Abstract
Relapse is common after hematopoietic cell transplantation for acute lymphoblastic leukemia (ALL). While 1200 cGy total body irradiation (TBI) and cyclophosphamide (Cy) is standard, attempts to lower relapse have led to the addition of a second chemotherapeutic agent and/or higher dose TBI. We examined transplantation outcomes in patients aged <18 years with ALL, in second or subsequent remission or in relapse at transplantation. Most transplants occurred in remission. Patients received grafts from an HLA-matched sibling or unrelated donor. Four treatment groups were created: 1) Cy + TBI≤1200 cGy (n=304), 2) Cy + etoposide + TBI≤1200 cGy (n=108), 3) Cy + TBI≥1300 cGy (n=327), and 4) Cy + etoposide + TBI≥1300 cGy (n=26). Neither TBI in excess of 1200 cGy nor the addition of etoposide resulted in fewer relapses. The 5-year probabilities of relapse were 30%, 28%, 35% and 31% for groups 1, 2, 3 and 4, respectively. However, transplant-related mortality was higher (35% vs. 25%, p=0.02) and overall survival lower (36% vs. 48%, p=0.03) after Cy + etoposide + TBI ≥1300 cGy compared to Cy + TBI ≥1300 cGy. Compared to the standard regimen neither TBI in excess of 1200cGy nor the addition of etoposide improves survival after HCT for ALL.
Keywords: TBI Dose, Leukemia Recurrence
INTRODUCTION
An accepted treatment for children with recurrent acute lymphoblastic leukemia (ALL) is allogeneic hematopoietic stem cell transplantation (HCT).(1-3) Transplant conditioning regimens often consist of total body irradiation (TBI), doses ranging from 1000 – 1400 cGy, with one or more chemotherapeutic agents. Although developed on empirical observations the standard conditioning regimen is cyclophosphamide (Cy, 120mg/kg and TBI, 1200cGy).(4) An earlier report from the Center for International Blood and Marrow Transplant Research (CIBMTR) showed non-irradiation containing regimens were associated with higher relapse compared to TBI-containing regimens for ALL.(5) Attempts to lower relapse risks after HCT by modulating transplant conditioning have included TBI dose greater than 1200 cGy and/or the addition of a second chemotherapeutic agent; the most common being etoposide.(6-8) Others have attempted to lower the intensity of the conditioning regimen relying on immune modulation (graft versus leukemia effect) for disease control.(9) Although reports with relatively few patients suggest acceptable leukemia-free survival; these regimens are used for fewer than 5% of pediatric ALL transplantations.(10)
A review of myeloablative transplant TBI-containing conditioning regimens for pediatric ALL reported to the CIBMTR identified four commonly used regimens: 1) TBI 1000 or 1200 cGy and Cy, 2) TBI 1000 or 1200 cGy, Cy and etoposide, 3) TBI 1320 – 1400 cGy and Cy, 4) TBI 1320 – 1400 cGy, Cy and etoposide. In the current analysis we sought to examine the effect of the four commonly used transplant conditioning regimens on leukemia relapse, transplant-related mortality and overall survival in 765 children and adolescents with ALL.
PATIENTS AND METHODS
Data Source
The CIBMTR is a voluntary working group of more than 400 transplant centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantation to a Statistical Center at the Medical College of Wisconsin in Milwaukee or the National Marrow Donor Program Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally. Patients and/or their guardians provided written informed consent. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Inclusion criteria
Included are patients with ALL and aged less than 18 years at transplantation who received grafts from an HLA-matched sibling or an unrelated donor. Unrelated donor grafts included bone marrow or umbilical cord blood. Transplants were performed in 1998 – 2007. All patients received myeloablative conditioning with TBI containing regimens (TBI ≥1000 cGy). Recipients of non-TBI containing regimens were excluded.
Outcomes
The primary outcome was relapse after transplantation. Relapse was defined as morphological reappearance of leukemic blasts. Other outcomes included: transplant-related mortality defined as death not related to leukemia recurrence and overall survival defined as death from any cause. Surviving patients were censored at last follow-up.
Statistical Analysis
Patient, disease, and transplant characteristics of the four treatment groups were compared with the Chi-square test for categorical variables. The probability of overall survival was calculated with the Kaplan-Meier estimator.(11) The probabilities of transplant-related mortality and relapse were calculated with the cumulative incidence estimator.(12) For transplant-related mortality, relapse was the competing event, and for relapse, transplant-related mortality was the competing event. 95% confidence intervals (CI) were derived from log transformation. Multivariate models were built using Cox proportional hazards regression models for transplant-related mortality, relapse and overall mortality.(13) Models were built using the backward stepwise selection procedure and confirmed with the use of forward stepwise selection procedure. The proportional-hazards assumption was tested for each variable individually; all variables met this assumption. P-value ≤0.05 was considered statistically significant.
The variable for transplant conditioning regimen: TBI ≤1200 cGy (1000 or 1200 cGy) and Cy vs. TBI ≤1200 cGy, Cy and etoposide vs. TBI ≥1320 (1320 or 1350 or 1400 cGy) and Cy vs. TBI ≥1320, Cy and etoposide were held in all steps of model building regardless of level of significance. Other variables tested were held in the final model when significant. Other variables tested include: patient age (≤10 years vs. >10 years), NCI risk score (standard risk vs. high risk), cytogenetic risk (standard risk vs. high risk) duration of first remission (≤ 36 months vs. > 36 months), patient performance score (90-100 vs. ≤ 80), donor and graft source (HLA-matched sibling [bone marrow/cord blood]) vs. HLA-matched unrelated donor bone marrow vs. HLA-mismatched unrelated donor bone marrow vs. unrelated cord blood), recipient CMV serostatus (positive vs. negative) and year of transplant (1998 – 1999 vs. 2000 – 2004 vs. 2005 – 2007). There was no significant transplant center effect on survival. All analyses were done using SAS 9.1 (Cary, NC).
RESULTS
Patient, Disease and Transplant Characteristics
Patient, disease, and transplant characteristics by treatment group are presented in Table 1. Sixteen of 412 (4%) patients in the TBI≤1200 cGy group received 1000 cGy and the remaining patients, TBI 1200 cGy. Eighty-four of 353 (24%) patients who received TBI ≥1320cGy received 1320 cGy, 145 of 353 (41%) received 1350 cGy and the remaining patients, 1400 cGy (124 of 353; 35%). Almost all patients received Cy 120 mg/kg regardless of TBI dose; 87% of those who received etoposide as a second agent received either 40 mg/kg or 60 mg/kg. While there were no significant differences in patient age, those that received etoposide in addition to TBI and Cy were more likely to have performance score less than 90. Disease characteristics including the National Cancer Index (NCI), cytogenetic risk, interval from diagnosis to transplantation and disease status at transplantation were similar across the treatment groups. There were differences in choice of conditioning regimen; recipients of TBI dose ≥1320, Cy and etoposide were more likely to have received HLA-matched sibling transplantation, less likely to have received umbilical cord blood transplantation, more likely to receive methotrexate containing graft-versus-host disease prophylaxis and more likely to be transplanted prior to 2005. The median follow-up of surviving patients in all treatment groups is 4 years.
Table 1.
Patient, Disease, and Transplant Characteristics
| Cyclophosphamide | Cyclophosphamide + etoposide | ||||
|---|---|---|---|---|---|
| TBI ≤1200 cGy | TBI ≥1320 cGy | TBI ≤1200 cGy | TBI ≥1320 cGy | p-value | |
| Number | 304 | 327 | 108 | 26 | |
| Age at Transplant | NS | ||||
| ≤ 10 | 171 (56) | 197 (60) | 71 (66) | 14 (54) | |
| 11-18 | 133 (44) | 130 (40) | 37 (34) | 12 (46) | |
| Sex | NS | ||||
| Male | 200 (65) | 214 (65) | 66 (61) | 14 (54) | |
| Female | 104 (32) | 113 (35) | 42 (39) | 12 (46) | |
| Performance Score | <0.0001 | ||||
| <90% | 47 (15) | 40 (12) | 28 (26) | 8 (31) | |
| ≥90% | 244 (80) | 253 (77) | 78 (72) | 17 (65) | |
| Not reported | 13 (4) | 34 (10) | 2 (2) | 1 (4) | |
| Recipient CMV Status | 0.002 | ||||
| Positive | 169 (56) | 145 (45) | 35 (32) | 11 (42) | |
| Negative | 133 (44) | 177 (54) | 71 (66) | 15 (58) | |
| Not reported | 2 (1) | 5 (1) | 2 (2) | — | |
| NCI Risk Score | NS | ||||
| Normal | 105 (35) | 124 (38) | 47 (44) | 8 (26) | |
| High | 164 (54) | 152 (46) | 47 (44) | 16 (58) | |
| Not reported | 35 (12) | 51 (16) | 14 (13) | 2 (16) | |
| Cytogenetic risk group | NS | ||||
| Intermediate Risk | 231 (76) | 229 (70) | 81 (75) | 13 (50) | |
| High Risk | 10 (3) | 17 (5) | 2 (2) | 3 (12) | |
| Not reported | 63 (21) | 81 (25) | 25 (23) | 10 (38) | |
| Duration of 1st remission | NS | ||||
| ≤36 mo | 201 (66) | 213 (65) | 66 (61) | 17 (65) | |
| >36 mo | 103 (34) | 114 (35) | 42 (39) | 9 (35) | |
| Disease status | NS | ||||
| 2nd complete remission | 227 (75) | 219 (67) | 78 (72) | 16 (62) | |
| 3rd complete remission | 55 (18) | 71 (22) | 21 (19) | 5 (19) | |
| Relapse | 22 (7) | 37 (11) | 9 (8) | 5 (19) | |
| Donor source | <0.0001 | ||||
| HLA - matched sibling | |||||
| Bone marrow | 103 (34) | 19 (6) | 15 (14) | 10 (38) | |
| Cord blood | 4 (1) | 6 (2) | 3 (3) | 1 (4) | |
| Unrelated donor | |||||
| Matched | 47 (15) | 66 (20) | 18 (17) | 4 (15) | |
| Mismatched | 82 (27) | 106 (32) | 29 (27) | 10 (38) | |
| Cord blood | 68 (22) | 132 (40) | 42 (39) | 3 (12) | |
| GVHD Prophylaxis | <0.0001 | ||||
| Cyclosporine + methotrexate | 186 (61) | 139 (43) | 56 (52) | 11 (42) | |
| Cyclosporine ± steroid | 77 (25) | 124 (38) | 26 (24) | 13 (50) | |
| Tacrolimus + methotrexate | 32 (11) | 54 (17) | 15 (14) | 0 (0) | |
| Tacrolimus ± other | 4 (1) | 7 (2) | 6 (6) | 1 (4) | |
| Methotrexate + other | 5 (2) | 3 (1) | 5 (5) | 1 (4) | |
| Year of Transplant | <0.0001 | ||||
| 1998-1999 | 73 (24) | 41 (13) | 30 (28) | 10 (38) | |
| 2000-2004 | 160 (53) | 173 (53) | 56 (52) | 14 (54) | |
| 2005-2007 | 71 (23) | 113 (35) | 22 (20) | 2 (8) | |
| Follow-Up of surviving patients; median (range), months |
50 (3-133) | 44 (2-119) | 46 (3-130) | 53 (34-93) | |
Relapse
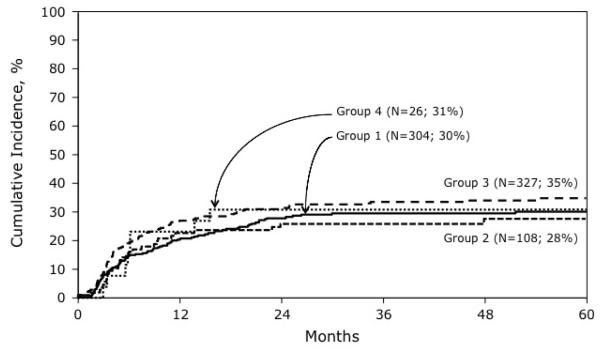
In multivariate analysis the risk of relapse was similar in all patients regardless of the conditioning regimen they received (Table 2). The 5-year probabilities of relapse for treatment groups 1, 2, 3 and 4 were 30% (95% CI 25-35), 28% (95% CI 19 – 37), 35% (95% CI 29 – 40), and 31% (95% CI 15 – 48), respectively (Figure 1). Relapse risks were similar after TBI (any dose) + Cy + etoposide compared to TBI (any dose) + Cy (HR 0.9, 95% CI 0.66 – 1.34, p=0.72). However, relapse risks were associated with gender, duration of first remission and disease status at transplantation. Risks were higher in females (HR 1.5, 95% CI 1.2-2.0, P=0.003), duration of first remission less than 36 months (HR 2.96, 95% CI 2.13 – 4.17, p<0.001) and those in third complete remission or relapse at transplantation (HR 1.6, 95% CI 1.2-2.2, P=0.001).
Table 2.
Results of multivariate analysis
| Hazard ratio 95% confidence interval |
P-value | |
|---|---|---|
| Relapse | ||
| Cyclophosphamide/etoposide/TBI ≤ 1200 cGy vs. Cyclophosphamide/TBI ≤ 1200 cGy | 0.97 (0.63 – 1.48) | 0.87 |
| Cyclophosphamide/etoposide/TBI ≥1320 cGy vs. Cyclophosphamide/TBI ≥ 1320 cGy | 1.01 (0.49 – 2.09) | 0.97 |
| Cyclophosphamide/TBI ≥1320 cGy vs. Cyclophosphamide/TBI ≤ 1200 cGy | 1.13 (0.85 – 1.50) | 0.41 |
| Cyclophosphamide/etoposide/TBI ≥1320 cGy vs. Cyclophosphamide/etoposide/TBI ≤ 1200 cGy | 1.19 (0.54 – 2.61) | 0.67 |
| Transplant-related mortality | ||
| Cyclophosphamide/etoposide/TBI ≤ 1200 cGy vs. Cyclophosphamide/TBI ≤ 1200 cGy | 1.06 (0.70 – 1.60) | 0.78 |
| Cyclophosphamide/etoposide/TBI ≥1320 cGy vs. Cyclophosphamide/TBI ≥ 1320 cGy | 2.36 (1.17 – 4.76) | 0.02 |
| Cyclophosphamide/TBI ≥1320 cGy vs. Cyclophosphamide/TBI ≤ 1200 cGy | 0.73 (0.53 – 1.01) | 0.06 |
| Cyclophosphamide/etoposide/TBI ≥1320 cGy vs. Cyclophosphamide/etoposide/TBI ≤ 1200 cGy | 1.63 (0.77 – 3.45) | 0.20 |
| Overall mortality | ||
| Cyclophosphamide/etoposide/TBI ≤ 1200 cGy vs. Cyclophosphamide/TBI ≤ 1200 cGy | 1.10 (0.82 – 1.50) | 0.52 |
| Cyclophosphamide/etoposide/TBI ≥1320 cGy vs. Cyclophosphamide/TBI ≥ 1320 cGy | 1.79 (1.07 – 2.99) | 0.03 |
| Cyclophosphamide/TBI ≥1320 cGy vs. Cyclophosphamide/TBI ≤ 1200 cGy | 0.87 (0.69 – 1.09) | 0.23 |
| Cyclophosphamide/etoposide/TBI ≥1320 cGy vs. Cyclophosphamide/etoposide/TBI ≤ 1200 cGy | 1.40 (0.81 – 2.43) | 0.23 |
Figure 1.
The probabilities of relapse by transplant conditioning regimen: Group 1: Cy + TBI ≤ 1200 cGy; Group 2: Cy + etoposide + TBI ≤ 1200 cGy; Group 3: Cy + TBI ≥ 1300 cGy; Group 4: Cy + etoposide + TBI ≥1300 cGy
Treatment Related Mortality
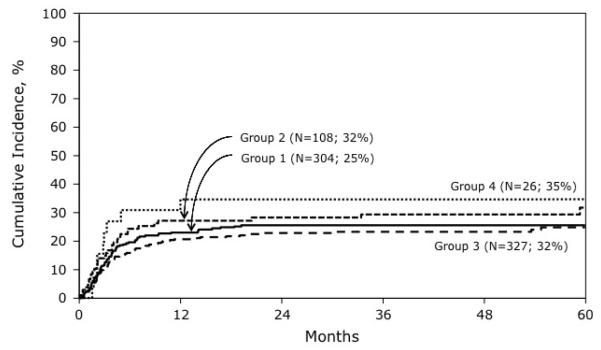
Transplant-related mortality risks differed by transplant conditioning regimen (Table 2). Compared to recipients of TBI ≥ 1320 cGy + Cy, those who received TBI ≥ 1320 cGy, Cy + etoposide experienced higher risks transplant-related mortality. The addition of etoposide to TBI ≤ 1200 cGy + Cy compared to TBI ≤ 1200 cGy + Cy alone was not associated with higher risks (HR 1.06, 95% CI 0.70 – 1.60; p=0.78). The 5-year cumulative incidence of transplant-related mortality for treatment groups 1, 2, 3 and 4 were 25% (95% CI 21 – 31), 32% (95% CI 23 – 41), 25% (95% CI 20 – 30), and 35% (95% CI 18-52), respectively (Figure 2). Transplant-related mortality risks were not higher in recipients of TBI (any dose) + Cy + etoposide compared to TBI (any dose) + Cy (HR 1.37, 95% CI 0.97 – 1.92, p=0.07). Age greater than 10 years (HR 1.93, 95% CI 1.45 – 2.56, p<0.001) led to higher risks of transplant-related mortality. Compared to recipients of HLA-matched sibling transplants, transplant-related mortality risks were higher after matched unrelated donor bone marrow (HR 3.26, 95% CI 1.77 – 6.02, p<0.001), mismatched unrelated donor bone marrow (HR 4.07, 95% CI 2.34 – 7.08, p<0.001) and umbilical cord blood (HR 5.30, 95% CI 3.04 – 9.25, p<0.001) transplants.
Figure 2.
The probabilities of transplant-related mortality by transplant conditioning regimen: Group 1: Cy + TBI ≤ 1200 cGy; Group 2: Cy + etoposide + TBI ≤ 1200 cGy; Group 3: Cy + TBI ≥ 1300 cGy; Group 4: Cy + etoposide + TBI ≥1300 cGy
Overall survival
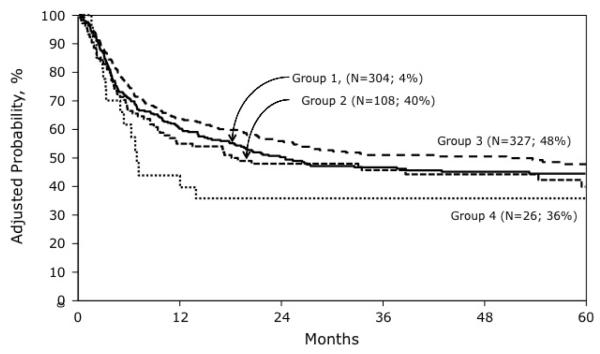
Overall mortality risks also differed by transplant conditioning regimen (Table 2). Recipients of TBI ≥ 1320 cGy who received etoposide + Cy had higher mortality risks compared to those who received Cy alone. Mortality risks were not higher in recipients of TBI ≤ 1200 cGy who received etoposide + Cy compared to Cy alone (HR 1.10, 95% CI 0.82 – 1.50; p=0.52). Overall mortality risks were not higher in recipients of TBI (any dose) + Cy + etoposide compared to TBI (any dose) + Cy (HR 1.24, 95% CI 0.96 – 1.59, p=0.09). Mortality risks were higher for patients older than 10 years (HR 1.05, 95% CI 1.22 – 1.85, p<0.001), duration of first remission ≤ 36 months (HR 1.69, 95% CI 1.35 – 2.11, p<0.001) and for those transplanted in third remission or in relapse (HR 1.41, 95% CI 1.14 – 1.74, p=0.002). Compared to recipients of HLA-matched sibling transplants, overall mortality risks were higher after mismatched unrelated donor bone marrow (HR 1.72, 95% CI 1.27 – 2.34, p<0.001) and umbilical cord blood (HR 1.87, 95% CI 1.36 – 2.57, p<0.001) transplants but not matched unrelated donor bone marrow transplants (HR 1.34, 95% CI 0.94 – 1.92, p=0.11). The 5-year probabilities of overall survival for treatment groups 1, 2, 3 and 4 were 44% (95% CI 38 – 50), 40% (95% CI 30 – 50), 48% (95% CI 42 – 54) and 36% (95% CI 19 –53) (Figure 3).
Figure 3.
The probabilities of overall survival by transplant conditioning regimen adjusted for patient age, duration of first complete remission, disease status at transplant and donor HLA-match: Group 1: Cy + TBI ≤ 1200 cGy; Group 2: Cy + etoposide + TBI ≤ 1200 cGy; Group 3: Cy + TBI ≥ 1300 cGy; Group 4: Cy + etoposide + TBI ≥1300 cGy
DISCUSSION
The current analysis sought to examine for an effect on relapse after transplantation with different TBI-containing myeloablative conditioning regimens for ALL in children and adolescents. Conditioning regimens were divided into four groups based on TBI dose and chemotherapeutic agents; neither TBI ≥ 1200 cGy nor addition of etoposide to Cy led to lower relapse risks. However, transplant-related and overall mortality risks were higher with TBI ≥ 1320 cGy + etoposide + Cy compared to TBI ≥ 1320 cGy + Cy alone. With lower dose TBI (1000 cGy or 1200 cGy) the addition of etoposide was not associated with higher mortality risks. The addition of a second chemotherapeutic agent for children and adolescents undergoing myeloablative TBI-based conditioning for enhanced leukemia control is not supported by these data. On the contrary, the addition of a second chemotherapeutic agent to TBI ≥ 1320 cGy + Cy increases mortality risks and should be avoided.
Our observations contrast those reported by Duerst and colleagues in their report on 41 children with ALL and AML who received TBI 1200 – 1400 cGy with etoposide and Cy.(14) They observed a single fatal regimen-related toxicity in their series; recurrent leukemia was the predominant cause of treatment failure. In the report by Duerst, the dose of etoposide was 30 mg/kg where as in the current analysis most patients (87%) received in excess of 30 mg/kg. The observed differences in mortality risks between the current analysis and the Deurst report may be explained by the dose of etoposide. As only 17 patients received 30 mg/kg of etoposide we were unable to test for an effect of etoposide dose on mortality risks. Others have reported lower relapse risks with TBI-containing regimens and etoposide alone. One such is a large series from the CIBMTR compared TBI dose (< 1300 cGy vs. ≥ 1300 cGy) with Cy or etoposide for children and adults with ALL in first or second complete remission.(15) In that report, for patients in second complete remission, relapse and mortality risks were lower for those that received TBI (any dose) and etoposide compared to TBI <1300 cGy and Cy. There is an on-going clinical trial through the International Berlin-Frankfurt-Muenster group for allogeneic transplantation in children and adolescents with ALL (NCT01423747). The recommended regimen is TBI 1200 cGy and etoposide 60 mg/kg for matched related and unrelated donor, and etoposide 40 mg/kg for mismatched related or unrelated donor transplants. We were unable to test for an effect of TBI + etoposide alone versus TBI + Cy alone as there were too few children who received etoposide alone. In another report that focused on pediatric ALL, Gassas and colleagues compared the addition of etoposide or Cy to TBI 1200 cGy and concluded both regimens were equally effective.(8) We tested for an effect when etoposide was added to TBI ≤ 1200 cGy + Cy and found none. It is plausible that the excess mortality risks observed with the addition of etoposide to higher dose TBI is the additive effect of higher dose TBI and a second chemotherapeutic agent. Though the true etiology for the excess mortality is not known, our observations suggest neither TBI dose in excess of 1200 cGy nor the addition of a second chemotherapeutic agent is necessary.
Several factors besides conditioning regimen were associated with leukemia relapse. Consistent with other reports, duration of first remission and disease status at transplantation were important predictors of relapse.(16) We did not observe significant differences in relapse risks with the addition of etoposide. Our observations contrast those reported by others in that TBI-containing regimens with etoposide alone was associated with superior leukemia-control post-transplant.15 The ages of patients included in the various studies differ; ours is limited to children and adolescents and the observed differences may be explained by differences in the biology of pediatric and adult ALL and/or differences in intensity of up-front chemotherapy regimens used to induce second remission.
In addition to differences in mortality risks by conditioning regimen, patient age and donor source had an adverse effect on survival. Older age and transplantation of grafts from unrelated donors led to higher mortality risks. While these factors predict mortality, patient age and donor source are not modifiable factors. The data presented here-in span the period 1998 – 2007. Concurrent with improvements in supportive care and donor selection survival rates are not different after HLA-matched sibling and matched unrelated donor transplantation.(17) In the absence of a suitably matched related donor, physicians should defer to recommended guidelines for selection of unrelated donors; i.e.; transplantation of bone from a 8/8 or 7/8 HLA-matched adult donor or mismatched umbilical cord blood unit with adequate cell dose.(18) It is noteworthy that the effect of transplant conditioning regimen on transplant-related and overall mortality was independent of patient age and donor source. Patients who received TBI ≥1320 cGy + etoposide + Cy were more likely to report performance scores less than 90. Since poor performance score predicts survival, this variable was retained in the final multivariate model implying the observed adverse effect on mortality is independent of performance score.
As with any study that uses data collected by a registry there could be several unknown or unmeasured factors that may have also influenced outcomes. However, we performed a carefully controlled analysis adjusting for patient, disease and transplant characteristics known to be associated with leukemia relapse and survival after transplantation. Our findings suggest the addition of etoposide to TBI ≥1320 cGy + Cy increases mortality risks and should be avoided for children and adolescents with ALL. Given the higher risks of second malignant neoplasm with TBI dose 1300 cGy or higher (19, 20), in the absence of data that demonstrate an advantage for either lower relapse or higher survival, TBI dose in excess of 1200 cGy must be avoided in children with ALL.
ACKNOWLEDGEMENTS
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chessells JM. The management of high-risk lymphoblastic leukaemia in children. Br J Haematol. 2000;108:204–216. doi: 10.1046/j.1365-2141.2000.01849.x. [DOI] [PubMed] [Google Scholar]
- 2.Borgmann A, von Stackelberg A, Hartmann R, et al. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003;101:3835–3839. doi: 10.1182/blood.V101.10.3835. [DOI] [PubMed] [Google Scholar]
- 3.Eapen M, Raetz E, Zhang MJ, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107:4961–4967. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bensinger W. Hi-dose preparative regimens. In: Appelbaum F, Forman SJ, Negrin RS, Blume KG, editors. Hematopoietic Cell Transplantation. Wiley-Blackwell Science; Chichester, United Kingdom: 1999. pp. 1316–1332. [Google Scholar]
- 5.Davies SM, Ramsay NK, Klein JP, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000;18:340–347. doi: 10.1200/JCO.2000.18.2.340. [DOI] [PubMed] [Google Scholar]
- 6.Woods WG, Ramsay NK, Weisdorf DJ, et al. Bone marrow transplantation for acute lymphocytic leukemia utilizing total body irradiation followed by high doses of cytosine arabinoside: lack of superiority over cyclophosphamide-containing conditioning regimens. Bone Marrow Transplant. 1990;6:9–16. [PubMed] [Google Scholar]
- 7.Snyder DS, Chao NJ, Amylon MD, et al. Fractionated total body irradiation and high-dose etoposide as a preparatory regimen for bone marrow transplantation for 99 patients with acute leukemia in first complete remission. Blood. 1993;82:2920–2928. [PubMed] [Google Scholar]
- 8.Gassas A, Sung L, Saunders EF, Doyle JJ. Comparative outcome of hematopoietic stem cell transplantation for pediatric acute lymphoblastic leukemia following cyclophosphamide and total body irradiation or VP16 and total body irradiation conditioning regimens. Bone Marrow Transplant. 2006;38:739–743. doi: 10.1038/sj.bmt.1705515. [DOI] [PubMed] [Google Scholar]
- 9.Pulsipher MA, Boucher KM, Wall D, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114:1429–1436. doi: 10.1182/blood-2009-01-196303. [DOI] [PubMed] [Google Scholar]
- 10.Verneris MR, Eapen M, Duerst R, et al. Reduced-intensity conditioning regimens for allogeneic transplantation in children with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2010;16:1237–1244. doi: 10.1016/j.bbmt.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein J, Moeschberger ML, editors. Survival Analysis: Statistical Methods for Censored and Truncated Data. Springer-Verlag; New York, NY: 2003. [Google Scholar]
- 12.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Cox D. Regression models and life-tables. Journal of the Royal Statistical Society. 1972;34:1187–1202. [Google Scholar]
- 14.Duerst RE, Horan JT, Liesveld JL, et al. Allogeneic bone marrow transplantation for children with acute leukemia: cytoreduction with fractionated total body irradiation, high-dose etoposide and cyclophosphamide. Bone Marrow Transplant. 2000;25:489–494. doi: 10.1038/sj.bmt.1702181. [DOI] [PubMed] [Google Scholar]
- 15.Marks DI, Forman SJ, Blume KG, et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12:438–453. doi: 10.1016/j.bbmt.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Oliansky DM, Camitta B, Gaynon P, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transplant. 2012;18:505–522. doi: 10.1016/j.bbmt.2011.12.585. [DOI] [PubMed] [Google Scholar]
- 17.Shaw PJ, Kan F, Woo Ahn K, et al. Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood. 2010;116:4007–4015. doi: 10.1182/blood-2010-01-261958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spellman SR, Eapen M, Logan BR, et al. A perspective on the selection of unrelated donors and cord blood units for transplantation. Blood. 2012;120:259–265. doi: 10.1182/blood-2012-03-379032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]