Abstract
Although numerous studies have been conducted on the use of ultrasonography (US) for the examination of thoracic structures, this procedure is not as widely accepted as abdominal US. The newer portable scanners can be used at the bedside to detect pleural malignancies and effusions, as well as peripheral lung nodules of the lung, even in seriously ill patients. Focal thickening of the pleura can be easily detected with US and further investigated with a US-guided biopsy. US guidance can also be used during percutaneous drainage of pleural effusion or transthoracic biopsy of peripheral lung lesions, thus reducing the incidence of procedure-related pneumothorax to almost zero. We review the current literature on thoracic US and present our clinical experience with the technique in large groups of patients with pleural and peripheral lung diseases.
Keywords: Ultrasonography, Chest, Pleura, Lung, Biopsy
Sommario
L'ecografia del torace non è ancora diffusa quanto quella addominale, nonostante una notevole quantità di studi ne attesti l'importanza. Le apparecchiature più recenti permettono di diagnosticare neoplasie, versamenti pleurici e noduli polmonari periferici al letto del paziente, anche nei casi più gravi. L'ispessimento pleurico focale è facilmente messo in luce dall'ecografia e può essere ulteriormente studiato tramite la biopsia ecoguidata. Questa può essere praticata anche su lesioni polmonari periferiche, riducendo quasi a zero il rischio di pneumotorace. In questo articolo esaminiamo la letteratura recente sull'ecografia del torace e presentiamo la nostra esperienza clinica su numerosi pazienti con patologia pleurica e della periferia polmonare.
Introduction
Ultrasonography (US) can be used to explore the surfaces of the lungs through the intercostal spaces, but the presence of the ribs and of air in the expanded lung reduces the value of this imaging modality in the examination of deeper thoracic structures. Nevertheless, US is considered a reliable, inexpensive, safe, and reproducible diagnostic method for the work-up of patients with diseases of the diaphragm (neoplasms, paresis), thoracic wall (abscesses, fistulas, neoplasms), lung (atelectasis, pulmonary consolidation), anterosuperior mediastinum (neoplasms, lymphoma, cysts), the region between the thorax and the abdomen, and above all, the pleurae (extrapleural masses, pleural effusions) [1].
Thanks to the recent diffusion of sophisticated US scanners equipped with color and power Doppler technology and special transducers for transesophageal and endobronchial examinations, US can now be used to investigate disorders involving the esophagus, bronchi, bronchial blood vessels, mediastinum, and the large vessels of the heart [2]. Although computed tomography is still the imaging method of choice for the diagnosis of these conditions, thoracic US can be considered an important supplementary tool in this setting [3]. Today, thoracic US is mainly used to guide transthoracic biopsy of peripheral lung lesions and the drainage of pleural effusions [4]. The increasingly widespread use of second-generation ultrasound contrast agents is further expanding the role of thoracic US, and it is producing promising results in the characterization of peripheral lung masses [5].
Technique
The US examination of the chest requires a scanner equipped with a sector or convex small array probe with medium to high frequency (3.5–7.5 MHz). In most cases, conclusive information can be obtained with a 3.5-MHz transducer. However, a high-frequency (8–10 MHz) linear array probe is needed to study the chest wall, the pleurae, and the superficial structures of the lung. Color Doppler technology is not essential during the initial thoracic US examination, but it is a must during minimally invasive procedures such as biopsy or drainage.
The thoracic US examination can be performed at the bedside, and no specific patient preparation is needed. However, patients with respiratory failure should receive drug therapy and oxygen to relieve their symptoms during the examination and reduce the risk of motion-related artifacts caused by labored breathing. Scans of the basal pleurae and the diaphragm should be obtained with the patient seated and then (when possible) in the supine position. In rare cases, the patient may be asked to stand during the examination (e.g., when the costophrenic recess is being explored for a possible effusion) [6,7]. Various scanning planes can be used: intercostal, longitudinal, transversal, and paravertebral scans can be used to explore the posterior chest wall, whereas the anterior wall is usually investigated with intercostal, longitudinal, supra- and parasternal, sub-xiphoid, and supraclavicular scans. Specific acoustic windows are exploited to improve visualization of the examined structures. On the right side of the body, the liver provides a good window for observing the basal pleurae and the diaphragmatic dome (The patient should be placed in the supine position and instructed to inhale deeply). The spleen provides a similar window for examination of structures in the left side of the chest. Each pathological finding must be documented in two perpendicular projections. In some cases, US can also be used to further explore thoracic lesions that have already been visualized on chest radiographs or on CT scans.
We currently perform most of our thoracic US examinations with a Toshiba SSA340 scanner (Toshiba, Tokyo, Japan) and both convex (3.5 Mhz) and linear (8 MHz) probes. In some cases, however, we use a multifrequency scanner (Esaote Technos MPX, Esaote, Genoa, Italy) with convex (3.5 MHz) and linear (8 MHz) transducers and special software for the analysis of second-generation ultrasound contrast agent signals.
Clinical applications
The thoracic structures that can be explored by US are (starting at the surface) (1) skin, (2) derma, (3) intercostal muscles and endothoracic membrane, (4) extrapleural fat and the parietal and visceral pleurae (Fig. 1). Once the US beam has penetrated the visceral pleura, it is completely dispersed by the air in the lungs. The elevated acoustic impedance generated at the interface between the superficial soft tissues and the air in the lung results in a thin (<3 mm) echogenic line known as the pleural line. The parietal pleura is immobile, whereas the visceral pleura moves during respiration (“gliding or sliding sign”). In healthy subjects, the marked difference in the acoustic impedance levels of soft tissue and air-filled structures usually produces two types of artifact: [8] the “comet-tail” artifact, which consists in parallel, hyperechoic reverberations extending vertically from the pleural interface to the opposite edge of the screen, and “reverberation artifacts,” which are concentric, horizontal hyperechoic lines representing the interface between the pleura and the thoracic wall (Fig. 2).
Fig. 1.
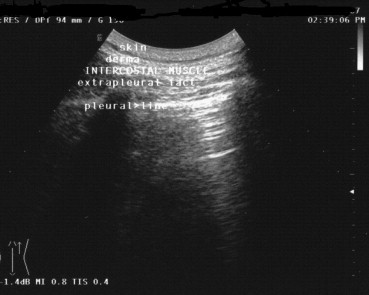
The structures that can be explored by thoracic US include (from the surface) (1) skin; (2) derma; (3) intercostal muscles, endothoracic membrane; (4) extrapleural fat, parietal and visceral pleura.
Fig. 2.
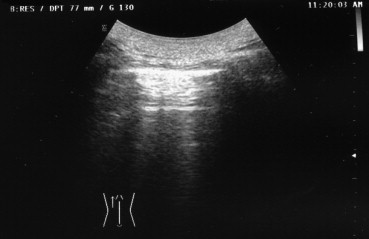
Reverberation artifacts (concentric, horizontally oriented, hyperechoic lines that represent the pleural-thoracic wall interface) in a normal subject.
Pleural and extrapleural diseases
Thoracic US is the “gold-standard” method for studying pleural effusions [9]. It is more sensitive than chest radiography or CT in the detection of small amounts of pleural fluid (less then 10 ml) (Fig. 3). Effusions appear as a sharply demarcated, dark or echo-free zone image associated with downward displacement of the pleural line. The underlying lung may be well aerated, consolidated, or atelectatic [10]. Various formulas have been elaborated to estimate the volume of a pleural effusion and of atelectatic lung tissue and to identify the characteristics of the pleural fluid. In routine, daily practice, we usually measure the maximal longitudinal and transversal diameters of the effusion [11].
Fig. 3.

A longitudinal-oblique posterobasal US scan reveals a small effusion in the left costodiaphragmatic recess.
An expert examiner is usually able to define the diagnostically relevant characteristics of an effusion. Four types of effusion fluid may be distinguished on US: (1) anechoic (similar to the contents of a simple cyst); (2) complex nonseptate (resembling that of a simple cyst with floating particles); (3) complex septate (similar to the contents of a complex cyst) (Fig. 4); (4) homogeneously hyperechogenic, which indicates a corpuscular effusion [3] (Fig. 5). US can also reveal several signs of inflammation (fibrous strands, mobile or immobile septa with encapsulated liquid), as well as pleural thickening. The echogenic characteristics of the fluid may be suggestive of empyema, hemothorax, or neoplastic effusion. These exudative effusions are usually associated with a blurred signal (due to their high protein content) and with a thickened, nodular pleura. However, in all these cases, the diagnosis needs to be confirmed by exploratory thoracentesis and biopsy. According to some authors, the presence within the effusion fluid of numerous, floating, echogenic particles that swirl in response to respiratory or cardiac movements (the “swirling pattern”) is strongly predictive of a malignant pleural effusion [12].
Fig. 4.
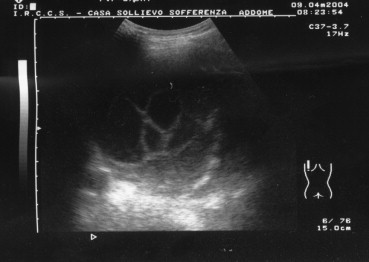
US appearance of a complex septate pleural effusion at the base of the lung.
Fig. 5.
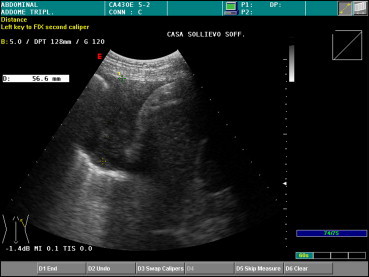
The basal effusion shown in Fig. 4 is uniformly hyperechogenic on US.
Effusions located close to the pulmonary wall, which are usually associated with solid pulmonary formations or pneumonia, are easy to detect [12]. Some effusions move freely when the patient's position changes; encapsulated effusions are less mobile. An encapsulated pleural effusion can be easily differentiated from a fibrothorax, which parallels the ribs and does not move with respiratory movements.
US can also be employed to continuously monitor the needle position during diagnostic and therapeutic thoracentesis procedures. It is particularly valuable in this setting due to its ability to detect minute amounts of fluid (less then 10 ml) within the costophrenic recesses [13].
Malignant pleural lesions (mesothelioma or metastasis) can be easily visualized on US, especially when they are associated with pleural effusions, as is often the case with diffuse pleural mesotheliomas (Fig. 6). These tumors may appear as a hyperechoic lesion (or, in rare cases, one with a mixed echo pattern) associated with thickening of the parietal and diaphragmatic pleura. In all cases, US is a valuable tool for guiding pleural biopsy, which is associated with a low rate of complications [14]. Several other lesions of the chest wall can also be detected with US, including cysts, abscesses, hematomas, benign tumors, and various lesions involving the ribs (metastases, bacterial osteomyelitis, tubercular lesions). In the presence of osteomyelitic involvement, the ribs present irregular margins and are often surrounded by anechoic infiltrates [2].
Fig. 6.

A mesothelioma is easily detected by US when it is associated with pleural effusion.
The findings that emerge from thoracic US are not always specific, but this imaging modality can still be helpful for assessing pleural changes, e.g., extension, interruption, displacement of the echoic pleural line, its immobility during respiratory movements. US-guided transthoracic cutting biopsy may also be of value for diagnosing lesions in the peripheral regions of the chest, which is still based primarily on chest radiography, CT, and MR imaging [15].
Pulmonary diseases
Pulmonary disease can also be detected by US as long as there is no air between the probe and the lesion and the beam reaches the pleura. Even a thin layer (1–2 cm) of air can seriously reduce the visualization of solid lesions, regardless of their size. In certain cases, US imaging can also reveal deeper-seated pulmonary lesions, e.g., when the surrounding parenchyma is consolidated, i.e., atelectatic, or when the lesion is surrounded by a pleural effusion, which acts as an acoustic window [16].
Uniformly anechoic pulmonary lesions, usually well circumscribed, may represent cysts (congenital, bronchogenic, parasitic, or pleural) or, more rarely, pulmonary infarcts. Lipomas, which are benign tumors, appear on US as localized nodules that are hypoechoic or anechoic (Fig. 7). The presence of echoes within a hypoechoic–anechoic cyst is indicative of a “complex” lesion, such as an abscess, a hematoma, a necrotic neoplasm, or a multivesicular hydatid cyst [17] (Fig. 8). Pulmonary abscesses appear as circumscribed, collections of corpuscular fluid. They can be detected easily with US when they are located in the peripheral regions of the lung, close to the parietal pleura, or associated with pleural adhesions [18].
Fig. 7.
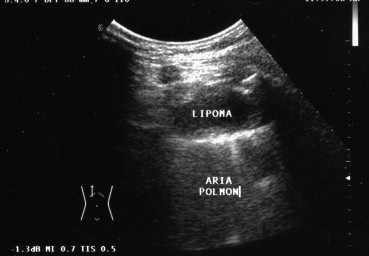
Pulmonary lipoma appears on US as a localized hypo-anechoic nodule.
Fig. 8.

Pulmonary echinococcal cyst with small daughter cysts (Type CE3b in the WHO Classification).
Nodular or ovoid solid lesions with blurred margins are suggestive of lung tumors. They are usually hypoechoic, but hyperechoic and anechoic patterns (due to the presence of colliquative necrosis) are not infrequent (Fig. 9) [19]. Histologically, peripheral pulmonary malignancies may be adenocarcinoma, squamous cell carcinoma, undifferentiated large-cell carcinoma, microcytoma, or metastatic lesions [20]. Although conventional US cannot discriminate between different pulmonary neoplasms [21], the use of US contrast agents has produced promising results. Recent studies suggest that contrast-enhanced US might help identify the nature of peripheral lung masses [22]. We have recently shown that US performed with the second-generation ultrasound contrast agent, SonoVue® (Bracco, Milan, Italy) can be used to differentiate malignant from benign neoplasms [17] (Fig. 10), although these preliminary findings need to be confirmed in a larger series of patients.
Fig. 9.

Hypoechoic lesions with blurred margins in a patient with a malignant tumor located in the periphery of the left lung.
Fig. 10.

US performed with the contrast agent SonoVue® (Bracco) reveals an adenocarcinoma of the right lung in the posterobasal region. Contrast enhancement of the lesion was detectable 90 seconds after intravenous administration of SonoVue®.
Pulmonary abscesses cannot be differentiated from tumors undergoing colliquative necrosis based on US findings alone, and the US diagnosis of solid pulmonary lesions also requires confirmatory studies, such as transthoracic cutting biopsy. However, US plays a critical role in the guidance of the latter procedure [23], and some authors [24] recommend it as a supplement to CT in the study of peripheral lung malignancies. US seems to be highly sensitive in defining local tumor extension, providing good visualization of invasion of the pleura and the chest wall. The mobility of the lesion during the various phases of breathing can also be evaluated.
The pneumoconioses, a group of a lung diseases caused by inhaled particles, are not associated with typical US patterns, although irregular pleural thickening is commonly detected in the advanced phases of the disease. The location of the lesion in the lung may be of help in the diagnostic work-up: the presence of subpleural nodules or of lesions in the dorsal apex of the lung are suggestive of silicosis, whereas peripheral lesions in the lower lobes are a characteristic feature of asbestosis [25].
US can detect interstitial lung disease if the interstitium of the peripheral lung is involved [26]. The typical US findings associated with pulmonary fibrosis include [27] (1) fragmentation and irregular thickening of the pleural line, especially in the lower lobes (findings that are independent of the severity of the disease) (Fig. 11); (2) attenuation of the physiological gliding sign, which is related to the stage of the disease; (3) immobility of the diaphragm, which is best visualized on a scan of the right chest made when the patient is in the supine position (end-stage disease); and (4) multiple reverberation artifacts (advanced stage). All these abnormalities can be detected in both lungs, which is a reflection of the diffuse nature of the fibrotic process [28].
Fig. 11.
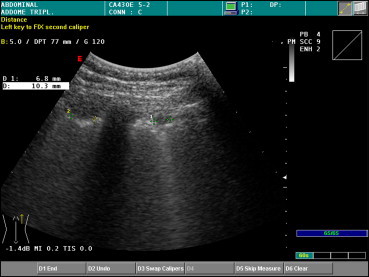
Micronodulation, fragmentation, and irregular thickening of the pleura associated with idiopathic pulmonary fibrosis.
The US findings associated with fibrothorax are a thickened pleural line associated with an attenuated gliding sign. Pulmonary sarcoidosis is associated with micronodulation, related to the thickness of connective tissue in the upper lobes of the lung. In some cases, small pleural effusions are also seen at the lung bases.
Lung atelectasis is easily diagnosed by US [29]. Two forms can be distinguished; a central form caused by airway obstruction and a peripheral form related to parenchymal compression by effusion fluid, air (pneumothorax), trauma, diffuse fibrous tissue, or malignant tumors. Within the boundaries of the atelectatic lesion, the consolidated parenchyma generates a homogeneous pattern that is isoechoic or hypoechoic with respect to the liver. Sometimes, fluid-filled tubular structures with echogenic walls are visible within the lesion; these images represent pulmonary blood vessels or mucus-filled bronchi (the so-called “fluid US bronchogram”) [30].
Inflammatory processes, such as pneumonia or pulmonary consolidation, that involve the peripheral regions of the lung can also be detected by US. Inflamed lung tissue usually appears as a hypoechoic or anechoic lesion, or, sometimes, as a hypo–hyperechoic image with blurred margins. Within the borders of the lesion are hyperechoic lines corresponding to bronchi filled with air (the “sonographic air bronchogram”) (Fig. 12). A less common finding is the presence of inflated hypoechoic tubular lines, which represent trapped vessels or edematous bronchi (the “sonographic fluid bronchogram”) [31]. Inflammatory diseases of the lung are frequently associated with basal pleural effusions, which can be detected by US. This modality can also be useful in the follow-up of a patient with pulmonary consolidation, since it allows visualization of changes involving the inflammatory process after therapy [32]. Although data based on the study of a small series of patients with bronchopneumonia suggest that contrast-enhanced US performed with SonoVue® can be helpful in this setting, the results are still inconclusive.
Fig. 12.

Laterobasal consolidation of the left lung: The hyperechoic line (arrow) represents air-filled bronchi (“US air bronchogram”).
The most characteristic US findings associated with pneumothorax are the absence of the physiological gliding sign and the absence of comet-tail artifacts during respiratory movements [33]. To investigate the potential contributions of US to the work-up of this disorder, we observed over 340 patients with either pneumothorax or hydro-pneumothorax over a 12-year period. In all subjects, chest radiography was performed before US imaging, but the sonographer was blinded to the radiographic diagnosis. The most common US findings in patients with pneumothorax were (a) absence of the gliding sign (all patients with pneumothorax), and (b) absence of ring-down artifacts (75% of cases). The main finding in patients with hydro-pneumothorax was the so-called “curtain sign.” The diagnosis of pneumothorax or hydro-pneumothorax made on the basis of posteroanterior radiographic findings was consistently confirmed by the US study [29].
Since then, we have regularly used US alone to rule out the possibility of pneumothorax following thoracentesis or pleuro-pulmonary biopsy. In light of our experience and reports in the literature [34], the diagnostic accuracy of US in most cases of pneumothorax and hydro-pneumothorax appears superior to that of conventional chest radiography. Except in a few dubious cases, the latter examination is no longer mandatory after invasive diagnostic or therapeutic procedures. However, in the presence of diffuse fibrothorax, diagnosis of pneumothorax by US imaging is very difficult because the gliding sign is not easily detectable in these patients [35].
A recent study [36] has emphasized the role of US in the diagnosis of acute pulmonary edema. Management of this condition is based mainly on clinical findings, but imaging confirmation is often sought by the attending physician. US might be even more informative than standard chest radiography in distinguishing acute pulmonary edema from an exacerbation of bronchopneumonia [37]. The presence of multiple comet-tail or vertical artifacts (more than 4–6 per costal space) is indicative of acute pulmonary edema [38]. In 68 consecutive patients presenting to our Emergency Department with clinical signs of acute pulmonary edema, this finding was detected in all cases [39]. This experience confirms the diagnostic accuracy of US imaging for the diagnosis of “wet lung” based on the observation of these artifacts [40] (Fig. 13).
Fig. 13.
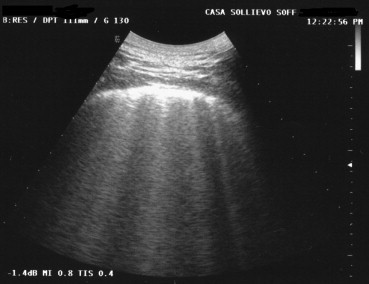
The presence of more than 4 comet-tail artifacts in a single intercostal space and the absence of horizontal artifacts is suggestive of “wet lung” in patients with acute pulmonary edema.
Invasive pleural and pulmonary procedures
Thoracic US is widely used to guide needle placement during thoracentesis procedures, reducing the risk of pneumothorax [41]. Pneumothorax reportedly occurs in 7–15% of patients who undergo blind thoracentesis, but the frequency drops to 0.5% when US needle guidance is used [42,43]. In the past 10 years, we have performed 1480 diagnostic or therapeutic thoracenteses under US guidance. In 270 (18%) cases, the purpose of the procedure was to examine the drainage fluid for tumor cells. There were only two cases of pneumothorax (0.1%), both of which resolved spontaneously within a few days of the procedure, and only one patient developed pulmonary edema.
As mentioned above, US can provide information on the characteristics of the effusion (simple, encapsulated, or organized), which is important for defining the nature of opacities seen on chest radiographs (e.g., differentiation of pleural effusion and pulmonary consolidation) [44]. Every emergency department should be equipped with an US scanner. It can play a key role in verifying clinical suspicion of massive pleural effusion in patients with acute respiratory distress. It also allows prompt drainage of the chest with a low rate of pneumothorax. In critically ill patients, even massive effusions can often be drained within a reasonably short time with a low-flow, low-pressure aspirator with a small-gauge needle (20 G).
Pleural lesions that can be evaluated with US-guided transthoracic biopsy include nodular thickening, which appears as hypoechoic micronodulation often combined with pleural line thickening; and pleural mesotheliomas, which appear as hyperechoic mixed lesions, often multiple forms, that are associated with serohematic effusions [45]. (Benign pleural lesions, such as fibromas or lipomas, are rare.) In cases of pneumonia, it is often impossible to determine the etiology based on bacteriological assessment of sputum and/or pleural effusion fluid, blood cultures, and serological examination of urine specimens [46]. The reported complication rates are low (pneumothorax in 1–2% of the cases). In cases of pleural empyema or peripheral pulmonary abscess, US guidance is a fundamental clinical tool.
US is capable of detecting lung tumors only when they are located in the peripheral regions of the organ [47]. Because there is no typical neoplastic pattern on US (or on other imaging methods), needle biopsy of these lesions is mandatory. All pulmonary lesions detected with US can be safely subjected to needle-aspiration. We currently use both aspiration and cutting 20–21 G needles (Histo-cut, Sterylab, Rho, Italy or Biomol Hospital Service, Aprilia, Italy). In our experience, the lung tumors most frequently diagnosed by US-guided needle-aspiration are adenocarcinomas (41%), squamous cell carcinomas (37%), large-cell anaplastic carcinomas (17%), microcytomas or metastases (5%). The diagnostic accuracy rates reported in the literature for US-guided transthoracic needle-aspiration biopsy range from 85–96% [48]. In our hands, the procedure was associated with even higher diagnostic accuracy (99%). In a series of 740 patients with peripheral lung tumors, we obtained cytological–histological diagnoses of carcinoma in 683 patients. The complication rate was only 0.7% (three cases of partial pneumothorax, which resolved spontaneously; one case of mild hemoptysis; and 1 case of complete pneumothorax, which was successfully managed with chest-tube drainage) [49]. The use of US-guided radiofrequency thermal ablation of lung tumors is attracting increasing attention [48]. For this purpose, US appears to be more valuable than computed tomography because it allows continuous, real-time visualization of all phases of the procedure.
The most recent application of US in the study of pleural and pulmonary diseases is endoluminal US [50]. This complex technique, which is currently performed only in a few highly specialized centers, includes (1) transesophageal US-endoscopy, which can reveal abnormalities (lymphadenopathy and neoplastic masses) between the aorta and the central pulmonary veins, eliminating the need for biopsy; (2) endobronchial US, which can be used to visualize intra- and subparietal lesions of the bronchi, as well as peribronchial extension of a bronchial mass, and to characterize the vascular pattern of these lesions [51]; (3) US during thoracoscopy, which can identify tiny nodules that are impossible to detect with others tools [52].
Ethical approval for this study was granted by the Medical Research Ethics Committee of our University, and informed consent was obtained from all patients.
Conflict of interest statement
None declared.
References
- 1.Simeone J.F., Mueller P.R., vanSonnenberg E. The uses of diagnostic ultrasound in the thorax. Clin Chest Med. 1984;5:281–290. [PubMed] [Google Scholar]
- 2.Beckh S., Bolcskei P.L., Lessnau K.D. Real-time chest ultrasonography: a comprehensive review for the pulmonologist. Chest. 2002;122:1759–1773. doi: 10.1378/chest.122.5.1759. [DOI] [PubMed] [Google Scholar]
- 3.McLoud T.C., Flower C.D. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156:1145–1153. doi: 10.2214/ajr.156.6.2028857. [DOI] [PubMed] [Google Scholar]
- 4.Muller N.L. Imaging of the pleura. Radiology. 1993;186:297–309. doi: 10.1148/radiology.186.2.8421723. [DOI] [PubMed] [Google Scholar]
- 5.Bokor D., Chambers J.B., Rees P.J., Mant T.G., Luzzani F., Spinazzi A. Clinical safety of SonoVue, a new contrast agent for ultrasound imaging, in healthy volunteers and in patients with chronic obstructive pulmonary disease. Invest Radiol. 2001;36:104–109. doi: 10.1097/00004424-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Eibenberger K.L., Dock W.I., Ammann M.E., Dorffner R., Hormann M.F., Grabenwoger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191:681–684. doi: 10.1148/radiology.191.3.8184046. [DOI] [PubMed] [Google Scholar]
- 7.Sperandeo M., Caturelli E., Sperandeo G., Camagna A. New technique of thoracentesis in massive hydrothorax. J Hepatol. 2002;36:209. [Google Scholar]
- 8.Mathis G. Thoraxsonography – part I: chest wall and pleura. Ultrasound Med Biol. 1997;23:1131–1139. doi: 10.1016/s0301-5629(97)00112-9. [DOI] [PubMed] [Google Scholar]
- 9.Doust B.D., Baum J.K., Maklad N.F., Doust V.L. Ultrasonic evaluation of pleural opacities. Radiology. 1975;114:135–140. doi: 10.1148/114.1.135. [DOI] [PubMed] [Google Scholar]
- 10.Yu C.J., Yang P.C., Wu H.D., Chang D.B., Kuo S.H., Luh K.T. Ultrasound study in unilateral hemithorax opacification. Image comparison with computed tomography. Am Rev Respir Dis. 1993;147:430–434. doi: 10.1164/ajrccm/147.2.430. [DOI] [PubMed] [Google Scholar]
- 11.Yang P.C., Luh K.T., Chang D.B., Wu H.D., Yu C.J., Kuo S.H. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159:29–33. doi: 10.2214/ajr.159.1.1609716. [DOI] [PubMed] [Google Scholar]
- 12.Chian C.F., Su W.L., Soh L.H., Yan H.C., Perng W.C., Wu C.P. Echogenic swirling pattern as a predictor of malignant pleural effusions in patients with malignancies. Chest. 2004;126:129–134. doi: 10.1378/chest.126.1.129. [DOI] [PubMed] [Google Scholar]
- 13.Wang H.C., Yu C.J., Chang D.B. Transthoracic needle biopsy of thoracic tumours by a colour Doppler ultrasound puncture guiding device. Thorax. 1995;50:1258–1263. doi: 10.1136/thx.50.12.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Targhetta R. Sonographic approach to pulmonary disease. JEMU. 1998;19:217–221. [Google Scholar]
- 15.Pang J.A., Tsang V., Hom B.L., Metreweli C. Ultrasound-guided tissue-core biopsy of thoracic lesions with Trucut and Surecut needles. Chest. 1987;91:823–828. doi: 10.1378/chest.91.6.823. [DOI] [PubMed] [Google Scholar]
- 16.El Fortia M., El Gatit A., Bendaoud M. Ultrasound wall-sign in pulmonary echinococcosis (new application) Ultraschall Med. 2006;27:553–557. doi: 10.1055/s-2006-927232. [DOI] [PubMed] [Google Scholar]
- 17.Sperandeo M., Sperandeo G., Varriale A. Contrast-enhanced ultrasound (CEUS) for the study of peripheral lung lesions: a preliminary study. Ultrasound Med Biol. 2006;32:1467–1472. doi: 10.1016/j.ultrasmedbio.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Yang P.C., Chang D.B., Yu C.J. Ultrasound-guided core biopsy of thoracic tumors. Am Rev Respir Dis. 1992;146:763–767. doi: 10.1164/ajrccm/146.3.763. [DOI] [PubMed] [Google Scholar]
- 19.de Gregorio M.A., D'Agostino H. Ultrasound in pneumology: the current situationArch Bronconeumol. 2003;39:535–536. doi: 10.1016/s0300-2896(03)75450-7. [DOI] [PubMed] [Google Scholar]
- 20.Yu C.J., Yang P.C., Chang D.B., Luh K.T. Diagnostic and therapeutic use of chest sonography: value in critically ill patients. AJR Am J Roentgenol. 1992;159:695–701. doi: 10.2214/ajr.159.4.1529829. [DOI] [PubMed] [Google Scholar]
- 21.Sheth S., Hamper U.M., Stanley D.B., Wheeler J.H., Smith P.A. US guidance for thoracic biopsy: a valuable alternative to CT. Radiology. 1999;210:721–726. doi: 10.1148/radiology.210.3.r99mr23721. [DOI] [PubMed] [Google Scholar]
- 22.Herth F.J., Becker H.D. Transthoracic ultrasound. Respiration. 2003;70:87–94. doi: 10.1159/000068420. [DOI] [PubMed] [Google Scholar]
- 23.Bungay H.K., Adams R.F., Morris C.M., Haggett P.J., Traill Z.C., Gleeson F.V. Cutting needle biopsy in the diagnosis of clinically suspected non-carcinomatous disease of the lung. Br J Radiol. 2000;73:349–355. doi: 10.1259/bjr.73.868.10844858. [DOI] [PubMed] [Google Scholar]
- 24.Sugama Y., Tamaki S., Kitamura S., Kira S. Ultrasonographic evaluation of pleural and chest wall invasion of lung cancer. Chest. 1988;93:275–279. doi: 10.1378/chest.93.2.275. [DOI] [PubMed] [Google Scholar]
- 25.Yang P.C., Luh K.T., Wu H.D. Lung tumors associated with obstructive pneumonitis: US studies. Radiology. 1990;174:717–720. doi: 10.1148/radiology.174.3.2406780. [DOI] [PubMed] [Google Scholar]
- 26.Heilo A. US-guided transthoracic biopsy. Eur J Ultrasound. 1996:141–151. [Google Scholar]
- 27.Decuzzi M., Sperandeo M., Varriale G., Giampaolo A., Tesse M.R. Transthoracic ultrasound in the evaluation of pulmonary fibrosis. Our experience. Ultrasound Med Biol. 2006;32:158. doi: 10.1016/j.ultrasmedbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein D., Meziere G., Biderman P., Gepner A., Barre O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–1646. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstein D., Meziere G., Biderman P., Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25:383–388. doi: 10.1007/s001340050862. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein D.A., Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest. 1995;108:1345–1348. doi: 10.1378/chest.108.5.1345. [DOI] [PubMed] [Google Scholar]
- 31.Targhetta R., Bourgeois J.M., Chavagneux R. Ultrasonic signs of pneumothorax: preliminary work. J Clin Ultrasound. 1993;21:245–250. doi: 10.1002/jcu.1870210406. [DOI] [PubMed] [Google Scholar]
- 32.Kirkpatrick A.W., Ng A.K., Dulchavsky S.A. Sonographic diagnosis of a pneumothorax inapparent on plain radiography: confirmation by computed tomography. J Trauma. 2001;50:750–752. doi: 10.1097/00005373-200104000-00029. [DOI] [PubMed] [Google Scholar]
- 33.Wernecke K., Galanski M., Peters P.E., Hansen J. Pneumothorax: evaluation by ultrasound-preliminary results. J Thorac Imaging. 1987;2:76–78. [PubMed] [Google Scholar]
- 34.Larscheid R.C., Thorpe P.E., Scott W.J. Percutaneous transthoracic needle aspiration biopsy: a comprehensive review of its current role in the diagnosis and treatment of lung tumors. Chest. 1998;114:704–709. doi: 10.1378/chest.114.3.704. [DOI] [PubMed] [Google Scholar]
- 35.Targhetta R., Bourgeois J.M., Chavagneux R., Balmes P. Diagnosis of pneumothorax by ultrasound immediately after ultrasonically guided aspiration biopsy. Chest. 1992;101:855–856. doi: 10.1378/chest.101.3.855. [DOI] [PubMed] [Google Scholar]
- 36.Sargsyan A.E., Hamilton D.R., Nicolaou S. Ultrasound evaluation of the magnitude of pneumothorax: a new concept. Am Surg. 2001;67:232–235. [discussion 235–236] [PubMed] [Google Scholar]
- 37.Lichtenstein D., Meziere G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331–1334. doi: 10.1007/s001340050771. [DOI] [PubMed] [Google Scholar]
- 38.Lichtenstein D.A., Lascols N., Meziere G., Gepner A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004;30:276–281. doi: 10.1007/s00134-003-2075-6. [DOI] [PubMed] [Google Scholar]
- 39.Sperandeo M., Varriale A., Filabozzi P. The role of thoracic echography in the pleuro-pulmonary disease: ambitious or standard project? Intern Emerg Med. 2007;2:S204. [Google Scholar]
- 40.Agricola E., Bove T., Oppizzi M. “Ultrasound comet-tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–1695. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 41.Tsai T.H., Yang P.C. Ultrasound in the diagnosis and management of pleural disease. Curr Opin Pulm Med. 2003;9:282–290. doi: 10.1097/00063198-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Liao W.Y., Chen M.Z., Chang Y.L. US-guided transthoracic cutting biopsy for peripheral thoracic lesions less than 3 cm in diameter. Radiology. 2000;217:685–691. doi: 10.1148/radiology.217.3.r00dc21685. [DOI] [PubMed] [Google Scholar]
- 43.Westcott J.L. Percutaneous transthoracic needle biopsy. Radiology. 1988;169:593–601. doi: 10.1148/radiology.169.3.3055026. [DOI] [PubMed] [Google Scholar]
- 44.Dynes M.C., White E.M., Fry W.A., Ghahremani G.G. Imaging manifestations of pleural tumors. Radiographics. 1992;12:1191–1201. doi: 10.1148/radiographics.12.6.1439021. [DOI] [PubMed] [Google Scholar]
- 45.Pan J.F., Yang P.C., Chang D.B., Lee Y.C., Kuo S.H., Luh K.T. Needle aspiration biopsy of malignant lung masses with necrotic centers. Improved sensitivity with ultrasonic guidance. Chest. 1993;103:1452–1456. doi: 10.1378/chest.103.5.1452. [DOI] [PubMed] [Google Scholar]
- 46.Zimmer T., Rost T., Patan M., Dulce M.C., Liehr R.M., Riecken E.O. Endoscopic ultrasound of pathological mediastinal findings. Radiologe. 1997;37:165–169. doi: 10.1007/s001170050190. [DOI] [PubMed] [Google Scholar]
- 47.Nahum Goldberg S., Dupuy D.E. Image-guided radiofrequency tumor ablation: challenges and opportunities-part I. J Vasc Interv Radiol. 2001;12:1021–1032. doi: 10.1016/s1051-0443(07)61587-5. [DOI] [PubMed] [Google Scholar]
- 48.Dupuy D.E., Zagoria R.J., Akerley W., Mayo-Smith W.W., Kavanagh P.V., Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 49.Sperandeo M. Ultrasonography guided biopsy in peripheral pulmonary lesions. In: Sperandeo M., editor. Thoracic echography. CIC International; Rome: 2004. pp. 33–34. [Google Scholar]
- 50.Miyazu Y., Miyazawa T., Kurimoto N., Iwamoto Y., Kanoh K., Kohno N. Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am J Respir Crit Care Med. 2002;165:823–827. doi: 10.1164/ajrccm.165.6.2108092. [DOI] [PubMed] [Google Scholar]
- 51.Hurter T., Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax. 1992;47:565–567. doi: 10.1136/thx.47.7.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimmer T., Patan M., Berdel W.E., Dulce M.C., Riecken E.O. Value of endoscopic ultrasound examination of the mediastinum in Hodgkin's and non-Hodgkin's lymphomasUltraschall Med. 1997;18:67–71. doi: 10.1055/s-2007-1000520. [DOI] [PubMed] [Google Scholar]


