055-9965.EPI-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benowitz NL, Dains KM, Hall S, Stewart SL, Wilson M, Dempsey D, et al. Smoking Behavior and Exposure to Tobacco Toxicants During 6 months of Smoking Progressively Reduced Nicotine Content Cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21(5):761–9. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P. Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3013. doi: 10.1158/1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob P, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B. 2011 doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob P, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per Milliliter Determination of the Tobacco-Specific Carcinogen Metabolite 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol in Human Urine Using Liquid Chromatography Tandem Mass Spectrometry. Anal Chem. 2008;80(21):8115–21. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng S, Kapur S, Sarkar M, Muhammad R, Mendes P, Newland K, et al. Respiratory retention of nicotine and urinary excretion of nicotine and its five major metabolites in adult male smokers. Toxicol Lett. 2007;173(2):101–6. doi: 10.1016/j.toxlet.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benowitz NL, Swan GE, Jacob P, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine*. Clin Pharmacol Ther. 2006;80(5):457–67. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 23.St Helen G, Novalen M, Heitjan DF, Dempsey D, Jacob P, III, Aziziyeh A, et al. Reproducibility of the Nicotine Metabolite Ratio in Cigarette Smokers. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1105–14. doi: 10.1158/1055-9965.EPI-12-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding YS, Zhang L, Jain RB, Jain N, Wang RY, Ashley DL, et al. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3366. doi: 10.1158/1055-9965.EPI-08-0320. [DOI] [PubMed] [Google Scholar]
- 25.Goniewicz ML, Havel CM, Peng MW, Jacob P, Dempsey D, Yu L, et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3421. doi: 10.1158/1055-9965.EPI-09-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]