CONSPECTUS
Engineered nanomaterials (ENMs) are a new class of environmental pollutants. Researchers are beginning to debate whether new modeling paradigms and experimental tests to obtain model parameters are required for ENMs or if approaches for existing pollutants are robust enough to predict ENM distribution between environmental compartments.
This Account outlines how experimental research can yield quantitative data for use in ENM fate and exposure models. We first review experimental testing approaches that are employed with ENMs. Then we compare and contrast ENMs against other pollutants. Finally, we summarize the findings and identify research needs that may yield global descriptors for ENMs that are suitable for use in fate and transport modeling.
Over the past decade, researchers have made significant progress in understanding factors that influence the fate and transport of ENMs. In some cases researchers have developed approaches toward global descriptor models (experimental, conceptual, and quantitative). We suggest the following global descriptors for ENMs: octanol-water partition coefficients, solid-water partition coefficients, attachment coefficients, and rate constants describing reactions such as dissolution, sedimentation, and degradation. ENMs appear to accumulate at the octanol-water interface and readily interact with other interfaces, such as lipid-water interfaces. Batch experiments to investigate factors that influence retention of ENMs on solid phases are very promising. However ENMs probably do not behave in the same way as dissolved chemicals, and therefore researchers need to use measurement techniques and concepts more commonly associated with colloids. Despite several years of research with ENMs in column studies, available summaries tend to discuss the effects of ionic strength, pH, organic matter, ENM type, packing media, or other parameters qualitatively rather than reporting quantitative values, such as attachment efficiencies, that would facilitate comparison across studies. Only a few structure-activity relationships have been developed for ENMs so far, but such evaluations will facilitate the understanding of the reactivities of different forms of a single ENM.
The establishment of predictive capabilities for ENMs in the environment would enable accurate exposure assessments that would assist in ENM risk management. Such information is also critical for understanding the ultimate disposition of ENMs and may provide a framework for improved engineering of nanomaterials that are more environmentally benign.
Introduction
Engineered nanomaterials (ENMs) are a potential new class of pollutants, and debate is growing regarding appropriate test methodologies and modeling paradigms for their behavior in the environment. The sources, fate and transport, and toxicity of ENMs have been a major focus of environmental health and safety research efforts across the globe over the past decade1. The potential for environmental exposure dictates a compelling need to understand and predict the disposition of ENMs in and on the environment2. There has been a call for harmonization of test protocols suitable for assessing the environmental fate of ENMs3. However, the first challenge may be to understand how outcomes from such harmonized testing can be parameterized into models capable of predicting ENM fate and transport in the environment.
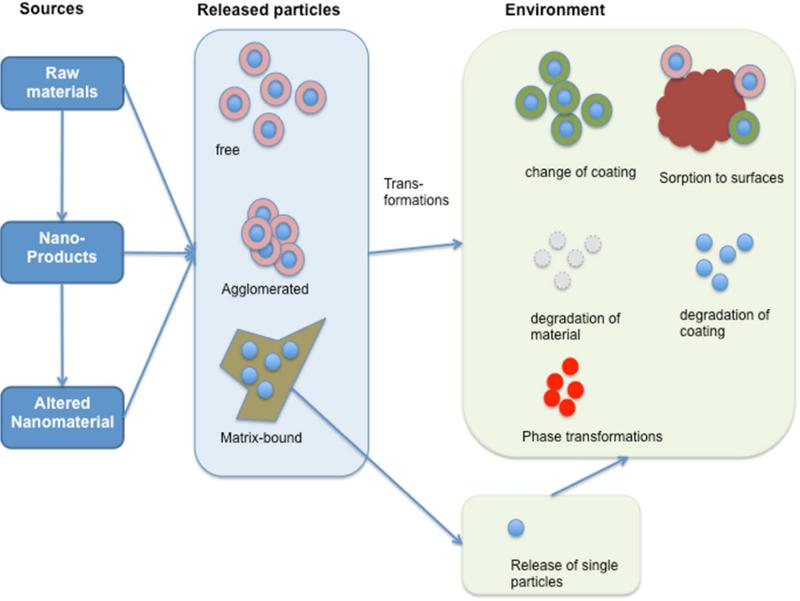
ENMs emitted into the environment originate from a variety of sources throughout the life cycle of ENM-containing products, and understanding ENM distribution is a critical need for performing risk assessments and developing regulations (Figure 1). Physical, chemical, and/or biological processes within the environment can transform the properties of ENMs4. Because analytical capabilities to quantifiably track these changes remain in their infancy5, models of these processes are needed to begin assessing ENM fate.
Figure 1.
ENMs are released from different sources in different forms that are changed by environmental transformation reactions.
At one level, understanding just the distribution of ENMs between environmental compartments is informative because it defines likely exposure pathways. Models describing the distribution of pollutants among compartments often assume pseudo-steady state conditions and use global descriptors such as partition coefficients6. Partition coefficients (also called “distribution coefficient” or “attachment coefficient”) usually refer to reversible equilibrium of a pollutant between two phases (e.g., air-water, water-solid), and can be related to thermodynamic principles. More complicated models incorporate fluxes (i.e., time-dependent movement of mass) within and between environmental compartments.
It remains unclear if new modeling paradigms and experimental tests to obtain model parameters are required for ENMs or if approaches for existing pollutants are robust enough to predict ENM distribution between environmental compartments. This article outlines issues related to the methods by which experimental research can yield quantitative data for use in ENM fate models. We first review experimental testing approaches employed with ENMs, then compare and contrast ENMs with other pollutants before summarizing potential global descriptors for ENMs that are suitable for use in fate and transport modeling. Table 1 summarizes various testing schemes that have been considered in order to understand and predict ENM fate and transport. These will be discussed in subsequent sections. For each testing approach, we identify and describe a potential ENM global descriptor. We describe the basic approaches and the use of likely outcomes in predicting the fate and transport of ENMs as well as discuss quantitative values where available.
Table 1.
Testing schemes that have been considered as a potential strategy to understand and predict ENM fate and transport.
| Testing Approach | Example Schemes | Potential Fate & Transport Outcomes | Global Descriptor |
|---|---|---|---|
| Solvent exchange | Measuring the distribution of ENMs between water containing ENMs and a solvent phase (octanol) | Single coefficient (Kow, % hydrophobicity) indicating potential to interact with hydrophobic phases (e.g., soil, lipids, tissue) | Kow |
| Surface affinity | Dynamic column tests using media coated with different surfaces (e.g., silica, iron oxide, hydrophobic material) | Single coefficient (α) from 0 to 1 indicating tendency to interact with different environmental surfaces (soils, suspended sediment) | α D |
| Sorption | Interaction of ions or NOM with ENMs | Changes in zeta potential, sorptive capacity factors | Freundlich isotherm K and 1/n values Or Ligand binding constants (L1) and conditional stability constants (K1) |
| Sediment retention | Measuring the retention of ENMs from water on natural soils or sediments | Single coefficient (KR or KD) indicating tendency to interact with environmental surfaces | KR or KD |
| Self-aggregation | Measuring aggregation kinetics of ENMs with themselves in water matrices with different ionic composition or NOM | Single coefficient (α) from 0 to 1 indicating the effect of water composition on the tendency of ENM to aggregate into large particles that could settle out of a water column | α |
| Electrostatic repulsion | Measuring zeta potential as an indicator of particle stability | Zeta potential of ENMs can be binned into likely to be stable or likely to aggregate | Enet (Net energy barrier) |
| Multidimensional parameter or high-throughput testing | Automated chemical additions of salts, organics, acids, or suspended sediment toquickly assess ENM stability | 3-D contour plots of key parameters (zeta potential, turbidity) to understand ENM stability in a series of water chemistries | STIFF diagram plotting parameters |
| Dissolution kinetics | Measuring dissolution of metallic ENMs as a function of dissolved oxygen, pH, and redox conditions | Thermodynamic conditional stability coefficients and surface area–dependent kinetic rate constants; solubility limits | kdissolution (Dissolution rate constant) |
| Weathering | Simulated photolysis, dissolution, and biodegradation of ENMs and/or their coatings | Changes in size, zeta potential, composition, coatings, etc. | ki, (multiple rate constants for different “i” mechanisms) |
ENM Fate and Transport Experimental Platforms
Octanol-Water Batch Partitioning
Distribution of pollutants between aqueous and organic phases is a starting point in predicting organic contaminants’ fate, bioavailability, and transport in the environment7. Octanol is typically used as a surrogate for organic-containing media ranging from sediments to lipid membranes. The octanol-water distribution coefficient,
| [1] |
where CO and CW are the concentrations of the contaminant in the octanol and aqueous phases, respectively, has become essential to testing and predicting the transport, bioaccumulation, and toxicity of many organic contaminants.
ENM partitioning between water and solvents initially was studied as a means of creating stable suspensions of C60 fullerenes, which have a high KOW value (Log KOW of 6.67)8. Log KOW values ranging from less than -1 to >2 have been reported for functionalized fullerenes9. Log KOW values of ~3 were measured for multiwall carbon nanotubes (MWCNTs) but were not found to predict MWCNT bioaccumulation in earthworms or oligochaetes10. Single wall carbon nanotubes (SWCNTs) coated with humic acid were not capable of partitioning into octanol; the humic acid functionalization prevented this phase change from occurring11. Recent work indicates that a wide range of ENMs can partition between octanol and water but, depending upon pH, a significant fraction of the total ENMs accumulate at the interface between the two phases12 (see Figure 2), which complicates extension of KOW as a global descriptor for ENM. The traditional KOW partitioning concept was therefore amended with an interface partition coefficient (KI) to describe this behavior12. Although termed “percentage hydrophobicity” or “hydrophobicity index” instead of KOW, the distribution of ENMs between water and octanol or other solvent phases has been incorporated into high-throughput toxicity and fate protocols13.
Figure 2.
Distribution of hematite nanoparticles into the octanol and aqueous phases and the interface at different pH values. Figure reprinted by permission of Taylor & Francis Ltd) from ref12.
Overall, these batch partition experiments appear to be useful in comparing functionality or other surface properties within a class of nanomaterials. The broader significance of KOW in predicting accumulation of ENMs in more hydrophobic environmental compartments (e.g., soil, biota) remains to be determined. The lipid-water distribution ratio may be a more appropriate descriptor for bioaccumulation of hydrophobic ionizable compounds14. For example, the lipid bilayer-water association coefficient (Klipw) for nC60 was larger than that for poly-hydroxylated fullerol at a given pH, which indicates greater propensity for nC60 to interact with lipid bilayers15. ENM number concentrations for gold particles of differing size explained the variability in Klipw values based upon mass concentrations (i.e., ENMs with the smallest diameter distributed most into the lipid bilayers)16.
Solid-Water Batch Tests
In batch sorption tests, ENMs, water, and soil, sewage sludge and other materials have been mixed and then the distribution of ENM concentrations between water and soil phases has been determined. Such solid–liquid partition of chemicals is commonly operationally defined as a partitioning coefficient (Kd; L/kg):
| [2] |
where Msolid is the solid-phase (sorbed) concentration of a compound (mg/kg) and Maq is the dissolved phase concentration (mg/L). High Kd values thus indicate preferential partitioning to the solid phase, but they do not give information on specific retention mechanisms.
The primary difference between Kd and KOW lies in how the coefficients are applied. Extrapolating KOW from water-solvent tests to field conditions would require considering the fraction of organic matter (foc) present in sediments or soils or other phases, as has been widely done for organic pollutants17. In contrast, Kd is usually determined on a specific sediment or soil, or can be adjusted between samples using foc.
Classical colloidal flocculation models predict continuous aggregation over time, and thus an equilibrium distribution may not be applicable to ENMs. However, recent work with bacterial cells and suspended lipid bilayers is showing that steady-state distributions vary with changes in solution or surface concentrations of ENMs. The distribution kinetics can be fit with an empirical model:15,18,19
| [3] |
where Clip(t) is the ENM mass per unit mass of lipid (mg ENM/kg lipid) as a function of time, t is time (h), ka is the association constant (mg/L/h), kd is the disassociation constant (1/h), C is the initial ENM mass concentration (mg/L), and Clip,0 is the maximum ENM mass that can distribute to the lipid bilayer (per unit mass of lipid). These studies suggest that although physical transport processes likely initially control aggregation kinetics, a steady-state condition appears to exist on some surfaces that prevents further aggregation and provides a potential justification for using partition coefficients.
Cornelis and co-workers presented a screening tool for ENMs called the retention coefficient (Kr)20-22, that was later used by other authors25. Ag or CeO2 ENMs are retained to different extents on soils with varying properties and an empirical relationship could be developed between ENM retention and the clay content, phosphate concentration and pH of soils.
Many other studies have investigated the distribution of ENMs between water and solid phases23. The distribution of MWCNTs between water and peat soil, for example, was measured and the data fit with a Freundlich isotherm24. Increasing salt concentration drove more MWCNTs to the solid phase and forced a linear isotherm relationship, which suggests that the overall forces that drive the solid-phase distribution of MWCNTs (aggregation and peat sorption) are uniform with varying MWCNT concentrations.
To evaluate the potential removal of ENMs in wastewater treatment plants (WWTPs), batch sorption tests have been conducted using ENMs and biomass from activated sludge basins25-27. Such work confirmed that the nanosilver frequently used in consumer products would likely accumulate in biosolids28,29,30. Previous batch tests to obtain liquid-biomass distribution parameters for organic chemicals relied upon freeze-dried biomass, which has been shown not to be valid for ENMs because freeze-drying releases NOM-like surfactants31. Observations in such batch experiments are consistent with ENM removal in continuously loaded biological reactors32,33 and suggest that such simple screening batch experiments may be useful in assessing differences in removal potential of different classes of ENMs. The observed distribution coefficients for ENMs between wastewater and biomass surfaces vary as a function of size, density and surface functionality and it would be useful to find a global descriptor capable of predicting such distribution instead of conducting batch experiments.
Studies using batch experiments to obtain partition or distribution coefficients generally acknowledge that ENMs probably do not behave like dissolved chemicals, and try to interpret findings based upon measurements of ENM size, surface charge, density, or coating properties (hydrophobicity). A need exists to examine this growing number of databases and attempt to parameterize quantitative structure activity relationships or at least linear least-squares fitting to rank the relative importance of these parameters.
Column Testing
In contrast to the batch partitioning experiments with ENMs described above, a far more developed literature exists on dynamic column tests to ascertain the affinity of ENMs for soil or other surfaces34,35. An already available excellent review of ENM column test results emphasizes the theoretical framework for these tests and experimental findings36. The experimental apparatus usually involves a small column packed with quartz, sand, or soil particles into which an aqueous solution containing ENMs is pumped. Data analysis using the Carman-Kozeny equation,
| [4] |
where f is porosity of the media, η is a physical transport efficiency function, L is the length of packed column, and dc is the diameter of the collector media, as well as the measured concentrations of ENMs in the inflow (C) and outflow (Co), can be used to back-calculate an attachment/deposition efficiency or stickiness coefficient (αD). The advantage of this approach is that the calculation of α allows separation of physical transport processes (sedimentation, interception, diffusion) that lead to collisions between the ENMs and surface from forces that lead to ENM attachment to the surface and retention in the column (e.g., electrostatic, van der Waals, hydrophobic). However, calculation of αD requires assumptions regarding the shape of the collector and ENMs along with idealized flow conditions within the column.
Parameterization of the above models can be used to predict the transport distance of ENMs in the subsurface, or potentially to compare the relative affinity of different types of ENMs for variable surfaces. Migration of ENMs in the subsurface can pose significant environmental risk (e.g., migration from septic tanks into rivers or infiltration into drinking water wells)35. As an example of relative ENM affinity for different surfaces, ENMs were exposed to bare or coated glass beads in column experiments and αD values were calculated. Values for αD for ENMs with hydrophobic coatings increased two to four times on hydrophobic compared against clean glass beads, whereas citrate coated ENMs saw no change in αD values37.
Despite several years of research with ENMs and columns36, available summaries tend to discuss the effects of ionic strength, pH, NOM, ENM type, packing media, or other parameters qualitatively rather than quantitatively reporting C/Co or αD values, which would facilitate comparison across all studies.
Aggregation/Sedimentation Tests
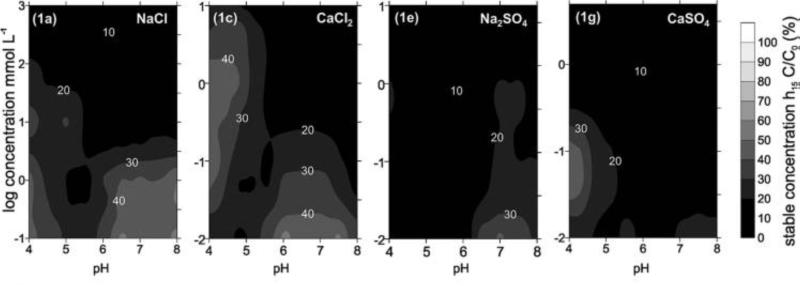
Most of the published data on aggregation involve homo-aggregation of ENMs, that is, aggregation with themselves. Increasing ionic strength and the presence of divalent cations tend to quicken the aggregation rate. An excellent review on aggregation theory that includes a summary of available ENM aggregation work is available36. The most common approach to study ENM aggregation employs dynamic light scattering (DLS). In a new approach38 that leverages high-throughput screening of ENM stability in complex mixtures of varying ionic strength/composition and organics, contour plots of ENM concentration in solution illustrate the effects of these parameters and predict the stability of ENMs in a test water relative to a control water (Figure 3).
Figure 3.
Contour plots of particle stability expressed as the residual concentration in supernatant as function of pH and medium composition. Reprinted with permission from38, copyright (2011) American Chemical Society.
Two types of traditional aggregation studies can be distinguished: 1) the particle size of a suspension is followed directly over time, usually using DLS, and the aggregation rate constants are derived, and 2) the aggregated particles are allowed to settle; only the supernatant is analyzed, and the “stable” fraction is quantified. Whereas the first approach allows mechanistic information and parameters39 such as the critical coagulation concentration (CCC) to be obtained, the second approach allows determination of more environmentally relevant information, e.g., the amount of an ENM that remains stable in suspension and can be transported over long distances. Decades of aggregation modeling accounting for physical collision of colloids based upon diffusion, mixing-induced velocity gradients, or differential settling transport mechanisms as well as favorable chemical interactions based upon a “stickiness factor” (∝) has led to some modeling advances for natural colloids40,41, but recent attempts to incorporate ENMs into these models have highlighted challenges, often due to the lack of ENM characterization data, related to aggregate break-up, fractal dimensions, and coatings42.
Potentially far more important to the fate of ENMs in natural systems than homo-aggregation is their interaction with clay, cellular debris, and other natural colloids/particles (heteroaggregation). Water contains a huge number of natural nano-sized particles (1 to 100 nm), an estimated 1010 to >1015 per m3.43 Quik et al.23 proposed that a first-order removal rate could approximate removal of ENMs from the water column that contained suspended natural colloids. To our knowledge, few other experimental reports address heteroaggregation of ENMs and natural colloids. Simulations suggested that nano-TiO2 aggregation at environmentally relevant nano-TiO2 concentrations was governed by heteroaggregation and that homo-aggregation only played a minor role39.
Transformation Reactions
ENMs can undergo a wide range of transformations during manufacturing, incorporation into products, product use, and release, as well as within the environment and organisms4,44. A few examples of major transformation are highlighted here (see also Figure 1). Elemental metals (e.g., Ag0, Cu0), metal oxides (e.g., ZnO, CeO2), and quantum dots (e.g., CdSe) can be oxidized/reduced or dissolve in water to form soluble ions or cause formation of reactive oxygen species (ROS) on the ENM surface45. Fullerenes can be oxidized by sunlight (photolysis), ozone, and even atmospheric oxygen to produce peroxy-fullerenes or hydroxylated fullerenes46. CNTs can undergo catalytic oxidation47. Dissolution of surface coatings can completely change particle behavior, as observed, e.g., for siloxane-AlOOH-TiO2 nanocomposites48.
All the transformation reactions listed above are not equilibrium processes. Therefore, elucidation of the kinetics should be the primary goal of any investigation. However, to date only a few studies provide quantitative data on reaction kinetics that can be used to predict the behavior of a certain ENM under environmental conditions. In the meantime, research has commenced on investigating “end-members”, such as bare elemental silver versus silver sulfide44.
Modeling ENMs in the Environment
Quantitative Structure-Activity Relationships (QSAR)
A quantitative structure activity relationship (QSAR) is a statistical model that relates a set of structural descriptors of a compound to its physical, chemical, or biological activity. Parameters that, e.g., account for hydrophobicity and steric effects are used as descriptors, whereas activities include chemical measurements and biological assays. According to the QSAR paradigm, the unknown activity of a new compound can be interpreted using mathematical models if the molecular parameters have been calculated for a group of known compounds49. The first applications of QSAR to ENMs, so-called nano-QSARs or QNAR50, have been published, e.g., to predict CNT water solubility and KOW values51 as well as the Young's modulus (a measure of the stiffness of an elastic material) of 29 different ENMs52.
Correlations between the chemical and physical parameters and properties of ENMs have also been developed. For example, the partitioning constant of C60 between water and organic compounds was correlated to the gap between ELUMO and EHOMO53. The partitioning coefficient between CeO2 and soils was found to depend on clay content, phosphate concentration, and pH20. The binding of 13 different polyaromatic hydrocarbons to CNTs could be best described using the solubility of the subcooled liquid and the polarizability54. CCCs for CNTs were a function of the atomic percentage of surface oxygen.55 The aqueous stability of 10 different CNT-types in the presence of humic acids was related to the oxygen content of the CNTs and their outer diameter56. Deposition data for a variety of ENMs were used to develop an empirical correlation between measurable ENM properties and the sticking coefficient (α) in the presence of DOM and polyelectrolytes57.
Although to date only a few structure-activity relationships have been developed for ENMs, such evaluations will be very helpful in understanding the different reactivities of different forms of a single ENM.
Multi-media Models
Multimedia mass-balance models can provide a powerful framework for understanding the behavior of pollutants in the environment6, and first attempts to apply them to ENMs have been published39,42,58,59. The big challenge is how to incorporate the specific ENM properties described in this paper into mass-flow and environmental fate models. Thus far, limited use of material-specific descriptors has been made, and models have predicted the fate of rather generic ENMs, e.g. “nano-Ag”. The published models included to some extent dissolution of ENM and agglomeration/sedimentation in natural waters60,62. Removal during wastewater treatment (a combination of particle-solid partitioning/agglomeration/sedimentation processes) was treated using transfer coefficients that were derived from batch or lab-scale experiments58.
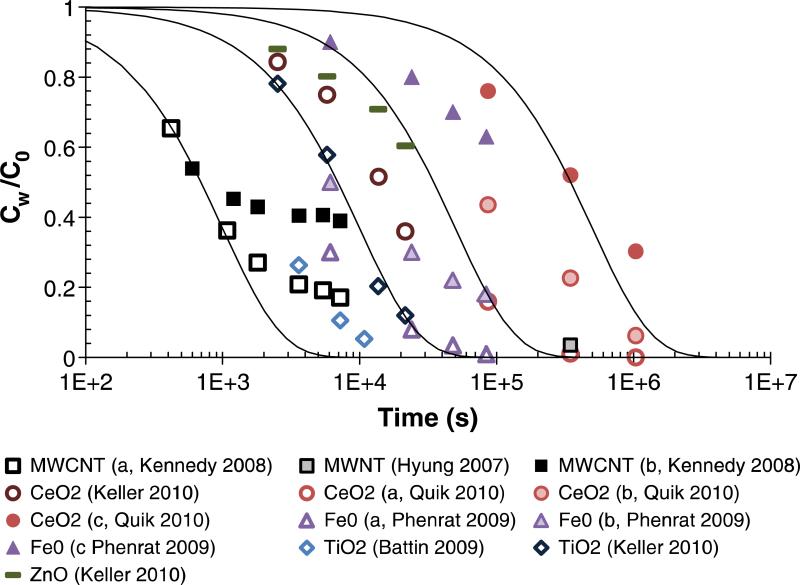
Arvidsson et al.42 presented a first approach to including aggregation in environmental fate models. Koelmans et al.60 incorporated calculations into fate models accounting for ENM sedimentation fluxes, removal rates due to aggregation or degradation, and ENM burial in deeper sediment layers, coupled to a material flow model for calculating the input into water. Gottschalk et al.61 coupled a material flow model58 to a geographical model of all Swiss rivers and used two scenarios with different ENM removal from the water column to account for the variability in ENM properties. Quik et al.23 suggested that using first-order reactions to describe sedimentation and ENM dissolution might be sufficient to amend existing fate models for conventional chemicals (see Figure 4). Recently, a multimedia fate model was developed using attachment efficiencies and heterogeneous aggregation models to predict the fate of nano TiO2 in the Rhine River, and is among the first environmental models to include ENM aggregation with complex suspended particulate matter39.
Figure 4.
Fraction of ENMs remaining in suspension over time (filled symbols, with NOM; open symbols, without NOM). The lines represent a first-order removal model. Reprinted from23, copyright (2011) with permission from Elsevier.
Similarities of ENMs to Other Pollutants
ENMs highlight the basic question of at what scalar values do pollutants behave more like molecules and at which more like particles (Figure 5). Brownian motion dominates the movement of molecules and smaller colloids, whereas sedimentation and diffusive forces affect the movement of larger colloids and particles. As Figure 5 suggests, at some point very small ENMs may take on the attributes of large molecules, such as proteins. Understanding these size-dependent thresholds may lead to approaches to model the fate of ENMs in heterogeneous environments similar to those applied to chemical pollutants.
Figure 5.
Conceptual paradigm for the tendency of ENMs to behave more like aqueous molecules or colloids in solution as a function of their relative size.
Transport phenomena are only one example in which very small ENMs potentially behave more like a chemical than a colloid. Another important phenomenon is the distribution of pollutants between two phases. Classically, aqueous chemicals are viewed as reaching equilibrium between different phases driven in part by thermodynamics associated with organizing water molecules versus energy associated with partitioning to a surface (i.e., partition coefficients), whereas colloidal interactions usually consider the net energy of attraction/repulsion with a surface. Furthermore, equilibrium is defined as reversible, which is clearly true for many hydrophobic chemicals. In contrast, colloids associated at or between different phases require external energy inputs (e.g., mixing, heat) to disassociate from the interface.
In contrast to many organic chemicals, ENM may come in a myriad of different forms. A “generic nano-TiO2” for example does not exist as many different mineralogical forms exist (see Figure 6) that may be functionalized and undergo different aging and transformation reactions during use and in the environment. Each of these different may is expected to have different reactivities and it remains open if we can find global descriptors that allow to describe all different form or if we need different sets for different form. However, concepts how to deal with different reactivities of various forms of one compound have been established for classical chemicals. Metal speciation is necessary to understand the different reactivities and effects of different forms of the same metal. Whereas, for example, Cr(III) is largely insoluble and of low toxicity, Cr(VI) is much more soluble and toxic; uncomplexed metals such as Cu2+ or Pb2+ bind very strongly to mineral surfaces at neutral or basic pH, whereas the same metals complexed to organic ligands may exhibit the opposite behavior and do not adsorb at the same pH. Advanced environmental fate models for metals incorporate such processes. Similarly, the different forms of an ENM can be considered “species” of an ENM that need to be evaluated differently. Figure 6 compares metal species and “ENM species”. For many metals, a theoretical equilibrium between different species can be calculated using the stability constants of different complexes, which cannot be done for ENM. However, kinetic constraints often result in non-equilibrium conditions for metals.
Figure 6.
Environmental fate models for ENM need to incorporate the different reactivities of the different forms of a specific ENM. This is similar for metals where it is necessary to understand speciation in order to predict the different reactivities of different forms of a metal.
Summary and Future Research Needs
Over the last decade significant progress has been made in understanding factors influencing the fate and transport of ENMs. In some cases approaches toward global descriptor models (experimental, conceptual, and quantitative) have been developed. ENMs are seen as an opportunity for academics to push for more mechanistic frameworks for the prediction of pollutants in general, compared with the rather empirical approaches used by regulatory agencies. No better example exists than modeling of the environmental fate of colloids. Should classical flocculation and filtration models be used instead of partition coefficients? Arguments for each exist, but significant commonalities also exist. Fundamental models allow separation of physical transport from an interaction term (an empirical α value) that allows for kinetic modeling, whereas distribution coefficients (KD) attempt to look at the longer-term outcome, which may approach some type of steady-state phase distribution of the ENMs. The commonality is that both KD and α are fitted empirical values; the difference is that classical kinetic modeling argues that aggregation continues, whereas distribution coefficients reach some type of steady state. However, even this potential difference subsides if a long-enough time frame is given to the kinetic modeling approach because over time the number concentration of particles in solution decreases to a point at which the probability of collisions is quite low. Both approaches benefit from the selection of common dosimetry (i.e., number concentrations). Moving forward, these communities should understand how application of fundamental models can inform simpler distribution or global descriptor models that are more readily applied to large-scale environmental systems.
Acknowledgements
The authors acknowledge personal discussions with many of the authors cited in this work that have contributed to the insights in this paper. Furthermore, the authors’ ability to think about this topic over the past decade would not have been possible without funding from various sponsors, e.g., USEPA (RD831713 and RD833322), DOE (DE-FG02-08ER64613), NIH (DE-FG02-08ER64613), WERF, SNF, EU-FP7, and BAFU.
Biography
Paul Westerhoff holds a PhD (1995) in environmental engineering from the University of Colorado at Boulder. He is a Professor in the School of Sustainable Engineering and the Built Environment and Associate Dean for Research in the Ira A. Fulton Schools of Engineering at Arizona State University. His research focuses on emerging water quality issues and their treatment using physicochemical processes. He has more than 120 peer-reviewed publications.
Bernd Nowack holds an MSc (1992) and a PhD (1995) in environmental sciences from ETH Zürich. He is the leader of the “Environmental Risk Assessment and Management” group at Empa, the Swiss Federal Laboratories for Materials Science and Technology. His current research focuses on engineered nanomaterials, particularly qualitative risk assessment, quantitative exposure modeling, release of nanomaterials from products, and nanomaterial behavior and effects in the environment. He has published 80+ peer-reviewed publications and is Associate Editor of the journal Environmental Pollution.
References
- 1.Wiesner MR, Lowry GV, Jones KL, Hochella MF, Di Giulio RT, Casman E, Bernhardt ES. Decreasing Uncertainties in Assessing Environmental Exposure, Risk, and Ecological Implications of Nanomaterials. Environmental Science & Technology. 2009;43:6458–6462. doi: 10.1021/es803621k. [DOI] [PubMed] [Google Scholar]
- 2.Gottschalk F, Nowack B. Release of engineered nanomaterials to the environment. J. Environ. Monitoring. 2011;13:1145–1155. doi: 10.1039/c0em00547a. [DOI] [PubMed] [Google Scholar]
- 3.Stone V, Nowack B, Baun A, van den Brink N, von der Kammer F, Dusinska M, Handy R, Hankin S, Hassellöv M, Joner E, Fernandes TF. Nanomaterials for environmental studies: Classification, reference material issues, and strategies for physicochemical characterisation. Sci. Total Environ. 2010;408:1745–1754. doi: 10.1016/j.scitotenv.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Nowack B, Ranville JF, Diamond S, Gallego-Urrea JA, Metcalfe C, Rose J, Horne N, Koelmans AA, Klaine SJ. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012;31:50–59. doi: 10.1002/etc.726. [DOI] [PubMed] [Google Scholar]
- 5.von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, Koelmans AA, Horne N, Unrine JM. Analysis of engineered nanomaterials in complex matrices (environment and biota): General considerations and conceptual case studies. Environ. Toxicol. Chem. 2012;31:32–49. doi: 10.1002/etc.723. [DOI] [PubMed] [Google Scholar]
- 6.MacLeod M, Scheringer M, McKone TE, Hungerbuhler K. The State of Multimedia Mass-Balance Modeling in Environmental Science and Decision-Making. Environmental Science & Technology. 2010;44:8360–8364. doi: 10.1021/es103297w. [DOI] [PubMed] [Google Scholar]
- 7.Burnison BK. Review of bioconcentration, bioaccumulation and KOW techniques. Water Quality Research Journal of Canada. 1998;33:213–230. [Google Scholar]
- 8.Jafvert CT, Kulkarni PP. Buckminsterfullerene's (C-60) octanol-water partition coefficient (K-ow) and aqueous solubility. Environmental Science & Technology. 2008;42:5945–5950. doi: 10.1021/es702809a. [DOI] [PubMed] [Google Scholar]
- 9.Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H, Sarna T, Hamblin MR. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radical Biology and Medicine. 2007;43:711–719. doi: 10.1016/j.freeradbiomed.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen EJ, Huang QG, Weber WJ. Relevance of Octanol-Water Distribution Measurements to the Potential Ecological Uptake of Multi-Walled Carbon Nanotubes. Environ. Toxicol. Chem. 2010;29:1106–1112. doi: 10.1002/etc.149. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Shi QH, Liang HJ, Steuerman DW, Stucky GD, Keller AA. Enhanced Environmental Mobility of Carbon Nanotubes in the Presence of Humic Acid and Their Removal from Aqueous Solution. Small. 2008;4:2166–2170. doi: 10.1002/smll.200800753. [DOI] [PubMed] [Google Scholar]
- 12.Hristovski K, Westerhoff P, Posner J. Octanol-water Partitioning of Engineered Nanomaterials. J. Environ. Sci. Health, Part A - Toxic/Hazardous Substance & Environmental Engineering. 2011;46:636–647. doi: 10.1080/10934529.2011.562859. [DOI] [PubMed] [Google Scholar]
- 13.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 14.Escher BI, Schwarzenbach RP, Westall JC. Evaluation of liposome-water partitioning of organic acids and bases. 2. Comparison of experimental determination methods. Environmental Science & Technology. 2000;34:3962–3968. [Google Scholar]
- 15.Hou WC, Moghadam BY, Westerhoff P, Posner JD. Distribution of Fullerene Nanomaterials between Water and Model Biological Membranes. Langmuir. 2011;27:11899–11905. doi: 10.1021/la2017837. [DOI] [PubMed] [Google Scholar]
- 16.Hou WC, Moghadam BY, Corredor C, Westerhoff P, Posner J. Distribution of Functionalized Gold Nanoparticles between Water and Lipid Bilayers as Model Cell Membranes. Env. Sci. Tech. 2012;46:1869–1876. doi: 10.1021/es203661k. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzenbach RP, Gschwend PM, Imboden DM. Environmental Organic Chemistry. John Wiley and Sons; New York: 1993. [Google Scholar]
- 18.Wilhelm C, Gazeau F, Roger J, Pons JN, Bacri J-C. Interaction of Anionic Superparamagnetic Nanoparticles with Cells: Kinetic Analyses of Membrane Adsorption and Subsequent Internalization. Langmuir. 2002;18:8148–8155. [Google Scholar]
- 19.zhang W, Kalive M, Capco DG, Chen Y. Adsorption of hematite nanoparticles onto Caco-2 cells and the cellular impairments: effect of particle size. Nanotechnology. 2010;21:355103. doi: 10.1088/0957-4484/21/35/355103. [DOI] [PubMed] [Google Scholar]
- 20.Cornelis G, Ryan B, McLaughlin MJ, Kirby JK, Beak D, Chittleborough D. Solubility and Batch Retention of CeO2 Nanoparticles in Soils. Environmental Science & Technology. 2011;45:2777–2782. doi: 10.1021/es103769k. [DOI] [PubMed] [Google Scholar]
- 21.Cornelis G, Kirby JK, Beak D, Chittleborough D, McLaughlin MJ. A method for determination of retention of silver and cerium oxide manufactured nanoparticles in soils. Environ. Chem. 2010;7:298–308. [Google Scholar]
- 22.Cornelis G, Doolette C, Thomas M, McLaughlin MJ, Kirby JK, Beak DG, Chittleborough D. Retention and Dissolution of Engineered Silver Nanoparticles in Natural Soils. Soil Sci. Soc. Am. J. 2012;76:891–902. [Google Scholar]
- 23.Quik JTK, Vonk JA, Hansen SF, Baun A, Van De Meent D. How to assess exposure of aquatic organisms to manufactured nanoparticles? Environment International. 2011;37:1068–1077. doi: 10.1016/j.envint.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LW, Petersen EJ, Huang QG. Phase Distribution of C-14-Labeled Multiwalled Carbon Nanotubes in Aqueous Systems Containing Model Solids: Peat. Environmental Science & Technology. 2011;45:1356–1362. doi: 10.1021/es1026097. [DOI] [PubMed] [Google Scholar]
- 25.Kiser MA, Westerhoff P, Benn T, Wang Y, Perez-Rivera J, Hristovski K. Titanium Nanomaterial Removal and Release from Wastewater Treatment Plants. Environ. Sci. Tech. 2009 doi: 10.1021/es901102n. [DOI] [PubMed] [Google Scholar]
- 26.Westerhoff P, Song G, Hristovski K, Kiser A. Occurrence and Removal of Titanium at Full Scale Wastewater Treatment Plants: Implications for TiO2 Nanomaterials. J. Environ. Monit. 2011;13:1195–1203. doi: 10.1039/c1em10017c. [DOI] [PubMed] [Google Scholar]
- 27.Brar SK, Verma M, Tyagi RD, Surampalli RY. Engineered nanoparticles in wastewater and wastewater sludge - Evidence and impacts. Waste Management. 2010;30:504–520. doi: 10.1016/j.wasman.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P. The Release of Nanosilver from Consumer Products Used in the Home. Journal of Environmental Quality. 2010;39:1875–1882. doi: 10.2134/jeq2009.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics (vol 42, pg 4133, 2008). Environ. Sci. Tech. 2008;42:7025–7026. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- 30.Kiser A, Ryu H, Jang G, Hristovski K, Westerhoff P. Biosorption of nanoparticles on heterotrophic wastewater biomass. Water Research. 2010;44:4105–4114. doi: 10.1016/j.watres.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 31.Kiser A, Ladner D, Hristovski K, Westerhoff P. Nanomaterial transformation and association with fresh and freeze-dried wastewater activated sludge: Implications for testing protocol and environmental fate. Env. Sci. Tech. 2012 doi: 10.1021/es300339x. DOI: 10.1021/es300339x. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Westerhoff P, Hristovski KD. Fate and biological effects of silver, titanium dioxide, and C60 (fullerene) nanomaterials during simulated wastewater treatment processes. J Hazardous Materials. 2012;201-202:16–22. doi: 10.1016/j.jhazmat.2011.10.086. [DOI] [PubMed] [Google Scholar]
- 33.Limbach LK, Bereiter R, Mueller E, Krebs R, Gaelli R, Stark WJ. Removal of oxide nanoparticles in a model wastewater treatment plant: Influence of agglomeration and surfactants on clearing efficiency. Environmental Science & Technology. 2008;42:5828–5833. doi: 10.1021/es800091f. [DOI] [PubMed] [Google Scholar]
- 34.Brant J, Lecoanet H, Wiesner MR. Aggregation and Deposition Characteristics of Fullerene Nanoparticles in Aqueous Systems. J. Nanoparticle Research. 2005;7:545–553. [Google Scholar]
- 35.Lecoanet HF, Bottero JY, Wiesner MR. Laboratory Assessment of the Mobility of Nanomaterials in Porous Media. Env. Sci. Tech. 2004;38:5164–5169. doi: 10.1021/es0352303. [DOI] [PubMed] [Google Scholar]
- 36.Petosa AR, Jaisi DP, Quevedo IR, Elimelech M, Tufenkji N. Aggregation and Deposition of Engineered Nanomaterials in Aquatic Environments: Role of Physicochemical Interactions. Environmental Science & Technology. 2010;44:6532–6549. doi: 10.1021/es100598h. [DOI] [PubMed] [Google Scholar]
- 37.Song JE, Phenrat T, Marinakos S, Xiao Y, Liu J, Wiesner MR, Tilton RD, Lowry GV. Hydrophobic Interactions Increase Attachment of Gum Arabic- and PVP-Coated Ag Nanoparticles to Hydrophobic Surfaces. Environmental Science & Technology. 2011;45:5988–5995. doi: 10.1021/es200547c. [DOI] [PubMed] [Google Scholar]
- 38.Ottofuelling S, Von der Kammer F, Hofmann T. Commercial Titanium Dioxide Nanoparticles in Both Natural and Synthetic Water: Comprehensive Multidimensional Testing and Prediction of Aggregation Behavior. Env. Sci. Tech. 2011;45:10045–10052. doi: 10.1021/es2023225. [DOI] [PubMed] [Google Scholar]
- 39.Praetorius A, Scheringer M, Hungerbuhler K. Development of Environmental Fate Models for Engineered Nanoparticles-A Case Study of TiO(2) Nanoparticles in the Rhine River. Environ Sci Technol. 2012;46:6705–13. doi: 10.1021/es204530n. [DOI] [PubMed] [Google Scholar]
- 40.Lee DG, Bonner JS, Garton LS, Ernest ANS, Autenrieth RL. Modeling coagulation kinetics incorporating fractal theories: A fractal rectilinear approach. Water Research. 2000;34:1987–2000. doi: 10.1016/s0043-1354(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 41.Thomas DN, Judd SJ, Fawcett N. Flocculation modelling: A review. Water Research. 1999;33:1579–1592. [Google Scholar]
- 42.Arvidsson R, Molander S, Sanden BA, Hassellov M. Challenges in Exposure Modeling of Nanoparticles in Aquatic Environments. Hum. Ecol. Risk Assess. 2011;17:245–262. [Google Scholar]
- 43.Filella M, Buffle J. Factors controlling the stability of submicron colloids in natural waters. Colloids Surf. A. 1993;73:255–273. [Google Scholar]
- 44.Lowry GV, Gregory KB, Apte SC, Lead JR. Transformations of Nanomaterials in the Environment. Environmental Science & Technology. 2012;46:6893–6899. doi: 10.1021/es300839e. [DOI] [PubMed] [Google Scholar]
- 45.Auffan M, Rose J, Wiesner MR, Bottero JY. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009;157:1127–1133. doi: 10.1016/j.envpol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Brunet L, Lyon DY, Hotze EM, Alvarez PJJ, Wiesner MR. Comparative Photoactivity and Antibacterial Properties of C-60 Fullerenes and Titanium Dioxide Nanoparticles. Environmental Science & Technology. 2009;43:4355–4360. doi: 10.1021/es803093t. [DOI] [PubMed] [Google Scholar]
- 47.Allen BL, Kotchey GP, Chen YN, Yanamala NVK, Klein-Seetharaman J, Kagan VE, Star A. Mechanistic Investigations of Horseradish Peroxidase-Catalyzed Degradation of Single-Walled Carbon Nanotubes. Journal of the American Chemical Society. 2009;131:17194–17205. doi: 10.1021/ja9083623. [DOI] [PubMed] [Google Scholar]
- 48.Labille J, Feng JH, Botta C, Borschneck D, Sammut M, Cabie M, Auffan M, Rose J, Bottero JY. Aging of TiO2 nanocomposites used in sunscreen. Dispersion and fate of the degradation products in aqueous environment. Environ. Pollut. 2010;158:3482–3489. doi: 10.1016/j.envpol.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Puzyn T, Leszczynska D, Leszczynski J. Toward the Development of “Nano-QSARs”: Advances and Challenges. Small. 2009;5:2494–2509. doi: 10.1002/smll.200900179. [DOI] [PubMed] [Google Scholar]
- 50.Fourches D, Pu DQY, Tassa C, Weissleder R, Shaw SY, Mumper RJ, Tropsha A. Quantitative Nanostructure-Activity Relationship Modeling. Acs Nano. 2010;4:5703–5712. doi: 10.1021/nn1013484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toropov AA, Leszczynska D, Leszczynski J. Predicting water solubility and octanol water partition coefficient for carbon nanotubes based on the chiral vector. Comput. Biol. Chem. 2007;31:127–128. doi: 10.1016/j.compbiolchem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Toropov AA, Leszczynski J. A new approach to the characterization of nanomaterials: Predicting Young's modulus by correlation weighting of nanomaterials codes. Chemical Physics Letters. 2006;433:125–129. [Google Scholar]
- 53.Wang Z, Chen JW, Sun Q, Peijnenburg W. C(60)-DOM interactions and effects on C(60) apparent solubility: A molecular mechanics and density functional theory study. Environment International. 2011;37:1078–1082. doi: 10.1016/j.envint.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 54.Kah M, Zhang XR, Jonker MTO, Hofmann T. Measuring and Modelling Adsorption of PAHs to Carbon Nanotubes Over a Six Order of Magnitude Wide Concentration Range. Environmental Science & Technology. 2011;45:6011–6017. doi: 10.1021/es2007726. [DOI] [PubMed] [Google Scholar]
- 55.Smith B, Wepasnick K, Schrote KE, Cho H-H, Ball WP, Fairbrother DH. Influence of Surface Oxides on the Colloidal Stability of Multi-Walled Carbon Nanotubes: A Structure,àíProperty Relationship. Langmuir. 2009;25:9767–9776. doi: 10.1021/la901128k. [DOI] [PubMed] [Google Scholar]
- 56.Schwyzer I, Kaegi R, Sigg L, Smajda R, Magrez A, Nowack B. Long-term colloidal stability of 10 carbon nanotube types in the absence/presence of humic acid and calcium. Environmental Pollution. 2012;169:64–73. doi: 10.1016/j.envpol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Phenrat T, Song JE, Cisneros CM, Schoenfelder DP, Tilton RD, Lowry GV. Estimating Attachment of Nano- and Submicrometer-particles Coated with Organic Macromolecules in Porous Media: Development of an Empirical Model. Environ. Sci. Technol. 2010;44:4531–4538. doi: 10.1021/es903959c. [DOI] [PubMed] [Google Scholar]
- 58.Gottschalk F, Sonderer T, Scholz RW, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- 59.O'Brien N, Cummins E. Nano-Scale Pollutants: Fate in Irish Surface and Drinking Water Regulatory Systems. Human and Ecological Risk Assessment. 2010;16:847–872. [Google Scholar]
- 60.Koelmans AA, Nowack B, Wiesner MR. Comparison of manufactured and black carbon nanoparticle concentrations in aquatic sediments. Environ. Pollut. 2009;157:1110–1116. doi: 10.1016/j.envpol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Gottschalk F, Ort C, Scholz RW, Nowack B. Engineered nanomaterials in rivers - exposure scenarios for Switzerland at high spatial and temporal resolution Environ. Pollut. 2011;159:3439–3445. doi: 10.1016/j.envpol.2011.08.023. [DOI] [PubMed] [Google Scholar]