Abstract
Patients with neurodegenerative disease show distinct patterns of personality change, some of which may be traced to focal neurologic damage, while others may be mediated by cultural reactions to functional impairment. While such changes are early and pervasive in behavioral variant frontotemporal dementia (bvFTD), and milder changes are seen in Alzheimer’s (AD), no study has examined all Big 5 factors of personality in mild cognitive impairment (MCI) patients. Also, the influence of culture and ethnicity on disease-related personality changes has seldom been examined. Premorbid and current personality were measured in 47 Greek patients with bvFTD, AD, and MCI according to informant reports using the TPQue5, a 5-factor inventory in the Greek language and accounting for Greek cultural factors. bvFTDs showed greater decreases in conscientiousness than ADs and MCIs. ADs and MCIs showed increased neuroticism, while the bvFTD patients were rated as having become much less neurotic in the course of their disease. The pattern of personality change in MCIs was very similar to that of ADs, supporting recent evidence that personality changes occur as early as the MCI disease stage. In all groups, personality changes were similar to those previously described in non-Mediterranean cultures, supporting the hypothesis that they may result directly from disease-specific neurologic processes.
Keywords: personality, frontotemporal dementia, Alzheimer’s disease, mild cognitive impairment, Big Five
INTRODUCTION
Personality traits are mostly consistent in adulthood and old age, although small shifts in personality occur across the life span 1–3. While there are many personality theories in use today, the Five Factor Theory is perhaps the most comprehensive and widely used theory of normal personality, and its factors (Openness, Conscientiousness, Extraversion, Agreeableness, and Neuroticism) seem to emerge quite consistently across cultures 4–6. This model has also frequently been used to measure personality of adults in later life 7–10. While several longitudinal studies have revealed substantial consistency in Big 5 personality traits continuing into old age, there do appear to be small but significant group changes in certain traits, such as increases in neuroticism and agreeableness and decreased Extraversion and Openness in healthy older subjects 11. Other studies reveal increased Agreeableness and Conscientiousness as well as a decrease in Openness in later life 3, 12.
Though these small shifts may occur on a group level as a function of normal aging, large changes in personality are not typical, and may signify underlying neurologic disease 10. In particular, uniform, unidirectional changes in personality occurring in the majority of individuals with a specific medical condition or disease are likely to derive from processes unique to the disease itself. These may be culturally-mediated reactions (e.g., aphasic patients who are unable to communicate in social interactions may become more introverted) or direct neurologic sequelae (e.g., patients with focal damage to frontal structures that mediate social dominance may become more introverted). For instance, recent studies have demonstrated that patients with neurodegenerative disease show distinct patterns of personality change that are disease-specific, and which can be directly traced to focal neurologic damage 13, 14. Personality change is a core and early feature of behavioral variant frontotemporal dementia (bvFTD) 15. Recent studies suggest that change may occur in all five personality factors in bvFTD, with agreement across studies that significant decreases in Conscientiousness occur in relation to healthy controls, and with mixed findings suggesting that bvFTD patients may also have decreased Openness, Extraversion and Neuroticism in comparison to both normal controls and Alzheimer’s disease (AD) patients14, 16. Studies using different personality measures have found decreased Agreeableness in bvFTD 14, 17, 18, a change that has been linked to medial orbitofrontal volume loss.
Personality change in AD patients is less striking when compared to bvFTD, as the frontal and anterior medial temporal areas primarily affected in bvFTD are relatively spared in AD. However, AD patients often present with significant changes in personality, showing increased Neuroticism and a decrease in Extraversion, Openness, and Conscientiousness,7–9 while results on loss of Agreeableness are mixed19,20. There is some evidence these changes wax and wane in predictable patterns over the course of the disease 21. While some of these recent studies suggest that such changes may occur early in the disease process and may be observable even at the MCI stage22, studies have not yet examined all of the Big 5 factors in MCI patients.
The influence of culture and ethnicity on these age-related and disease-related personality changes has seldom been examined. There have been efforts to measure the personality of healthy Greek adults across the adult lifespan, and to compare their personality traits with those of other cultures. These have primarily utilized Big Five-based measures such as the NEO-PI or the Traits Personality Questionnaire (TPQue) 23, a Greek-language five-factor self-report questionnaire developed by Tsaousis to explicitly account for ethnic and cultural characteristics found among Greek people. However, to our knowledge in Greece there has been no research focusing on personality change in older adults suffering from neurodegenerative diseases.
This study examined personality changes appearing in patients with AD, bvFTD and MCI compared to their premorbid personality using the TPQue5, the shortened version of a Greek measure of the five factor model of personality 24. Based on the findings of previous studies in other cultures, we expected to find that when current and premorbid states of our subjects are compared to derive a measure of personality change, separate, predictable patterns of personality changes would occur for the three diagnostic groups. We predicted AD patients would show a significant increase in Neuroticism and a decrease in Extraversion, Conscientiousness, and Openness. Also, we hypothesized that bvFTD patients would show decreases in these four factors as well as Agreeableness, while no significant personality changes were expected in any of the five factors for the MCI group.
METHODS
Subjects
A total of 42 patients, of whom 8 were diagnosed with behavioural variant frontotemporal dementia (bvFTD) according to the Neary consensus criteria 15, 17 with Alzheimer’s disease (AD) according to NINCDS-ADRDA criteria 25, and 17 with Mild Cognitive Impairment (MCI) according to the Petersen criteria 26 were recruited into the study. These criteria were used in order to exclude MCI patients whose pattern of cognitive deficits was inconsistent with early AD, such as executive MCI. MCI patients were included only if memory deficits were their primary domain of impairment, though patients were not excluded if they had some additional non-memory impairments. Age, education, and sex distribution within subject groups can be found in Table 1.
Table 1.
Demographic, functional, and neuropsychological characteristics of sample
| Mean (SD) | MCI | AD | bvFTD | F-value | P-value |
|---|---|---|---|---|---|
|
| |||||
| Demographics | |||||
| sex (m/f) | 5/12 | 5/12 | 2/6 | x2=0.06 | n.s. |
| Age | 71.3 (8.3) | 74.1 (7.2) | 67.6 (5.5) | 2.10 | n.s. |
| # years educ | 12.6 (4.7) | 8.5 (4.8) | 6.2 (0.7) | 6.87 | 0.0028 |
|
| |||||
| General/Functional Measures | |||||
| ADCS-ADL | 72.4 (3.7) | 58.9 (14) * | 48.2 (19.9) * | 7.68 | 0.0018 |
| GDS (max=30) | 7.6 (5.9) | 5.9 (5.3) | 5.9 (5.7) | 1.70 | n.s. |
| NPI total | 15.8 (16.8) | 20.8 (18.9) | 30.7 (9) | 1.62 | n.s. |
| MMSE (max=30) | 27.2 (1.3) | 20.2 (4.8)* | 17.6 (6.5)* | 21.86 | <0.0001 |
| ACE-R (max=100) | 81.2(10.1) | 59.3 (13.3) | 48.9(6.5) | 0.10 | n.s. |
|
| |||||
| Memory | |||||
| CVLT-MS 1–4 Regist (max=36) | 27 (5.6) | 17.7 (7.2) * | 17.9 (8.5) * | 5.63 | 0.0077 |
| CVLT Imm Rec (max=9) | 4 (2.9) | 1.9 (2.4) | 2.7 (2.9) | 1.37 | n.s. |
| CVLT Del Rec (max=9) | 3.1 (3) | 0.7 (1.6) * | 1.7 (1.9) | 3.99 | 0.0277 |
|
| |||||
| Benson Fig Recall (max=17) | 5.5 (3.6) | 2.9 (3.7) | 5.6 (4.8) | 0.81 | n.s. |
|
| |||||
| Visuospatial | |||||
| Benson Figure Copy (max=17) | 16 (2.1) | 13 (3.8) * | 14.9 (2.5) | 3.83 | 0.0315 |
| Clock Drawing Test | 6.6 (0.8) | 4 (2.5) * | 3.4 (2.7) * | 5.99 | 0.0056 |
|
| |||||
| Language | |||||
| Boston Naming (max=15) | 12.8 (2.3) | 10 (3.1) | 9.8 (3.6) | 0.71 | n.s. |
| Lexical Fluency | 10.6 (3.4) | 6.2 (3.3) | 3.5 (3.1) | 2.02 | n.s. |
| Semantic Fluency | 11 (4.1) | 7.8 (3.3) | 5.7 (4.9) | 0.24 | n.s. |
|
| |||||
| Executive | |||||
| Modified Trailmaking Speed | 14.4 (14.3) | 1.6 (3.6) | 0.7 (1.9) | 3.20 | 0.0528 |
| Stroop Time (sec) | 42.7 (14.4) | 109.6 (64.6) * | 140.7 (65.9) * | 6.90 | 0.0030 |
p<0.05 compared to MCIs using Tukey-Kramer post-hoc test controlling for age and MMSE
ABBREVIATIONS: ADCS-ADL – Alzheimer’s Disease Cooperative Study – Activities of Daily Living Inventory; GDS – Geriatric Depression Scale; NPI – Neuropsychiatric Inventory; MMSE – Mini Mental Status Examination; ACE-R – Addenbrooke’s Cognitive Examination – Revised; CVLT-MS – California Verbal Learning Test – Mental Status Edition;
Procedures
Patients were identified by diagnosis from a pool of clinic patients and were then recruited as study participants, consenting to undergo additional research tests, and agreeing for their clinical data to be analysed for research purposes. The study was approved by appropriate authorities at Athens General Hospital to assure ethical treatment of participants. A complete multidisciplinary diagnostic evaluation was performed, including a neurological examination, clinical interview with patients and caregivers, and neuropsychiatric evaluation. Subjects also underwent a comprehensive battery of functional, neuropsychological, social cognition, and personality tests.
There is substantial evidence that patients with AD and bvFTD are likely to provide inaccurate self-assessments due to insight-related cognitive deficits, thus their self-reports were not deemed reliable, and personality evaluation was obtained from a caregiver proxy informant (see a more detailed rationale for this procedure elsewhere27). Caregiver informants completed the TPQue5 twice, first describing the subjects’ current and then premorbid personality traits. For the retrospective account, informants were asked to describe the subjects “before they became ill.”
Measures
The TPQue5 24 is a measure of the Big Five Factor model of personality, including scales measuring Openness, Conscientiousness, Extraversion, Agreeableness and Neuroticism as well as lie and social desirability scales. It is a short version (101 items) of the TPQue 23, which was developed and validated specifically for use with Greek adults. Factor analysis of the TPQue5 short form demonstrates that it maintains the original factors, and displays good internal consistency (alphas for split-half reliability ranging from .73 [Openness] to .85 [Neuroticism] and excellent test-retest reliability (ICCs ranging from .71 [Agreeableness] to .86 [Conscientiousness]). The TPQue5 was preferable to the TPQue for this study because our aging and low-education population responds better to its short length.
The control sample developed to standardize the TPQue5 consisted of 735 primarily young Greek university students ranging in age from 18 to 55 years of age (mean=27.1, SD=9.7). Standardized, gender-specific T-scores were generated for all subjects based on this normative sample23, 24.
Neuropsychological testing included measures of all major cognitive domains (Table 1), which have been detailed elsewhere28. Functional and neuropsychiatric measures included the Activities of Daily Living (ADCS-ADL), Geriatric Depression Scale (GDS) 29, Neuropsychiatric Inventory (NPI) Total Score 30, Mini Mental State Examination (MMSE), and Addenbrooke’s Cognitive Examination (ACE-R)31.
Data Analyses
General linear models were used to compare the three groups for possible confounding effects of age, education, and disease severity (represented as MMSE score), and chi-square tests were used to test for group differences in gender. Though gender and age were equally represented across all diagnostic groups, education level and MMSE showed significant group differences, thus were included as confounding covariates in all analyses of personality, neuropsychological, neuropsychiatric, and functional measures.
All interpersonal trait scores are reported in the form of standardized T-scores that were calculated based on comparison to the Greek normative sample described earlier23, 24. Thus, scores were considered clinically abnormal if they fell more than 1.5 SD from the mean, i.e., scores below 35 (6th %ile) or above 65 (94th %ile). Statistically significant differences between scores (e.g., significant change over time, or between groups) are also reported regardless of whether the scores fell outside of this clinically normal range.
To appropriately handle the repeated nature of the personality data, a random coefficients model using SAS Proc Mixed 32 was used to generate estimates of the five personality scores for each diagnostic group for both the premorbid stage (informants’ retrospective account) and current stage of the disease.
RESULTS
Premorbid Scores
All premorbid personality factor scores of the MCI, AD, and bvFTD groups fell within the clinically normal range (i.e., T-score range of 35 – 65), suggesting that according to the informants’ retrospective reports, all subjects began within the range of normal personality (Table 2). MCI subjects were at the upper limit of normal Conscientiousness (T=64.0 (SD=3.0)), and upon statistical comparison, had significantly higher Conscientiousness scores than bvFTD subjects (T=52.5 (SD=3.9)) (p<0.05). No other personality factors showed premorbid differences across disease groups. It is unclear whether this difference reflects a true difference in premorbid personality across diagnostic groups, or whether it is due to informants’ uncertainty in estimating the time of symptom onset in the bvFTD patients (i.e., their “before” ratings may have reflected a time at which the bvFTD patients were already losing Conscientiousness, albeit at a clinically insignificant level.). Also, this sample diverged substantially from the expected average group T-score around 50, which suggests that our patient sample may be fundamentally different from the younger, highly educated control subjects on which the TPQue5 is standardized. Because of this, caution should be used in interpreting patient group scores found to be clinically abnormal (i.e., falling outside of the +/− 1.5 SD range described earlier).
Table 2.
Premorbid and current personality characteristics across Big 5 factors
| Mean T-score (SD) | Premorbid | Current | Pr > I t I |
|---|---|---|---|
|
| |||
| Openness | |||
| AD | 36.7 (2.3) | 36.1 (2.3) | 0.5930 |
| bvFTD | 35.5 (3.4) | 36.1 (3.4) | 0.7321 |
| MCI | 38.4 (2.7) | 37.2 (2.7) | 0.3022 |
|
| |||
| Conscientiousness | |||
| AD | 60.1 (2.6) | 42.6 (2.6) * | <.0001 |
| bvFTD | 52.5 (3.9) • | 28.2 (3.9) ¤ * | <.0001 |
| MCI | 64.0 (3.0) | 54.5 (3.0) ¤ | 0.0011 |
|
| |||
| Extraversion | |||
| AD | 51.2 (3.3) | 33.9 (3.3) | <.0001 |
| bvFTD | 47.8 (4.8) | 25.0 (4.8) | <.0001 |
| MCI | 46.7 (3.7) | 35.9 (3.8) | 0.0005 |
|
| |||
| Agreeableness | |||
| AD | 53.5 (3.3) | 47.2 (3.3) * | 0.0411 |
| bvFTD | 54.2 (4.9) | 48.4 (4.9) | 0.1906 |
| MCI | 62.1 (3.8) | 58.7 (3.8) | 0.2694 |
|
| |||
| Neuroticism | |||
| AD | 44.6 (2.8) | 53.8 (2.9) | 0.0014 |
| bvFTD | 42.9 (4.2) | 36.0 (4.2) ¤ * | 0.0834 |
| MCI | 43.9 (3.2) | 53.3 (3.2) | 0.0011 |
Different from MCI current
Different from AD current
Different from MCI premorbid
Personality Change and Group Differences
Openness
Subjects’ scores for Openness to new experiences were at the lower limit of normal for both retrospective premorbid and current ratings, ranging from T=35.5 to 38.4. No group’s scores changed significantly between the “before” and “current” timepoints, and no disease group had significantly different scores from any other disease group (Figure 1).
Figure 1.
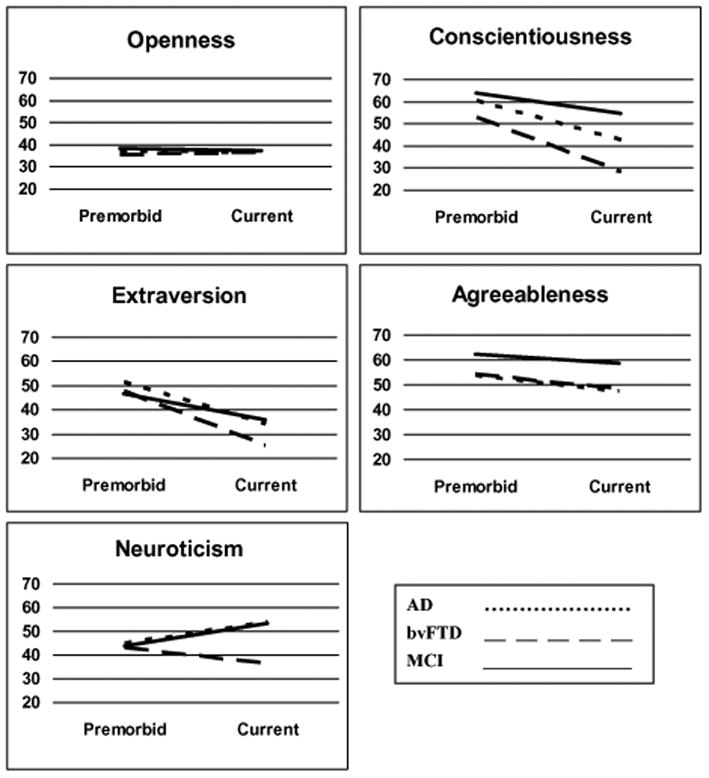
T-scores of Big Five personality factors before illness onset (Premorbid) and at time of initial clinical presentation (Current) for patients with Alzheimer’s disease (AD), behavioral variant frontotemporal dementia (bvFTD), and mild cognitive impairment (MCI).
Conscientiousness
Current scores for Conscientiousness were within clinically normal range for both MCI and AD patients, while bvFTD patients’ current scores were abnormally low (T=28.2). All groups’ scores dropped significantly at the current timepoint (p<0.001), and both AD and bvFTD patients’ current scores were significantly lower than those of MCI patients. bvFTD patients were also rated significantly lower in Conscientiousness than AD patients (p<0.05).
Extraversion
Subjects’ scores for Extraversion were within normal limits for the three groups’ retrospective premorbid ratings. There was a statistically significant drop in all groups’ scores between the premorbid and current state of disease, leading to clinically low scores for the AD and bvFTD patients current ratings. No significant differences were statistically noted among the different disease groups.
Agreeableness
Premorbid scores for Agreeableness were within a clinically normal range for all disease groups. While all groups’ Agreeableness dropped between premorbid and current timepoints, this change was only significant for the AD patients (p<0.05). AD patients also scored significantly lower than MCI patients at the current timepoint.
Neuroticism
Although all groups started within a normal range for retrospective premorbid ratings, AD and MCI patients were rated as significantly more neurotic currently (p<0.005) whereas bvFTD patients were rated as less neurotic than they had been premorbidly. The current ratings for bvFTD patients’ Neuroticism were significantly lower than either AD or MCI patients.
DISCUSSION
As expected, examination of personality change in Greek AD, bvFTD, and MCI patients with a Big Five measure revealed significant personality changes on most factors, with divergent patterns across groups. The bvFTD patients’ decrease in conscientiousness was significantly bigger than the decrease noted for the AD and MCI patients. Also, the changes noted in neuroticism for the three groups followed divergent paths: a significant increase was observed for the AD and MCI groups, while the bvFTD patients were rated as having become much less neurotic in the course of their disease. MCI patients unexpectedly presented with a decrease in conscientiousness and extraversion, as well as an increase in neuroticism, a pattern of change very similar to that of AD patients. These results suggest that predictable patterns of personality change do occur as a result of the disease process. These changes were seen in a Mediterranean sample that diverges culturally from the European and North- and South-American populations in which such personality changes have previously been observed, which supports the argument that neurologic factors superseding cultural environment may be involved. Though non-biological factors (e.g., a psychological reaction to becoming ill) may have influenced these group-level changes in personality across cultures, degenerative changes in specific neuroanatomic regions likely play a significant role 13, 14
Personality change in bvFTD
The bvFTD group showed significant decreases in conscientiousness and extraversion, which are findings common to other neurodegenerative diseases 7–9. Though there was a quantitative drop in agreeableness in this group, these changes were not statistically significant in our sample, despite evidence from previous reports indicating that bvFTD patients become significantly more disagreeable in the course of their disease 14, 17, 18. The previous research has occurred primarily with fairly high-functioning bvFTD patients, while the mean MMSE of this sample averaged 17.6/30, suggesting patients were at a moderate level of disease severity. If loss of agreeableness occurs early in the disease as a result of behavioural disinhibition, but becomes less apparent as patients become more apathetic with advancing disease, then this may cause the group average for agreeableness to be diminished in a sample comprised of more advanced bvFTD patients. The wider standard deviations on personality measures seen in our bvFTD patients than in the other groups might be evidence that this group included patients at many levels of disease severity.
Unlike the other two groups, bvFTD patients’ neuroticism decreased to a clinically abnormal level compared to levels typically seen in controls. Gradual lack of reactivity to social or emotional context is very characteristic of bvFTD patients. They lose sensitivity to criticism, exhibit diminished insight 33 and self-awareness 27 as well as self-conscious emotional responding 34 even in the context of a most dramatic event. Thus, they are less likely to appear anxious or depressed, or to experience shyness or feelings of guilt. This decrease in Neuroticism has also been seen in a sample of bvFTD patients in Great Britain 14.
Our results reflect a clear uniformity in our groups’ premorbid personality factor scores, within the range of normal personality. However, we saw unexpectedly high levels of conscientiousness and low levels of openness across groups at the premorbid stage, even to the point where bvFTDs were significantly lower than MCIs in conscientiousness premorbidly. This difference could be the reflection of a random true difference in premorbid personality across diagnostic groups, either due to a systematic bias in the individuals represented in this relatively small sample, or due to a disease-related difference in premorbid personality in which some individuals who later develop bvFTD have a lifelong pattern of poor conscientiousness. However, it may also be that this result is due to informants’ uncertainty in estimating the time of symptom onset in the bvFTD patients. This explanation seems more plausible considering the recent findings showing that Greek caregivers appear to underestimate the timing of bvFTD patients’ first symptoms, placing them approximately 6 years later than caregivers in the USA. The authors suggest that the initial behavioural symptoms of bvFTD are ignored or are more likely to be accepted as a normal part of life in Greece 35. This retrospective bias among Greek caregivers may have exerted a systematic effect on the estimates of change in this study.
Personality change in AD and MCI
Confirming our hypotheses concerning the AD group, decreases were found in conscientiousness, extraversion, and agreeableness, while neuroticism significantly increased. The MCI group showed a very similar pattern of change to the AD patients, contrary to our expectations.
MCI patients presented with a significant change in three out of five personality factors: a decrease in conscientiousness and extraversion, as well as an increase in neuroticism, supporting recent research suggesting personality change in MCI may occur earlier than previously expected 21, 35. While the patients diagnosed with MCI do not yet show clear clinical symptoms that would allow prediction of underlying neuropathology, this group was comprised only of patients with an amnestic-only or amnestic-plus pattern of cognitive deficits, and were selected in order to decrease the likelihood that their symptoms were caused by a non-Alzheimer’s neuropathology. Furthermore, the fact that the MCI group’s pattern of personality change, particularly the increase noted in neuroticism, more closely matches the pattern seen in the AD than the bvFTD group, provides support for the hypothesis that this group is primarily composed of patients with an underlying AD pathology. In fact, the distinction between pre-AD MCI patients and those already diagnosed with AD is not necessarily clear in clinical practice. There may have also been a diagnostic bias at work in which patients with a higher level of education had the cognitive reserve necessary to compensate and remain functional in their everyday life, explaining the higher mean level of education in the MCI group, while lower-educated patients may have been more likely to meet criteria for AD early on.
Looking at the patterns of personality change of the AD and bvFTD groups, it is possible to observe specific traits differentiating them. In particular the direction of personality change between the two groups is opposite for the trait of neuroticism, with a significant increase for the AD patients and a decrease for the bvFTD patients. Recent studies have examined the intrinsically connected brain network involved in evaluation of personally salient information in AD and bvFTD patients. Higher connectivity in this “salience network”, which has been associated with higher levels of anxiety in healthy adults 36, has been seen in AD patients, while bvFTD patients show a corresponding decrease of intrinsic connectivity in this network 37. While anxiety and neuroticism are not synonymous, trait neuroticism represents one’s ability to manage negative emotions, thus these two constructs overlap and may share neural circuitry. The divergence of neuroticism between AD and bvFTD patients may be a clinical marker for this altered functional connectivity, thus may be very useful for differential diagnosis.
No significant differences were found for openness in any of the patient groups. Previous findings for this factor in bvFTD and AD have been mixed 9, 14, 38. Researchers have found normal decreases in openness observed in the healthy elderly 1, 10, 11. However, our subjects’ scores for openness to new experiences were at the lower limit of normal for both current and retrospective premorbid ratings, with no significant differences between disease groups, which might be explained by the culturally specific representation of the elderly in Greece. Openness to experience reflects qualities such as appreciation of art and beauty, receptivity to the inner world of imagination, openness to new experiences on a practical level, openness to inner feelings and emotions, intellectual curiosity, and readiness to re-examine one’s own values as well as those of authority figures. In Greek culture, the elderly would rarely be seen by their family as carrying those traits, since their retreat from an active professional life is usually followed by a retreat from social activity in general. They become less exposed to new, everyday stimuli and therefore are presented with less opportunity to express these traits. Taking into account the fact that our study participants tended to have low to moderate levels of education, were over 60 years old, and the majority were female (71%), we believe that these findings for openness might best be explained by these cultural factors, and are less likely to have been directly caused by focal neurodegeneration.
Limitations and Future Directions
This study is limited by the fact that sample sizes were fairly small, thus we were unable to detect smaller effect sizes. Also, due to resource limitations, we were unable to collect personality data on a healthy older control group for this study. While our patients’ T-scores were derived by standardizing their performance against a large sample of normal Greek adults 23, 24, this highly educated and younger control sample was not ideal for this purpose. However, this lack of an age- and education-matched normal control group did not impact our ability to compare the “before” and “after” timepoints to assess clinical change within patients, nor did it affect our comparisons across disease groups, which were the primary aims of our study. Furthermore, clinical staging for these patients was performed using the MMSE, which is less likely to accurately incorporate the behavioral deficits seen in bvFTD than other staging tools like the Clinical Dementia Rating Scale (CDR). Another limitation is the lack of neuroanatomical data for these patients, which may have provided more evidence for why our results did not entirely confirm our literature-based hypotheses. Finally, our patients’ education level differences, although taken into account in our data analyses, could have influenced diagnostic group assignment. A further investigation of personality change of these diagnostic groups over the course of the disease, as well as the disease’s effect on personality for patients with different neurodegenerative conditions, would help clarify these issues. A follow-up of our MCI group would allow us to test our hypothesis that these surprisingly early changes for certain personality traits represent a prodrome of AD. If this is the case, personality testing may be a useful new tool supporting early prediction of AD.
Conclusions
This is the first effort to compare personality changes in bvFTD, AD, and MCI in the Greek population. Our finding that MCI patients present with decreased conscientiousness and extraversion, as well as increased neuroticism, support recent research suggesting personality change in MCI may occur earlier than previously expected, and may represent a prodrome of AD. The discovery that neuroticism changes in opposite directions in bvFTD and AD may prove to be a useful differential diagnostic marker. Our study did not find that agreeableness decreased significantly for bvFTD patients as expected, but further investigation is necessary to better understand how ratings of agreeableness may be subject to cultural, educational, or selection bias factors. Though personality change has long been neglected as a potential clinical marker differentiating neurodegenerative diseases, this study suggests that further investigation of these changes in various cultural milieus is warranted.
Acknowledgments
Sources of financial support: No direct financial support was provided for this specific study.
References
- 1.McCrae RR, Costa PT, Jr, Pedroso de Lima M, Simoes A, Ostendorf F, Angleitner A, Marusic I, Bratko D, Caprara GV, Barbaranelli C, Chae JH, Piedmont RL. Age differences in personality across the adult life span: Parallels in five cultures. Developmental Psychology. 1999;35(2):466–477. doi: 10.1037//0012-1649.35.2.466. [DOI] [PubMed] [Google Scholar]
- 2.Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin. 2000;126(1):3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: A meta-analysis of longitudinal studies. Psychological Bulletin. 2006;132(1):1–25. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- 4.McCrae RR, Terracciano A. Personality profiles of cultures: Aggregate personality traits. Journal of Personality and Social Psychology. 2005;89(3):407–425. doi: 10.1037/0022-3514.89.3.407. [DOI] [PubMed] [Google Scholar]
- 5.Heine SJ, Buchtel EE. Personality: The universal and the culturally specific. Annual Review of Psychology. 2009;60:369–394. doi: 10.1146/annurev.psych.60.110707.163655. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt DP, Allik J, McCrae RR, Benet-Martinez V. The geographic distribution of the big five personality traits: Patterns and profiles of human self-description across 56 nations. Journal of Cross-Cultural Psychology. 2007;38(2):173–212. [Google Scholar]
- 7.Siegler IC, Dawson DV, Welsh KA. Caregiver ratings of personality change in alzheimer’s disease patients: A replication. Psychology and Aging. 1994;9(3):464–466. doi: 10.1037//0882-7974.9.3.464. [DOI] [PubMed] [Google Scholar]
- 8.Siegler IC, Welsh KA, Dawson DV, Fillenbaum GG, Earl NL, Kaplan EB, Clark CM. Ratings of personality change in patients being evaluated for memory disorders. Alzheimer’s Disease and Associated Disorders. 1991;5:240–250. doi: 10.1097/00002093-199100540-00003. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee A, Strauss M, Smyth KA, Whitehouse PJ. Personality changes in alzheimer’s disease. Archives of Neurology. 1992;49:486–491. doi: 10.1001/archneur.1992.00530290070014. [DOI] [PubMed] [Google Scholar]
- 10.Lautenschlager NT, Forstl H. Personality change in old age. Current Opinion in Psychiatry. 2007;20(1):62–66. doi: 10.1097/YCO.0b013e3280113d09. [DOI] [PubMed] [Google Scholar]
- 11.Allemand M, Zimprich D, Hertzog C. Cross-sectional age differences and longitudinal age changes of personality in middle adulthood and old age. Journal of Personality. 2007;75(2):323–358. doi: 10.1111/j.1467-6494.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 12.Roberts BW, Mroczek D. Personality trait change in adulthood. Current Directions in Psychological Science : A Journal of the American Psychological Society. 2008;17(1):31–35. doi: 10.1111/j.1467-8721.2008.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sollberger M, Stanley CM, Wilson SM, Gyurak A, Weiner MW, Miller BL, Rankin KP. Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia. 2009;47(13):2812–2827. doi: 10.1016/j.neuropsychologia.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahoney CJ, Rohrer JD, Omar R, Rossor MN, Warren JD. Neuroanatomical profiles of personality change in frontotemporal lobar degeneration. The British Journal of Psychiatry : The Journal of Mental Science. 2011;198(5):365–372. doi: 10.1192/bjp.bp.110.082677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51(699071142):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 16.Pose M, Catagnola G, Gleichgerrcht E, Torralva T, Lopez P, Torrente F, Manes F. Personality in frontotemporal dementia [abstract] Neurology. 2010 Apr [Google Scholar]
- 17.Rankin KP, Kramer JH, Mychack P, Miller BL. Double dissociation of social functioning in frontotemporal dementia. Neurology. 2003;60(2):266–271. doi: 10.1212/01.wnl.0000041497.07694.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rankin KP, Rosen HJ, Kramer JH, Schauer GF, Weiner MW, Schuff N, Miller BL. Right and left medial orbitofrontal volumes show an opposite relationship to agreeableness in FTD. Dementia and Geriatric Cognitive Disorders. 2004;17(4):328–332. doi: 10.1159/000077165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pocnet C, Rossier J, Antonietti J, von Gunten A. Personality changes in patients with beginning alzheimer’s disease. Canadian Journal of Psychiatry. 2011;56(7):408–417. doi: 10.1177/070674371105600704. [DOI] [PubMed] [Google Scholar]
- 20.Wahlin TR, Byrne GJ. Personality changes in alzheimer’s disease: A systematic review. International Journal of Geriatric Psychiatry. 2011;26:1019–1029. doi: 10.1002/gps.2655. [DOI] [PubMed] [Google Scholar]
- 21.Sollberger M, Neuhaus J, Ketelle R, Stanley CM, Beckman V, Growdon M, Jang J, Miller BL, Rankin KP. Interpersonal traits change as a function of disease type and severity in degenerative brain diseases. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82(7):732–739. doi: 10.1136/jnnp.2010.205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ausen B, Edman G, Almkvist O, Bogdanovic N. Personality features in subjective cognitive impairment and mild cognitive impairment -- early indicators of dementia? Dementia and Geriatric Cognitive Disorders. 2009;28:528–535. doi: 10.1159/000255104. [DOI] [PubMed] [Google Scholar]
- 23.Tsaousis I. The traits personality questionnaire (TPQue): A greek measure for the five factor model. Personality and Individual Differences. 1999;26:271–283. [Google Scholar]
- 24.Tsaousis I, Kerpelis P. The traits personality questionnaire 5 (TPQue5) European Journal of Psychological Assessment. 2004;20(3):0–191. [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. 1984;34(784246267):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 27.Rankin KP, Baldwin E, Pace-Savitsky C, Kramer JH, Miller BL. Self-awareness and personality change in dementia. Journal of Neurology, Neurosurgery and Psychiatry. 2005;75(5):632–639. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sollberger M, Stanley CM, Ketelle R, Beckman V, Growdon M, Jang J, Neuhaus J, Kramer JH, Miller BL, Rankin KP. Neuropsychological correlates of dominance, warmth, and extraversion in neurodegenerative disease. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2011 doi: 10.1016/j.cortex.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yesavage JA. Geriatric depression scale: Consistency of depressive symptoms over time. Perceptual and Motor Skills. 1991;73(3 Pt 1):1032. doi: 10.2466/pms.1991.73.3.1032. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JL. The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology. 1997;48(5):S10–6. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 31.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The addenbrooke’s cognitive examination revised (ACE-R): A brief cognitive test battery for dementia screening. International Journal of Geriatric Psychiatry. 2006;21(11):1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- 32.SAS. User guide Version 9.3. 2011. [Google Scholar]
- 33.Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini M, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sturm VE, Ascher EA, Miller BL, Levenson RW. Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion. 2008;8(6):861–869. doi: 10.1037/a0013765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papatriantafyllou JD, Viskontas IV, Papageorgiou SG, Miller BL, Pavlic D, Bingol A, Yener G. Difficulties in detecting behavioral symptoms of frontotemporal lobar degeneration across cultures. Alzheimer Disease and Associated Disorders. 2009;23(1):77–81. doi: 10.1097/WAD.0b013e318182d874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW. Divergent network connectivity changes in behavioural variant frontotemporal dementia and alzheimer’s disease. Brain : A Journal of Neurology. 2010;133(Pt 5):1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strauss ME, Pasupathi M. Primary caregivers’ descriptions of alzheimer patients’ personality traits: Temporal stability and sensitivity to change. Alzheimer Disease and Associated Disorders. 1994;8(3):166–176. doi: 10.1097/00002093-199408030-00003. [DOI] [PubMed] [Google Scholar]


