Abstract
Aim
To evaluate the prevalence, severity, and hemodynamic features of nonalcoholic fatty liver disease (NAFLD) in nonobese diabetics.
Methods
We studied 100 consecutive nonobese (body mass index [BMI] < 30) patients with type 1 (n = 17) or type 2 (n = 83) diabetes and no known causes of liver disease. Steatosis was diagnosed and graded with ultrasonography. Digital sonographic images of the liver and right kidney were analyzed with dedicated software (HDI-Lab), and the liver/kidney ratio of grey-scale intensity was calculated as an index of the severity of the steatosis. Severity scores ranging from 0 (none) to 5 (severe) were compared with sonographic and Doppler findings (right liver size, portal vein diameter and flow velocity, hepatic and splenic arterial pulsatility indices, hepatic-vein flow profile and A- and S-wave velocities).
Results
The prevalence of steatosis was 24% in type I and 80% in type II diabetes (grade 1 in 17%, grade 2 in 34%, grade 3 in 33%, grade 4 in 9%, grade 5 in 7%). In patients with steatosis (especially those with grades 4–5 disease), hepatic volume was increased (p < 0.005). Portal vein diameter was increased in grade 5 steatosis. The hepatic artery pulsatility index was significantly increased, particularly in grades 4 and 5 (p < 0.0001); portal and A-wave velocities were significantly reduced in grades 3–5 (p < 0.001); and the hepatic vein flow profile was altered in 27% (biphasic: 20%, flat: 7%) patients with steatosis, although there was no correlation with severity.
Conclusions
NAFLD is very frequent in nonobese diabetics with type 2 but not type 1 disease, and it is associated with hepatomegaly and liver hemodynamic alterations only when it is severe.
Keywords: Steatosis, Diabetes, NAFLD, Doppler, Sonography, Splanchnic hemodynamics
Sommario
Scopo
Valutare la prevalenza e la gravità della steatosi nei pazienti diabetici non-obesi e le alterazioni emodinamiche epatiche associate.
Metodi
Sono stati studiati 100 pazienti diabetici non-obesi (BMI < 30 kg/m2), 17 di tipo I e 83 di tipo II, privi di cause note di epatopatia. Mediante eco-color-Doppler sono stati valutati: un'immagine digitale di confronto tra fegato e corticale del rene destro, analizzata tramite un programma dedicato per la valutazione del grado di steatosi (rapporto fegato/rene dell'intensità dei grigi), le dimensioni del lobo epatico destro, il calibro della vena porta (PV), la velocità della vena porta (PBV), gli indici di pulsatilità arteriosa epatica (PI-L) e splenica (PI-S), il profilo flussimetrico e la velocità delle onde a (HV-a) e s (HV-s) delle vene sovraepatiche.
Risultati
La prevalenza di steatosi è stata del 24% nel diabete di tipo I e dell'80% nel tipo II ed è risultata prevalentemente di grado lieve-moderato. Nella steatosi il volume epatico è risultato aumentato (p < 0,005), in particolare nei gradi superiori a 3, e il diametro della vena porta è risultato aumentato nel grado 5. Il PI-L è risultato aumentato in tutti i gradi di steatosi, in particolare in quelli di grado 4 e 5 (p < 0,0001); la PBV è risultata ridotta nei gradi 3, 4 e 5 (p < 0,001); la HV-a è risultata ridotta nei pazienti con grado 3, 4 e 5. Il profilo flussimetrico delle vene sovraepatiche è risultato alterato nel 27% (20% bifasico, 7% appiattito).
Conclusioni
La steatosi epatica è un reperto molto frequente nel diabete di tipo II senza obesità ed è associata a epatomegalia e alterazioni emodinamiche epatiche nelle forme più gravi.
Non-alcoholic fatty liver disease (NAFLD) is an important clinical entity that affects approximately 20% of the general population [1]. The prevalence of NAFLD is much higher in obese patients (60–95%) [2]. Many studies suggest that NAFLD often represents the hepatic component of the metabolic syndrome, which is characterized by obesity, hyperinsulinemia, peripheral insulin resistance, diabetes, and hypertension [3–6]. The reported prevalence of NAFLD in patients with diabetes is 40–80%, and it is frequently associated with obesity – mainly abdominal – hypertriglyceridemia, and high-normal levels of alanine aminotransferase (ALT) [7,8].
NAFLD has long been considered a benign disease, but recent studies show that in some cases it evolves into nonalcoholic steato-hepatitis (NASH) [9]. Fifty percent of NASH patients develop liver fibrosis, 15% develop cirrhosis, and 3% experience terminal liver failure [10,11]. The relation between liver steatosis and cardiovascular diseases has also been emphasized [12,13].
A probable diagnosis of NAFLD is usually based on the finding of a bright liver at sonography (US) and alterations in liver function tests. In many cases, a definitive diagnosis is not obtained, as it requires liver biopsy. The relationships between sonographic findings and pathology are controversial. There are very few reports on the hepatic hemodynamic alterations associated with NAFLD, which can be demonstrated noninvasively by Doppler sonography. In a small group of patients with NASH, Magalotti et al. [14] demonstrated decreased blood flow velocity in the portal vein and altered hepatic-vein flow-velocity waveforms in 25–30% of patients. Improvement was observed after treatment based on dietary modifications, increased physical activity, and administration of metformin.
The aim of our study was to evaluate the prevalence, severity, and hepatic hemodynamic features of NAFLD in nonobese patients with diabetes and to identify possible correlations between the hemodynamic changes and the severity of sonographic steatosis.
Patients and methods
The study population consisted of 100 nonobese (body mass indices [BMI] < 30 kg/m2) patients with diabetes (60 males and 40 females, aged 65 ± 7 years). The mean BMI was 26 ± 2.6 kg/m2. Seventeen patients had type 1 diabetes, and 83 had type 2 diabetes. Exclusion criteria were the following: present or past ethanol abuse, other causes of liver disease (viral hepatitis, hemochromatosis, Wilson disease, autoimmune hepatitis), heart failure, and/or kidney disease.
We also examined a control group of 20 normal subjects of similar age, sex, BMI, without sonographic evidence of steatosis.
Grade of steatosis
The degree of steatosis was defined according to a quantitative classification system developed by our group (unpublished data, J Hepatol 2008, abstract 921) based on comparative analysis of digital sonographic images of the liver and right kidney. Briefly, US images of both organs were acquired with an ATL 5000 scanner (ATL Ultrasound, City), transferred to a personal computer, and analyzed with dedicated software (HDI-Lab). The grey-scale intensity of selected regions of interest was measured, and a liver/kidney (L/K) ratio was calculated, which has been shown to display direct correlation with the degree of steatosis measured by histology. In the present study, steatosis was assigned a score ranging from 0 (L/K < 1.3 – no steatosis) to 5 (L/K > 3.3 – severe steatosis), as shown in Table 1.
Table 1.
A five-point scale for ultrasound grading of hepatic steatosis.
| Grade | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| L/K ratioa | 0.86–1.29 | 1.3–1.8 | 1.81–2.3 | 2.31–2.8 | 2.81–3.3 | >3.3 |
Liver/kidney ratio – ratio of grey-scale intensity in the liver to that of the right kidney based on digital B-mode US images of the 2 organs.
Doppler sonography
All Doppler sonographic studies were performed by operators with identical levels of skill. Inter- and intraobserver reproducibility data have been reported elsewhere [15,16]. Color Doppler sonography was used to assess splanchnic hemodynamic parameters. We used an HDI 5000 scanner (ATL, Seattle, Washington) with a broadband curved-array transducer (C5-2 4OR). Each result recorded was the mean of three measurements.
-
-
Liver size was measured as the longitudinal diameter of the right lobe on the midclavicular line (normal values, <12.6 cm) [17].
-
-
Portal vein diameter (PV) was measured at the hilum during deep inspiration.
-
-
Portal vein flow velocity (PBV) was measured by placing the sample volume in a standardized position along the vessel and setting its dimension to >50% of the diameter of the vein. An insonation angle of 30–60° was used [18]. The PBV was calculated as the mean of maximal velocities.
-
-
The hepatic arterial pulsatility index (PI-L) was measured in the left branch of the hepatic artery and was calculated according to the formula: (peak systolic velocity – end diastolic velocity/mean velocity) [15].
-
-
The splenic arterial pulsatility index (PI-S) was measured in the main branches of the splenic artery and calculated according to the formula: (peak systolic velocity – end diastolic velocity/mean velocity) [15,19].
-
-
The velocities of hepatic-vein a (HV-a) and s (HV-s) waves (cm/s) were measured by placing the sample volume over the middle hepatic vein, 1.5–2.0 cm from the inferior vena cava, with an insonation angle of 30–60°. The hepatic-vein waveform pattern was classified as triphasic, biphasic, or monophasic, as defined by Bolondi et al. [20].
Statistical analysis
The results were expressed as means ± standard deviations (SD). The significance of correlations was evaluated with linear regression analysis and the Spearman rank correlation test. ANOVA and the Student's t-test for unpaired data were used for intergroup comparisons. Statistical significance was defined as p < 0.05.
Results
The overall prevalence of fatty liver in our population of nonobese patients with diabetes was 76% (24% among those with type I disease, 80% in those with type II). There was no difference in the prevalence between male and female patients. Our quantitative evaluation revealed the following grades of steatosis: grade 1 in 17% of the patients, grade 2 in 34%, grade 3 in 33%, grade 4 in 9%, and grade 5 in 7%. Patients with fatty liver had significantly higher BMIs than those without fatty liver (26.8 ± 2 vs. 25 ± 2.3 kg/m2, p < 0.001), but there were no differences in the BMIs between patients with different grades of steatosis.
Liver size was normal in patients without steatosis, while it was increased in those with moderate-to-severe steatosis (grades 2–5) (Fig. 1).
Fig. 1.
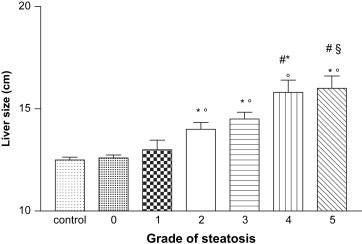
Liver sizes in patients with different grades of steatosis. * p < 0.001 vs control group; ° p < 0.001 vs grade 0 and 1; # p < 0.05 vs grade 2; § p < 0.05 vs grade 3.
The only significant hemodynamic alteration noted in the diabetic patients without steatosis was an increase in the PI-S compared with that of the healthy controls (Table 2). In diabetic patients with fatty liver, the PV (p = 0.0002) and PI-L (p < 0.0001) were significantly increased, while the PBV (p = 0.003) and HV-a (p = 0.0002) were significantly decreased compared with those of normal subjects. No differences were found in the HV-s and PI-S of the two groups (Table 2). Fig. 2 summarizes the correlations between the hemodynamic parameters we measured and the severity of the patients' steatosis. Considering the different grades of steatosis, PV was increased in patients with severe steatosis (grade 5 vs. grade 1 and vs. patients without steatosis) (Fig. 2A) while the PBV was decreased in patients with moderate-severe steatosis (grades 3– 5 vs. grade 0) (Fig. 2B). The PI-L was significantly increased in all groups of patients with steatosis compared with that of the nonsteatotic diabetic patients (Fig. 2C). The HV-a was significantly decreased only in patients with moderate-severe steatosis (grades 3– 5 vs. grade 0; p < 0.05) (Fig. 2D). Hepatic-vein waveform pattern was monophasic in 7% and biphasic in 20% of the patients with steatosis. No differences in PI-S and HV-s were found between patients with different grades of steatosis and patients without steatosis.
Table 2.
Hemodynamic parameters in controls and nonobese diabetic patients without and with steatosis.
| Vessel | Parameter | Control group | Diabetics without steatosis | Diabetics with steatosis |
|---|---|---|---|---|
| Portal vein | PV (mm) | 10.3 ± 1.1 | 9.9 ± 1.38 | 11.4 ± 1.75* ° |
| PBV (cm/s) | 35 ± 5 | 34.9 ± 6.4 | 30.8 ± 5.6* ° | |
| Hepatic artery | PI-H | 1.02 ± 0.2 | 1.1 ± 0.09 | 1.63 ± 0.43* ° |
| Hepatic vein | HV-a | 13 ± 4 | 13.3 ± 3.5 | 4.2 ± 11.1* ° |
| HV-s | −27 ± 6.2 | −24.2 ± 4.9 | −22.78 ± 11 | |
| Splenic artery | PI-S (cm/s) | 0.96 ± 0.1 | 1.16 ± 0.26° | 1.1 ± 0.27° |
PV, Portal vein diameter; PBV, Portal vein flow velocity, (PBV); PI-H, hepatic arterial pulsatility index; HV-a, hepatic-vein A-wave velocity; HV-s, hepatic-vein S-wave velocity; splenic arterial pulsatility index (PI-L). * p < 0.005 vs diabetics without steatosis; ° p < 0.005 vs controls.
Fig. 2.
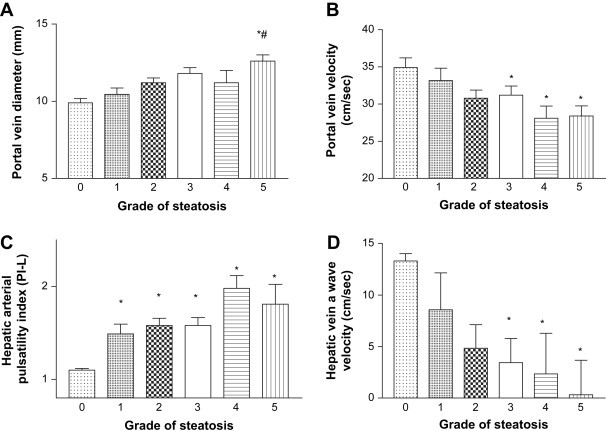
Portal vein diameter (A), portal vein blood flow velocity (B), hepatic arterial pulsatility index (C), and hepatic-vein a-wave velocity (D) in nonobese diabetic patients with different grades of steatosis. A) * p < 0.005 vs grade 0; # p < 0.005 vs grade 1; B) * p < 0.05 vs grade 0; C) * p < 0.05 vs grade 0; D) * p < 0.005 vs grade 0.
Discussion
Our findings indicate that 80% of nonobese patients with type II diabetes, but only in 24% of those with type I diabetes, have NAFLD, and that only severe forms are associated with hepatomegaly and altered liver hemodynamics. NAFLD has become an important clinical problem in Western countries: its prevalence in the general population ranges from 16% to 35%, and the figures for obese patients are close to 100%. The prevalence of NAFLD in nonobese patients with diabetes is not well known. Steatosis was present in 24% of the patients with type I diabetes we examined, a rate similar to that observed in the general population (24%), but the prevalence among patients with type II diabetes was over 3 times higher (76%), confirming the role of insulin resistance in the development of hepatic steatosis [4].
However, most patients had mild or moderate steatosis at the sonographic evaluation. Although the severity of steatosis did not correlate with the BMI, patients with fatty livers did have higher BMIs than their counterparts without steatosis, suggesting that BMI also plays a role in the pathophysiology of steatosis in diabetes. None of our patients had liver biopsies, so we cannot differentiate between NAFLD and NASH.
The evaluation of hemodynamic alterations detectable with Doppler sonography may be useful for the diagnosis of NAFLD, but it can also reveal aspects of the pathophysiology of this disease. Few data are available on the hemodynamic alterations associated with NAFLD. In a rabbit model of steatosis, moderate fatty liver infiltration has been shown to cause significant reductions in portal and total hepatic blood flow and microcirculation, along with significant increases in hepatic artery flow and portal pressure [21]. Magalotti et al. [14] showed that patients with NASH have decreased portal blood flow velocity, increased intrahepatic arterial resistance, and abnormalities in the Doppler waveforms of the hepatic veins. In our study the PI-L was increased in all patients with steatosis, and these changes were particularly evident in those with severe NAFLD. This increase may be secondary to the steatosis itself (due to the volumetric effect), or it might be one of the pathophysiological mechanisms of NAFLD. With reference to the latter possibility, it would be very interesting to compare the PIs-L of patients with simple steatosis and those with NASH. The only information we have at present is that hepatic arterial resistance indices decrease after treatment with metformin in patients with NASH [14].
The PBV was slightly decreased only in patients with severe (grades 4 and 5) steatosis. This reduction could be the result of a decrease in splanchnic inflow or an increase in resistance. Diminished inflow is difficult to justify, but increases in intrahepatic resistance could be caused by compression of the sinusoidal bed by steatotic hepatocytes.
The last hemodynamic parameter we considered was the hepatic-vein flow pattern. Abnormal waveforms were observed in 22 (30%) of the 76 patients with steatosis (monophasic patterns in 6, biphasic patterns in 16). None of the patients with mild steatosis (grades 1–2) had alterations in the pattern, suggesting that increased stiffness is probably involved, with a mechanism similar to cirrhosis, which involves a decrease in the a-wave velocity.
In conclusion, liver steatosis is a common finding in patients with type 2 diabetes (but not type 1), and it is usually mild to moderate. Even mild forms of steatosis are usually associated with increases in the hepatic arterial resistance, while alterations in portal and hepatic-vein hemodynamics are found mainly in severe steatosis. Future studies should disclose whether hemodynamic alterations have prognostic and/or diagnostic value, and in particular whether they can be used to detect the onset NASH.
Conflict of interest statement
The authors have no conflict of interest.
Footnotes
SIUMB 2007 – Award for the best poster presented at the 19th National Congress of the SIUMB.
References
- 1.Bedogni G., Miglioli L., Masutti F., Castiglione A., Crocè L.S., Tiribelli C. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology. 2007;46(5):1387–1391. doi: 10.1002/hep.21827. [DOI] [PubMed] [Google Scholar]
- 2.de Moura Almeida A., Cotrim H.P., Barbosa D.B., de Athayde L.G., Santos A.S., Bitencourt A.G. Fatty liver disease in severe obese patients: Diagnostic value of abdominal ultrasound. World J Gastroenterol. 2008;14(9):1415–1418. doi: 10.3748/wjg.14.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchesini G., Brizi M., Morselli-Labate A.M., Bianchi G., Bugianesi E., McCullough A.J. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107(5):450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 4.Marchesini G., Brizi M., Bianchi G., Tomassetti S., Bugianesi E., Lenzi M. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 5.Maruhama Y., Abe R., Okuguchi F., Oikawa S., Ohneda A., Goto Y. Interactions of obesity and glucose-stimulated insulin secretion in familial hypertriglyceridemia. Diabetes. 1978;27(6):682–693. doi: 10.2337/diab.27.6.682. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S., Saccoccio G., Masutti F., Crocè L.S., Brandi G., Sasso F. Prevalence and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132(2):112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 7.Angelico F., Del Ben M., Conti R., Francioso S., Feole K., Fiorello S. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90(3):1578–1582. doi: 10.1210/jc.2004-1024. [Epub 2004 Dec 14] [DOI] [PubMed] [Google Scholar]
- 8.Leite N.C., Salles G.F., Araujo A.L., Villela-Nogueira C.A., Cardoso C.R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2008 Apr 1 doi: 10.1111/j.1478-3231.2008.01718.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Sheth S.G., Gordon F.D., Chopra S. Nonalcoholic steatohepatitis. Ann Intern Med. 1997;127(8 Pt 1):658. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl. 1):S99–S112. doi: 10.1002/hep.20973. [review] [DOI] [PubMed] [Google Scholar]
- 11.Falchuk K.R., Fiske S.C., Haggitt R.C., Federman M., Trey C. Pericentral hepatic fibrosis and intracellular hyalin in diabetes mellitus. Gastroenterology. 1980;78(3):535–541. [PubMed] [Google Scholar]
- 12.Targher G., Bertolini L., Padovani R., Rodella S., Zoppini G., Zenari L. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29(6):1325–1330. doi: 10.2337/dc06-0135. [DOI] [PubMed] [Google Scholar]
- 13.Fan J.G. Impact of non-alcoholic fatty liver disease on accelerated metabolic complications. J Dig Dis. 2008;9(2):63–67. doi: 10.1111/j.1751-2980.2008.00323.x. [DOI] [PubMed] [Google Scholar]
- 14.Magalotti D., Marchesini G., Ramilli S., Berzigotti A., Bianchi G., Zoli M. Splanchnic haemodynamics in non-alcoholic fatty liver disease: effect of a dietary/pharmacological treatment. A pilot study. Dig Liver Dis. 2004;36(6):406–411. doi: 10.1016/j.dld.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Sacerdoti D., Merkel C., Bolognesi M., Amodio P., Angeli P., Gatta A. Hepatic arterial resistance in cirrhosis with and without portal vein thrombosis: relationships with portal hemodynamics. Gastroenterology. 1995;108(4):1152–1158. doi: 10.1016/0016-5085(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 16.Bolognesi M., Sacerdoti D., Merkel C., Bombonato G., Enzo E., Gatta A. Effects of chronic therapy with nadolol on portal hemodynamics and on splanchnic impedance indices using Doppler sonography: comparison between acute and chronic effects. J Hepatol. 1997;26(2):305–311. doi: 10.1016/s0168-8278(97)80046-3. [DOI] [PubMed] [Google Scholar]
- 17.Niederau C., Sonnenberg A. Liver size evaluated by ultrasound: ROC curves for hepatitis and alcoholism. Radiology. 1984;153(2):503–505. doi: 10.1148/radiology.153.2.6385110. [DOI] [PubMed] [Google Scholar]
- 18.Sabbà C., Merkel C., Zoli M., Ferraioli G., Gaiani S., Sacerdoti D. Interobserver and interequipment variability of echo-Doppler examination of the portal vein: effect of a cooperative training program. Hepatology. 1995;21(2):428–433. doi: 10.1002/hep.1840210225. [DOI] [PubMed] [Google Scholar]
- 19.Sacerdoti D., Gaiani S., Buonamico P., Merkel C., Zoli M., Bolondi L. Interobserver and interequipment variability of hepatic, splenic, and renal arterial Doppler resistance indices in normal subjects and patients with cirrhosis. J Hepatol. 1997;27(6):986–992. doi: 10.1016/s0168-8278(97)80141-9. [DOI] [PubMed] [Google Scholar]
- 20.Bolondi L., Li Bassi S., Gaiani S., Zironi G., Benzi G., Santi V. Liver cirrhosis: changes of Doppler waveform of hepatic veins. Radiology. 1991;100(2):586. doi: 10.1148/radiology.178.2.1987617. [DOI] [PubMed] [Google Scholar]
- 21.Seifalian A.M., El-Desoky A., Davidson B.R. Hepatic indocyanine green uptake and excretion in a rabbit model of steatosis. Eur Surg Res. 2001;33(3):193–201. doi: 10.1159/000049706. [DOI] [PubMed] [Google Scholar]


