Abstract
Innate immunity within the central nervous system (CNS) is primarily provided by resident microglia. Microglia are pivotal in immune surveillance and also facilitate the coordinated responses between the immune system and the brain. For example, microglia interpret and propagate inflammatory signals that are initiated in the periphery. This transient microglial activation helps mount the appropriate physiological and behavioral response following peripheral infection. With normal aging, however, microglia develop a more inflammatory phenotype. For instance, in several models of aging there are increased pro-inflammatory cytokines in the brain and increased expression of inflammatory receptors on microglia. This increased inflammatory status of microglia with aging is referred to as primed, reactive, or sensitized. A modest increase in the inflammatory profile of the CNS and altered microglial function in aging has behavioral and cognitive consequences. Nonetheless, there are major differences in microglial biology between young and old age when the immune system is challenged and microglia are activated. In this context, microglial activation is amplified and prolonged in the aged brain compared to adults. The cause of this amplified microglial activation may be related to impairments in several key regulatory systems with age that make it more difficult to resolve microglial activation. The consequences of impaired regulation and microglial hyper-activation following immune challenge are exaggerated neuroinflammation, sickness behavior, depressive-like behavior and cognitive deficits. Therefore the purpose of this review is to discuss the current understanding of age-associated microglial priming, consequences of priming and reactivity, and the impairments in regulatory systems that may underlie these age-related deficits.
Keywords: Brain, Microglia, Aging, Inflammation, Behavior
Increased inflammatory status in the aging brain
There is significant clinical and experimental evidence that inflammation within the central nervous system (CNS) increases with age. A hallmark of brain aging is increased oxidative stress and lipid peroxidation. Therefore, one hypothesis is that the accumulation of free radical damage over time leads to increased inflammation within the brain. Consistent with this premise, a myriad of microarray studies indicate that there is an overall increase in inflammatory and pro-oxidant genes while there is a reduction in growth and anti-oxidant genes in the brain of older rodents compared to adults (1, 2). Moreover, there are increased protein levels of several inflammatory cytokines, including interleukin (IL)-1β and IL-6, in the brain of aged rodents (2–8). In addition, there are reductions in several anti-inflammatory cytokines including IL-10 and IL-4 (9–12). There is significant interest in understanding why there is an age associated shift in the inflammatory profile of the brain. Recent evidence indicates that the resident glia are key contributors to the increased inflammatory state of the CNS.
Microglia
The majority of aging research focuses on understanding how age influences microglia biology. This is because microglia are dynamic cells of the CNS that play pivotal roles in development, plasticity and immune surveillance (for reviews see (13, 14)). During homeostasis, microglia perform essential functions including monitoring synapses (15), clearing up apoptotic debris (16, 17) and are involved in synaptic pruning (18). Surveying microglia have a ramified morphology with long and thin processes. These processes are highly motile and continuously survey the local microenvironment. The continuous sampling of the microenvironment ensures that microglia will quickly respond to any local disturbances in homeostasis (19, 20).
The distribution of microglia throughout the brain is diverse and varies amongst species. In humans, microglia comprise up to 16 % of the CNS cellular population and this is dependent on brain region. For instance, microglia are present at a higher density in white matter than gray matter in humans (Mittelbronn et al., 2001). In rodents, microglia comprise between 5–12 % of the CNS cellular population. Microglia in the rodent brain, however, are found at a higher density in the gray matter compared to white matter (21, 22).
Because of their similarity to macrophages, microglia are referred to as the resident innate immune cells of the CNS. This is because they can provide several macrophage related activities that provide an innate immunity as the first and main form of active immune defense in the brain. Consistent with these functional similarities to macrophages, microglia are derived from primitive yolk-sac myeloid cells and migrate to the CNS during embryonic development (23, 24). Once microglia reach a final differentiated state in the brain, microglia replication is limited. However, several studies report that microglia proliferate after traumatic CNS injury (25, 26). The effect of aging alone on microglial replication is controversial with the general consensus that there is not an increased number of microglia with age (27). The turnover of parenchymal microglia from bone marrow is also limited (28). For example, a recent study showed that microglia turnover from bone marrow derived myeloid cells ranged between nonexistent to 10 % over a 12 month period that microglial turnover was determined (24). Moreover, other studies examining myeloid cell trafficking in the context of CNS pathology indicate that self-renewal of microglia is likely from a CNS derived progenitor source, rather than from bone-marrow derived cells (29, 30). This is in contrast to CNS perivascular and meningeal macrophages that are renewed every 3–4 weeks from bone marrow derived circulating monocytes (31, 32). The limited replication and turnover of microglia make them a relatively stable population that is maintained throughout life. Therefore, aging is likely to have a profound effect on this relatively stable population of resident microglia.
Microglia are key mediators of the coordinated response to infection by the peripheral immune system and CNS. Microglia respond to and propagate inflammatory signals initiated at the periphery. Following activation, microglia produce pro-inflammatory cytokines including IL-1β, IL-6, and tumor necrosis factor alpha (TNFα) (14). These cytokines are essential for the induction and maintenance of the behavioral symptoms of sickness (reviewed by, (33)). These cytokines also promote the release of secondary inflammatory mediators including prostaglandins and nitric oxide (34–36). The activation of microglia and the production of cytokines is transient and microglia return to a surveying state as the immune stimulus is resolved.
Evidence of microglial priming in the aged brain
The increased inflammatory profile of the CNS with age is associated with microglial priming (Fig. 1). For example, there is increased expression of inflammatory markers including MHC II and complement receptor 3 (CD11b) in the aged brain of humans, rodents, canines, and non-human primates (2, 9, 27, 37–44). Many of these markers are present specifically on microglia of the aged brain, including MHC II (44). MHC II is relevant because it is conserved across species and is interpreted to indicate microglial priming. For example, approximately 25% of microglia from aged mice were MHC II positive, compared to only 2% of microglia from adult mice (44). Consistent with microglial priming, other inflammatory markers are also increased in models of aging. These include scavenger receptor CD68 (2, 45), CD11b and CD11c integrins (37, 46), Toll-like receptors (TLR) (27, 47), and co-stimulatory molecule CD86 (B7) (48) (Fig. 1).
Figure 1. Evidence of microglial priming in the aged brain.
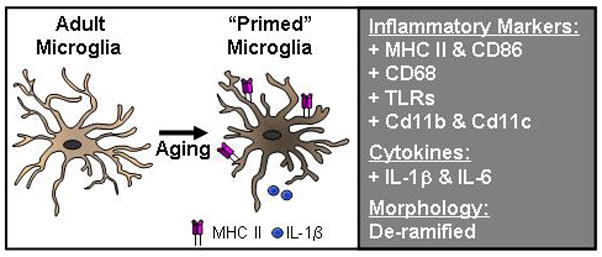
In normal aging there is increased mRNA and protein expression of several inflammatory markers on microglia. In older rodents and non-human primates these include proteins associated with antigen presentation, (MHC II and CD86), scavenger receptors (CD68), pattern associated recognition receptors (Toll-like receptors), and integrins (CD11b and CD11c). There are also detectable increases in inflammatory cytokines and decreases in anti-inflammatory cytokines in the aged brain. Last, in several aging models the morphology of the microglia is more de-ramifed. Collectively these findings are interpreted to indicate that microglia of the aged brain maintain a primed or activated immune profile.
Consistent with the increased inflammatory profile of microglia there is also evidence of an activated morphology in the brain of aged. Staining microglia against ionized calcium-binding adaptor protein-1 (Iba1), a protein expressed on the surface of microglia, indicate that microglia from non-diseased healthy brains in aged dogs, gerbils, and mice have shorter and less branched dendritic arbors than young adults (49, 50) (Fig. 1). This de-ramified morphology is comparable to the activated morphology of microglia. Moreover, a recent study showed that the de-ramified morphology of microglia in aged rats corresponded with higher protein expression of MHC II (27). These data are consistent with the hypothesis that MHC II is a marker for primed microglia. In addition, the higher inflammatory profile of microglia in the aged is also associated with a moderate increase in mRNA expression of pro-inflammatory cytokines TNFα, IL-1β and IL-6 (7). Not only were pro-inflammatory cytokine expression increased in this population but anti-inflammatory IL-10 and transforming growth factor beta (TGFβ) expression were also increased in aged microglia. Taken together, the increase in microglial associated mRNA and protein of inflammatory mediators indicates that they have a more primed, or inflammatory phenotype.
Astrocytes also have a more inflammatory profile with age
As described above, the majority of aging studies examine the effect of age on microglia. It is important, however, to briefly discuss that astrocytes become more inflammatory with age. For instance, there is increased expression of astrocytic glial fibrillary acidic protein (GFAP) in the brain of aged rodents and humans (2, 27, 51–54). In addition, vimentin, an intermediate filament protein, also increases with aging in humans (55). Furthermore, there is an increased hypertrophic morphology of hippocampal astrocytes with a shift from resting/stellate to activate in the brain of aged rats (27). The age related increases in GFAP and vimentin are similar to the activated astrocytic profile associated with inflammation and traumatic CNS injury (56, 57). Overall, these experimental and clinical data indicate that astrocytes have a more inflammatory profile with age.
There are many potential consequences of a more inflammatory astrocyte in the aged brain. First, astrocytes communicate directly with neurons and microglia, so an inflammatory astrocyte phenotype may directly effect immune to brain communication and microglia regulation (58). Second, astrocytes are integral to maintaining an intact blood brain barrier (BBB) (59). Age-related changes in astrocytes can affect BBB permeability, especially under inflammatory conditions and neurodegenerative diseases (60, 61). For example, Alzheimer’s disease is associated with increased occurrence of amyloid-β peptides that enter the brain from the periphery. Third, astrocytes secrete cytokines and chemokines that can function to recruit peripheral immune cells to the brain. For example, transforming growth factor (TGF) β1 signaling in the brain stimulates astrocytes to increase expression of MCP-1 (CCL2) (62), which is a key chemokine involved in the recruitment of peripheral monocytes. In fact, a recent report indicated that increased TGFβ signaling was detected in aged mice compared to adults with increased phosphorylated-Smad2 (63). Because TGFβ increases GFAP expression during differentiation (64), this may explain increased GFAP expression on astrocytes of the aged brain. Overall, astrocytes become more inflammatory with age, but the reasons why this shift in phenotype occurs or the potential consequences are unknown.
Increased inflammation within the aged brain influences cognition and neuronal plasticity
A modest increase in the inflammatory profile of the CNS in aging is associated with deficits in motor coordination, cognition and neuronal plasiticity. For example, aged mice had psychomotor deficits in psychomotor coordination and balance tests including rod walking and plank walking (65, 66). These deficits were associated with increased ex vivo IL-6 production and lipid peroxidation in the brain. Aged mice fed an antioxidant rich diet had reduced IL-6 ex vivo production that was associated with improved motor coordination (65).
Several studies report cognitive deficits associated with age. For instance, aged mice tested in the Morris water maze had learning/acquisition impairments compared to adult mice (67). In addition, another study reported age-associated memory impairments in the reversal task of the Morris water maze (68). Lowering CNS inflammation by treating with the anti-inflammatory agent luteolin resulted in better performance in the Morris water maze (68). Other tasks for cognition have given similar results. For example, in both the contextual fear conditioning test and radial arm maze, aged mice had memory impairments when compared to adult mice (69). It is important to point out, however, that not all aged mice perform poorly in memory tasks. One study found that performance in the Morris water maze varies by the individual rat and that not all aged rats showed learning deficits compared to adult rats (27). In addition, when aged rats were grouped into cognitive intact and cognitive impaired subsets, there was no correlation between baseline glial activation and cognitive impairment (27). Therefore, the mechanism for the development of impaired cognition remains unclear. Overall, there is variability associated with aging and cognitive impairments but all the studies discussed above indicate that aging is a risk factor for cognitive impairment.
These age-related deficits in cognition may be related to reductions neuronal plasticity. For instance, increased neuroinflammation with age has negative effects on neurogenesis, dendritic restructuring and long term potentiation (LTP). These are all important for cognitive function and establishment of memories. Impairments in both neurogenesis and LTP are reported in models of aging. For example, neurogenesis steadily decreases throughout life in mouse models of aging (70, 71). In an extensive study, reduced regenerative capacity indicative of senescence was reported in the mouse forebrain. There reductions were associated with declines in neuronal progenitor proliferation in the subventricular zone, neurogenesis in the olfactory bulb, and self-renewal potential (72). Moreover, additional reports of declined neurogenesis with age were associated with impairments in both the contextual fear conditioning test and radial arm maze (69). The increased amount of pro-inflammatory cytokines and oxidative stress present in the aged brain also has negative effects on LTP. Specifically, high IL-1β concentrations decrease LTP sustainability (73–76) and increased oxidative stress present in the aged reduced LTP (66). The correlation between increased neuroinflammation with age and reduced LTP is indicated by several reports (5, 48). For example, LTP was impaired in 9 and 15 month old rats and these ages corresponded with increases in brain levels of IL-1β and interferon gamma (IFNγ) (48). Lowering hippocampal IL-1β by minocycline treatment partially rescued this impairment in LTP (48), indicating that lowering inflammation by inhibiting microglial activity has beneficial effects on LPT. In similar studies, the anti-inflammatory agent rosiglitazone also restored LTP in aged rats (77, 78). Rosiglitazone, however, lowered the activation profile of astrocytes (decreased GFAP, decreased RANTES, and decreased TNF-α response in vitro), and not microglia, highlighting astrocytes as important CNS immune cells (78).
In addition to increased IL-1β and oxidative stress, decreases in neurotrophins also negatively impact LTP. Specifically, brain-derived neurotrophic factor (BDNF) is important for consolidation of hippocampus-dependent memory and maintaining LTP (79–81). This has implications for aging because decreased levels of BDNF transcripts and protein were found in the CA1 and CA3 regions of the hippocampus in aged rats (82–84). Overall, the increased inflammatory status of the brain and the decrease in neurotrophic support both contribute to decreased LTP and can result in impaired hippocampal dependent memory.
There is increasing evidence that cognition, neurogenesis, and LTP are influenced by peripheral growth factors. This is supported by studies using exercise to modulate the CNS environment. Exercise increases angiogenesis and thus provides more blood vessels and supporting endothelial cells for neurogenic niches to be formed (85). In aged mice, exercise enhanced learning, memory, and neurogenesis (71). These beneficial effects of exercise on the activity of neural progenitor cells and on cognitive impairments has been replicated by other groups (86–88), however, the mechanisms and pathways underlying these effects are still speculative. More recent reports showed that microglia have an important role in relaying the systemic effects of exercise to the CNS by providing a proneurogenic environment (89, 90). For example, microglia isolated from exercised animals were able to induce neurosphere formation in vitro. Furthermore, fractalkine receptor deficient microglia and microglia from aged mice had negative effects on neurosphere formation. This study indicates that microglia can exert dual roles on neural progenitor cell activity depending on their inflammatory state (89).
Furthermore, there is additional evidence that plasma derived factors has implications for brain aging. A recent article reports that plasma from young mice can ameliorate the decline seen in neural progenitor cells and neurogenesis in aged mice (69). This study used a parabiosis model in which two mice shared circulation. Aged mice paired with young mice showed improved memory as assessed by the fear conditioning test and an increase in neurogenesis and neural progenitor cells. Similarly, young mice sharing aged blood showed a decline in neurogenesis and number of neural progenitor cells. The authors then continued by attempting to pin-point the blood-born factors responsible to the impairments in aged mice. Their analysis reveled several chemokines, most significantly CCL11, believed to be the determinants in inhibiting neurogenesis (69).
Behavioral and cognitive consequences of impaired coordination between the immune system and the brain
Innate immune challenge leads to prolonged and exaggerated neuroinflammation
While aging alone reduces neuronal plasticity, major differences in microglial biology between young and old age occur when the immune system is challenged and microglia are activated. Thus, the increased markers of inflammation observed on microglia and astrocytes in the aged brain sets the stage for an increased or exaggerated immune response following stimulation. In support of this idea, exaggerated neuroinflammation in aging models has been reported following both peripheral and central immune activation (for reviews see (91–93)). For example, mixed glial cultures and coronal brain sections from aged mice produced elevated levels of IL-1β and IL-6 following lipopolysaccharide (LPS) stimulation compared to those established from adult mice (4, 10). LPS is a component of gram negative bacterial cell wall and is a potent activator of the innate immune system. In vivo, peripheral injection of LPS or E. coli caused prolonged and exaggerated neuroinflammation associated with increased IL-1β and IL-6 in aged rodents compared to young adults (2, 94). Similarly, central injection of LPS or GP120 caused amplified mRNA expression of IL-1β, IL-6 and TNFα in aged mice (95, 96). The increased expression of IL-6 mRNA was mirrored with enhanced IL-6 signaling in the aged brain after LPS injection (97). In addition, elevated mRNA expression of IL-1β, TNFα, and the inflammatory associated enzyme indoleamine 2,3-dioxygenase (IDO) was detected 24 and 72 h after LPS injection (84, 98), indicating that the neuroinflammation is both exaggerated and prolonged in the aged brain.
Several studies indicate that this exaggerated cytokine production is dependent on activation of microglia. For example, pretreatment with minocycline, an anti-inflammatory agent and reported microglial inhibitor (99, 100) attenuated the LPS induced amplification of TLR2, IL-1β, IL-6 and IDO in the hippocampus of aged mice (101). Moreover, a recent study showed that MHC II positive microglia were responsible for the robust increase of IL-1β following inter-peritoneal (i.p) injection of LPS. In this study, microglia were isolated from adult and aged mice 4 hours following LPS injection and analyzed by flow cytometry for intracellular IL-1β production. While LPS treated adult and aged mice exhibited a similar percentage of MHC II negative microglia expressing IL-1β, LPS treated aged mice had a significant increase in MHC II positive microglia expressing IL-1β. Furthermore, additional analysis of microglia from aged mice indicated that 95% of MHC II positive microglia were IL-1β positive, whereas only 31% of MHC II negative microglia were positive for IL-1β. These data support the hypothesis that primed MHC II positive microglia are highly responsive to immune challenge and provides a direct connection between heightened neuroinflammation and microglia. When microglia were isolated as a single population, other studies have reported similar age effects on microglial cytokine expression profile. Microglia isolated from aged mice 4 hours after LPS injection had increased mRNA levels of IL-1β, TLR2 and IDO compared to adult mice injected with LPS (44). In a similar study, elevated expression of TNFα, IL-1β, IL-6, and IL-12 mRNA were reported in aged LPS injected mice compared to adults (7). Other related studies indicated that microglia from aged mice stimulated ex vivo with LPS and Pam3CSK4, a TLR2 agonist, produced higher levels of IL-6 and TNFα than microglia from young mice (102). Overall, these studies indicate that immune challenge in the aged leads to exaggerated pro-inflammatory cytokine production by microgila (Fig. 2).
Figure 2. Neurobehavioral complications associated with microglial reactivity in the brain of aged.
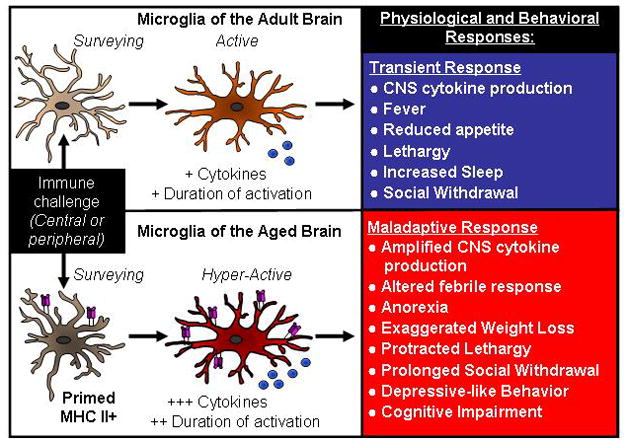
Under normal conditions, microglia interpret and propagate inflammatory signals that are initiated either peripherally or centrally. Microglial activation increase cytokine and secondary messenger release that lead to transient physiological and behavioral responses that are beneficial to the host organism (top panel). A consequence of microglial priming with age, however, is a hyperactive response to an immune challenge with amplified and prolonged production of cytokines. In several models of aging, an exaggerated cytokine response is associated with the development of cognitive, behavioral, and physiological complications that are interpreted to be maladaptive to the host organism (bottom panel).
Microglia activated by an innate immune challenge are also activated for a longer duration in the aged brain compared to adults. This results in a protracted production of inflammatory cytokines. For example, exaggerated microglial expression of IL-1β was continued up to 24 h after LPS injection in aged mice (103). Furthermore, elevated expression of hippocampal IL-1β has been reported for up to 72 h following LPS injection (84). This prolonged upregulation of inflammatory cytokines may be related to an impaired ability to “shut off” active microglia. In support of this notion, activated microglia from aged mice actually had higher levels of IL-10 production than those of adult mice (7, 44) and lower expression of TGFβ (7). IL-10 is an anti-inflammatory cytokine and can regulate IL-1β production. Thus, increased IL-10 but a maintained inflammatory response could imply that the aged brain has an impaired response to IL-10.
Primed microglia in the aged brain also produce a more robust response to peripheral stimulation induced by injury and stress. For example, minor abdominal surgery resulted in neuroinflammation and increased IL-1β levels 24 h post surgery in aged, but not adult mice (67) and mild psychological stress induced an amplified central cytokine response with increased IL-1β mRNA and MHC II protein expression in the hippocampus of aged but not adult mice (104). Overall, neuroinflammation is exaggerated in aged animals following central or peripheral stimulation, and many of these changes can be attributed to a hyperactive and primed microglial population.
It is also important to mention that microglial priming is detected in other models including neurodegenerative disease (for review see (105)). For example, both prion diseases (transmissible spongiform encephalopathies) and Alzheimer’s Disease show marked astrocyte and microglial activation (106–108), and evidence of microglial priming is apparent in both of these models of neurodegeneration. For example, in a murine model of pre-clinical prion disease, both central and peripheral inflammation exacerbated brain inflammation and neuronal death in prion disease mice compared to controls (109). This study was the first to demonstrate microglial priming under pre-symptomatic conditions as LPS challenge caused a marked increase in IL-1β and inducible nitric oxide synthase (iNOS) expression in microglia of prion disease mice compared to control mice (109). In a similar manner, microglial priming was also detected in transgenic mouse models of Alzheimer’s disease. In these models, systemic challenge with LPS caused a significant increase in CNS inflammation with exacerbated IL-1β and iNOS compared to non-transgenic controls (110, 111). The priming of microglia and their hyperactivation under pro-inflammatory conditions may contribute to or even amplify the neurodegenerative processes of prion and Alzheimer’s disease, making microglial priming an important research focus in the field of neurodegeneration. Overall, there are many examples of microglial priming in various models, but the focus on this review is on microglial priming under non-pathological conditions.
We refer to microglia of the aged brain as a primed or reactive microglial population. This is because they express increased markers of inflammation and when activated by an innate immune challenge (i.e., LPS) they have an amplified activation profile. Nonetheless, others described microglia of the aged brain as senescent or dystrophic (112). The terminology used depends on the context in which the microglia are examined and it is clear that microglia from older rodents also have other functional impairments. For example, there was reduced phagocytosis of beta-amyloid by microglia from older AD transgenic mice (113, 114). Microglia from aged rats showed delayed recruitment of phagocytic cells and less clearance of myelin after a toxin-induced demyelination lesion (115). In addition, in a focal laser injury model microglia from aged mice migrated at a slower velocity towards the site of injury and also aggregated at the injury site for a longer duration than that of adult mice (116). These functional impairments may be considered indicators of microglial senescence. Therefore the terminology of microglial priming or microglial senescence both reflect age-related differences in microglia function, but are related to the context in which they are examined.
Sickness and depressive-complications following innate immune challenge
As discussed above, inflammatory challenge in the elderly leads to exaggerated and prolonged inflammation in the brain. Prolonged exposure to cytokines such as IL-1β and IL-6 is accompanied by behavioral complications including prolonged sickness response, depression, and cognitive impairments (93). For example, our lab and others have shown that central and peripheral LPS challenge in aged mice caused prolonged and exaggerated sickness response characterized by protracted anorexia, lethargy, and social withdrawal (2, 95, 96) (Fig. 2). Aged rats also displayed an altered febrile response to E. coli infection. The aged rats had a delayed increase in core body temperature but this was followed by a significant and prolonged increase lasting up to three days (117). The exaggerated sickness response was likely caused by the exaggerated and prolonged production of IL-1β by MCH II positive microglia in aged mice (44) because a central administration of an IL-1 receptor antagonist rescued the behavior (118).
In addition to a prolonged sickness response, aged mice also developed depressive-like behavior following immune challenge (Fig. 2). To evaluate the depressive state of rodents, resignation behavior is determined in the trail suspension test (TST) and forced swim test (FST). Protracted depressive like behavior in the TST and FST were evident in aged mice 72 h after injection of LPS but not in adult mice (98). It is important to note that the depressive behavior was independent of general lethargy associated with the sickness response (98). These findings in animal models are consistent with clinical findings in which elderly patients exposed to infection or illness have an increased frequency of behavioral complications including depression and delirium compared to younger adults with similar peripheral insults (119). One potential reason for exaggerated depression after LPS injection in aged mice is heightened activation of the IDO pathway. IDO is an important enzyme in the degradation of tryptophan. This is important because inflammation-associated depression results from tryptophan degradation into kynurenine and subsequent monoamines which leads to the development of neuroactive metabolites and an imbalance in glutamate and serotonin neurotransmission (for reviews see (120, 121)). In comparisons between young and old mice, LPS caused a prolonged depressive phenotype that was not apparent in the adult matched controls. This depression was associated with increased inflammatory cytokine production in the brain, IDO upregulation, and serotonin turnover (98). In addition, microglia from aged mice injected with LPS had an exaggerated upregulation of IDO mRNA (44). Therefore a hyperactive microglial response with amplified IDO expression may underlie the prolonged depressive-like behavior in aged mice after peripheral LPS injection (98). In support of the role of IDO in inflammatory mediated depression, recent studies indicate that inhibiting IDO with 1-methyl tryptophan blocks development of inflammatory induced depression in adult mice (122, 123).
Cognitive impairments
There are also cognitive consequences of an exaggerated response to peripheral or central immune challenge. As discussed previously, aging alone is associated with some memory impairments. Following an immune challenge, however, cognitive impairments are exaggerated and become more apparent in the aged. These impairments are likely linked to increases in IL-6 and IL-1β because high levels of CNS IL-6 can inhibit memory formation and learning, cause neurodegeneration and exacerbate sickness behavior (124). These effects are similar to that of IL-1β as high IL-1β concentrations in the hippocampus are associated with impaired memory (125–127). For example, LPS injection in aged mice caused an amplified cytokine response in the hippocampus and this was paralleled by learning deficits in the radial arm water maze (128) and memory consolidation deficits in contextual fear conditioning (97). By inhibiting IL-6 signaling using an antagonist, contextual fear conditioning impairments were rescued in aged mice (97). Similar to aged mice, aged rats also had reduced long-term contextual memory in the contextual fear conditioning test and the Morris water maze following E. coli infection (94, 129). The anti-inflammatory agent resveratrol had beneficial effects on cognition in aged mice (130). In this study, mice received resveratrol supplemented in their diet and following LPS injection they were tested in the Morris water maze. Resveratrol lowered plasma and hippocampal IL-1β and this reduction in inflammation was paralleled by an attenuation of the memory deficits in aged mice (130).
Similar to the effects of inflammation on cognition, an exaggerated response in the aged brain may also impair cognition through impaired neuronal plasticity. For example, peripheral LPS injection caused amplified inflammation that lead to increased dendritic atrophy in the CA1 region of the hippocampus in aged mice (84). Adding to the effects of increased CNS inflammation, BDNF mRNA decreased following E. coli or LPS injection (82, 84). This decrease in BDNF was even more pronounced in aged rats that already had reduced baseline levels BDNF transcripts, making aged rats more prone to the behavioral deficits caused by decreased BDNF following injection (82, 84). Furthermore, the effects of inflammation on LTP sustainability also becomes apparent in aged rodents. For example, aged, but not adult, rats challenged with E. coli showed late phase LTP deficits and impaired hippocampal dependent memory (131). Furthermore, these impairments were rescued by central administration of the anti-inflammatory cytokine IL-1 receptor antagonists which blocks IL-1β signaling (131, 132). In a similar study, amyloid-β challenge inhibited LTP in aged but not adult rats (133). In summary, microglia from aged animals are primed and have an exaggerated immune response following activation which leads to heightened inflammation in the brain and reductions in LTP.
Impaired regulation of microglia in the aged brain
The cause of this amplified microglial activation with age may be related to impairments in several key regulatory systems that make it more difficult to resolve microglial activation (Fig. 3). Microglial activity can be modulated by anti-inflammatory cytokines including IL-10, TGFβ and IL-4. Although IL-10 was decreased in the brain of aged rodents under homeostatic conditions, following immune challenge, IL-10 levels were exaggerated in aged microglia compared to adults (44). IL-10 is a potent anti-inflammatory cytokine that should regulate IL-1β production and decrease inflammation. It is unclear why higher IL-10 is ineffective in reducing brain inflammation after LPS challenge in older mice. Microglia, however, do not express high levels of IL-10 receptor and do not appear to respond to the M2 promoting effects of IL-10 (134). Similar to IL-10, TGFβ is an anti-inflammatory factor that can regulate microglia (135). For example, TGFβ increases fractalkine receptor expression and reduces IL-1β mRNA in BV2 microglia stimulated with LPS (103). In adult mice, TGFβ mRNA increased in the brain 24 after LPS injection, however, this increase was not detected in aged mice (103). This lack of TGFβ mRNA induction following inflammatory challenge can therefore be a possible link to the prolonged microglial activation reported in aged mice. Taken together, deficits in IL-10 and TGFβ signaling pathways with age may lead to a reduced ability to shut off microglia.
Figure 3. Activated microglia from the aged brain are refractory to anti-inflammatory stimulus.
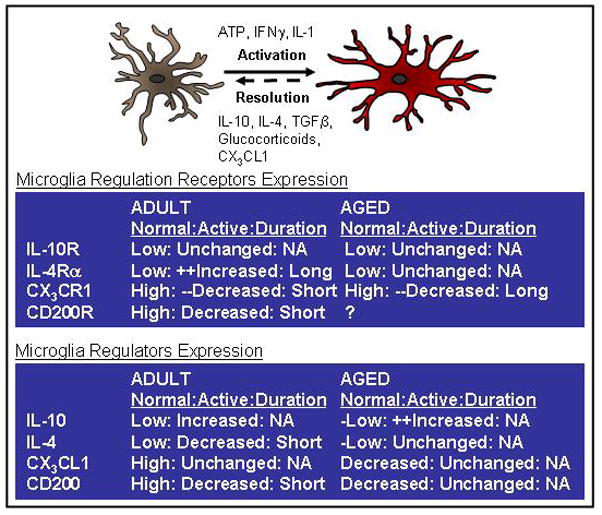
The activation of microglia is tightly regulated and there are several anti-inflammatory mediators that modulate microglial activation. For example, anti-inflammatory cytokines including IL-10, TGF-β, and IL-4 modulate the activation of microglia and are decreased in the brain with age. In addition neuronally derived ligands including CD200 and fractalkine (CX3CL1) are also decreased with age. The right panels depict the differences in expression of several regulatory proteins and receptors with age. There are also several regulatory systems in that the ligand and the receptor interaction that change when microglia become activated. For example, compared to adult microglia, aged microglia have prolonged reduction of CX3CR1 and fail to increase surface expression of the IL-4 receptor-α (bottom left panel). Taken together, the prolonged activation of microglia of the aged brain may be because they are less sensitive to the anti-inflammatory regulation that normally helps to resolve activation.
IL-4 is another anti-inflammatory cytokine that is influenced by age. For example, in aged rats there was reduced IL-4 levels in the brain and this corresponded with increased neuroinflammation and reduced LTP (11, 12). Induction of hippocampal IL-4 successfully restored LTP in the aged rats (136) suggesting an important role for IL-4 to modulate the CNS environment and retain LTP by lowering CNS inflammation. Similarly, IL-4 attenuated neuroinflammation and restored LTP in adult rats challenged with amyloid-β protein (137). In addition to reductions in IL-4 with age, there are also reductions in IL-4 sensitivity with age. For instance, recent work indicates that microglia from the brain of aged mice were less responsive to the anti-inflammatory effects of IL-4 (134). Following peripheral LPS stimulation, microglia from adult but not aged mice had upregulated expression of IL-4 receptor-alpha (Fig. 3). After isolation, microglia were stimulated with IL-4 ex vivo. Microglia from adult mice were responsive to IL-4 and shifted in phenotype towards an alternatively activated M2 state whereas microglia from aged mice retained a classically activated or M1 phenotype in the presence of IL-4 (134). Therefore, the failure to upregulate IL-4 receptor on microglia of aged mice was associated with decreased sensitivity to IL-4. Taken together, either reduced levels of IL-4 or a reduced sensitivity IL-4 in the aged brain impairs the ability to lower inflammation in the brain. This is potentially important because IL-4 has a role in maintaining memory and learning (138) and reparative processes after traumatic CNS injury (139).
In addition to regulation by anti-inflammatory cytokines, microglia are also regulated by neuronally derived proteins including fractalkine, CD200, and TREM2. These regulatory systems are important to keep microglia in a surveying state (reviewed by (13, 105). For example, fractalkine signaling is important for maintaining microglia in a resting state and also attenuating microglial activation following the removal of inflammatory stimulus. Neuronally expressed fractalkine ligand (CX3CL) can exist as either membrane bound or released as a free ligand. Both forms of fractalkine bind to the corresponding fractalkine receptor (CX3CR) which is expressed exclusively on microglia in the CNS. In vitro, the addition of fractalkine ligand to LPS stimulated glia cultures attenuated production of inflammatory mediators including IL-1β, IL-6, TNFα, inducible nitric oxide synthase (iNOS), and MHC II (140–142). The importance of fractalkine signaling is mirrored in vivo by transgenic fractalkine receptor knockout mice where microglial activity is dysregulated. Following peripheral LPS stimulation, fractalkine receptor knockout mice had amplified IL-1β production in the brain (143, 144) and amplified microglial expression of IL-1β, and the inflammatory associated enzymes IDO and kynurenine 3-monooxygenase (KMO) at 4 h after injection and prolonged induction of IL-1β, TLR2 and CD14 24 h after injection compared to heterozygote controls (144). The increased expression of IDO and KMO in microglia from fractalkine receptor knockout mice was associated with prolonged depressive like behavior following peripheral LPS challenge compared to wild-type and heterozygote mice (144). Blocking IDO activity in these mice blocked the development of depressive-like behavior (145) suggesting IDO is important for the development of depression in models where microglial regulation is impaired. Overall, these results indicate that fractalkine signaling is important to modulate the microglial response following activation and also returning microglia to homeostasis following cease of inflammation.
Fractalkine signaling has implications in aging because several studies indicate that fractalkine ligand was lower in the aged brain compared adult (89, 103, 142, 146). In addition, following LPS challenge, microglia from aged mice showed prolonged downregulation of the fractalkine receptor (103). In this study by Wynne et al., microglia from both adult and aged mice had downregulated fractalkine receptor at 4 h following LPS injection. Microglia from adult mice, however, had restored levels of fractalkine receptor at 24 h but this was not observed in microglia from aged mice. This failure to upregulate the receptor by 24 h corresponded with prolonged induction of IL-1β and an extended sickness response. Taken together, reduced levels of fractalkine ligand and prolonged downregulation of fractalkine receptor can combine to give severe impairments in fractalkine signaling in the aged brain, which ultimately leads to a dysregulated microglial population (Fig. 3).
Similar to fractalkine, CD200 regulation keeps microglia in a resting state and this regulation is also impaired in the aged brain. CD200 is membrane glycoprotein expressed on neurons and oligodendrocytes. The CD200 receptor (CD200R) is expressed exclusively on microglia and the binding of CD200 ligand to CD200R plays a pivotal role of modulating microglial activation (147, 148). For example, CD200-deficient mice show a greater extent of microglial activation in several models of inflammation (147, 149). Following a peripheral injection of LPS, microglia downregulated CD200R and this downregulation was associated with an increase in microglial activation (150). This is relevant because in aged rats, there was less CD200R mRNA and protein expression on microglia (9, 148) compared to adult rats. In addition, there are decreased levels of CD200 in aged rats compared to adults (148, 151) (Fig. 3). Enhancing CD200 signaling by injection of a mimetic of the neural cell adhesion molecule (NCAM) FGL or CD200 fusion protein rescued some of the age-related phenotypes. For example, FGL attenuated expression of pro-inflammatory cytokines in the brain following LPS injection in aged mice (152). In a similar manner, intrahippocampal injection of CD200 fusion protein decreased microglial activation in the hippocampus of aged rats, and this decrease in activation correlated with rescued LTP, which was impaired following LPS injection in aged rats (151). In addition, systemic treatment with FGL had positive effects on glial-synaptic interactions (153) which were also otherwise impaired in aged rats. These findings indicate that CD200 signaling is important for modulating microglial activity, and impairments in CD200 with age can have detrimental effects on microglia to neuron interactions.
Conclusions
In conclusion, there are inflammatory alterations in microglia biology with aging. Microglia of the aged brain are termed primed with a higher expression of MHC II and pro-inflammatory cytokines including IL-1β. This shift towards priming is associated with a prolonged and amplified response to an immune challenge. In addition, there are clear age associated deficits in memory and learning and neuronal plasticity. These deficits can worsen by inflammatory challenge. Aging is also associated with dysregulation of microglia, for example deficits in CD200 and fractalkine regulation. What causes these impairments and gives rise to a primed microglial population, however, remains to be elucidated. Therefore, a better understanding of the pathways by which microglia become dysregulated with age is needed to improve our understanding of neuroinflammatory complications associated with age and lead to the development of therapeutic interventions.
Acknowledgments
This work is supported by NIH grants R01-AG-033028 to JPG.
Footnotes
Competing interests
The authors of this manuscript declare that there are no actual or potential conflicts of interest. The authors affirm that there are no financial, personal or other relationships with other people or organizations that have inappropriately influenced or biased their research.
References
- 1.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 2.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 3.Maher FO, Martin DS, Lynch MA. Increased IL-1beta in cortex of aged rats is accompanied by downregulation of ERK and PI-3 kinase. Neurobiol Aging. 2004;25:795–806. doi: 10.1016/j.neurobiolaging.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Experimental neurology. 2003;182:135–141. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 5.Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. The Journal of biological chemistry. 1998;273:12161–12168. doi: 10.1074/jbc.273.20.12161. [DOI] [PubMed] [Google Scholar]
- 6.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 7.Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 8.Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64:721–728. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- 9.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 11.Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, Mills KH, Lynch MA. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. The Journal of biological chemistry. 2005;280:9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- 13.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 14.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 15.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell stem cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 19.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 21.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 22.Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101:249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- 23.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 24.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Progress in neurobiology. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 26.Wohl SG, Schmeer CW, Witte OW, Isenmann S. Proliferative response of microglia and macrophages in the adult mouse eye after optic nerve lesion. Investigative ophthalmology & visual science. 2010;51:2686–2696. doi: 10.1167/iovs.09-4537. [DOI] [PubMed] [Google Scholar]
- 27.VanGuilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, Warrington JP, Sonntag WE, Freeman WM. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J Neuroinflammation. 2011;8:138. doi: 10.1186/1742-2094-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 30.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 31.Bechmann I, Priller J, Kovac A, Bontert M, Wehner T, Klett FF, Bohsung J, Stuschke M, Dirnagl U, Nitsch R. Immune surveillance of mouse brain perivascular spaces by blood-borne macrophages. Eur J Neurosci. 2001;14:1651–1658. doi: 10.1046/j.0953-816x.2001.01793.x. [DOI] [PubMed] [Google Scholar]
- 32.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 33.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 34.Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–7179. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marty V, El Hachmane M, Amedee T. Dual modulation of synaptic transmission in the nucleus tractus solitarius by prostaglandin E2 synthesized downstream of IL-1beta. The European journal of neuroscience. 2008;27:3132–3150. doi: 10.1111/j.1460-9568.2008.06296.x. [DOI] [PubMed] [Google Scholar]
- 36.Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89:535–548. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- 37.Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- 38.Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- 39.Streit WJ, Sparks DL. Activation of microglia in the brains of humans with heart disease and hypercholesterolemic rabbits. J Mol Med. 1997;75:130–138. doi: 10.1007/s001090050097. [DOI] [PubMed] [Google Scholar]
- 40.Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- 41.Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89:687–699. doi: 10.1016/s0306-4522(98)00334-0. [DOI] [PubMed] [Google Scholar]
- 42.Nicolle MM, Gonzalez J, Sugaya K, Baskerville KA, Bryan D, Lund K, Gallagher M, McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning- impaired rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- 43.Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- 44.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong AM, Patel NV, Patel NK, Wei M, Morgan TE, de Beer MC, de Villiers WJ, Finch CE. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neuroscience letters. 2005;390:76–80. doi: 10.1016/j.neulet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 46.Stichel CC, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28:1507–1521. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 47.Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146:248–254. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- 49.Choi JH, Lee CH, Hwang IK, Won MH, Seong JK, Yoon YS, Lee HS, Lee IS. Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. The Journal of veterinary medical science / the Japanese Society of Veterinary Science. 2007;69:1131–1136. doi: 10.1292/jvms.69.1131. [DOI] [PubMed] [Google Scholar]
- 50.Hwang IK, Lee CH, Li H, Yoo KY, Choi JH, Kim DW, Suh HW, Won MH. Comparison of ionized calcium-binding adapter molecule 1 immunoreactivity of the hippocampal dentate gyrus and CA1 region in adult and aged dogs. Neurochemical research. 2008;33:1309–1315. doi: 10.1007/s11064-007-9584-6. [DOI] [PubMed] [Google Scholar]
- 51.Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE. GFAP mRNA increases with age in rat and human brain. Neurobiol Aging. 1993;14:421–429. doi: 10.1016/0197-4580(93)90100-p. [DOI] [PubMed] [Google Scholar]
- 52.Unger JW. Glial reaction in aging and Alzheimer’s disease. Microscopy research and technique. 1998;43:24–28. doi: 10.1002/(SICI)1097-0029(19981001)43:1<24::AID-JEMT4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 53.Cotrina ML, Nedergaard M. Astrocytes in the aging brain. Journal of neuroscience research. 2002;67:1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- 54.Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging. 2003;24(Suppl 1):S123–127. doi: 10.1016/s0197-4580(03)00051-4. discussion S131. [DOI] [PubMed] [Google Scholar]
- 55.Porchet R, Probst A, Bouras C, Draberova E, Draber P, Riederer BM. Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer’s disease. Proteomics. 2003;3:1476–1485. doi: 10.1002/pmic.200300456. [DOI] [PubMed] [Google Scholar]
- 56.Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. The Journal of pathology. 2004;204:428–437. doi: 10.1002/path.1645. [DOI] [PubMed] [Google Scholar]
- 57.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochemical research. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 58.Tichauer J, Saud K, von Bernhardi R. Modulation by astrocytes of microglial cell-mediated neuroinflammation: effect on the activation of microglial signaling pathways. Neuroimmunomodulation. 2007;14:168–174. doi: 10.1159/000110642. [DOI] [PubMed] [Google Scholar]
- 59.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 60.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Popescu BO, Toescu EC, Popescu LM, Bajenaru O, Muresanu DF, Schultzberg M, Bogdanovic N. Blood-brain barrier alterations in ageing and dementia. J Neurol Sci. 2009;283:99–106. doi: 10.1016/j.jns.2009.02.321. [DOI] [PubMed] [Google Scholar]
- 62.Weiss JM, Berman JW. Astrocyte expression of monocyte chemoattractant protein-1 is differentially regulated by transforming growth factor beta. J Neuroimmunol. 1998;91:190–197. doi: 10.1016/s0165-5728(98)00183-0. [DOI] [PubMed] [Google Scholar]
- 63.Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation. 2010;7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Sampaio e Spohr TCL, Martinez R, Da Silva EF, Neto VM, Gomes FCA. Neuro–glia interaction effects on GFAP gene: a novel role for transforming growth factor-β1. European Journal of Neuroscience. 2002;16:2059–2069. doi: 10.1046/j.1460-9568.2002.02283.x. [DOI] [PubMed] [Google Scholar]
- 65.Richwine AF, Godbout JP, Berg BM, Chen J, Escobar J, Millard DK, Johnson RW. Improved psychomotor performance in aged mice fed diet high in antioxidants is associated with reduced ex vivo brain interleukin-6 production. Brain Behav Immun. 2005;19:512–520. doi: 10.1016/j.bbi.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Hayashi Y, Yoshida M, Yamato M, Ide T, Wu Z, Ochi-Shindou M, Kanki T, Kang D, Sunagawa K, Tsutsui H, Nakanishi H. Reverse of age-dependent memory impairment and mitochondrial DNA damage in microglia by an overexpression of human mitochondrial transcription factor a in mice. J Neurosci. 2008;28:8624–8634. doi: 10.1523/JNEUROSCI.1957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jang S, Dilger RN, Johnson RW. Luteolin inhibits microglia and alters hippocampal-dependent spatial working memory in aged mice. The Journal of nutrition. 2010;140:1892–1898. doi: 10.3945/jn.110.123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2010;31:151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 71.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain research. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 74.Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neuroscience letters. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 75.Kelly A, Lynch A, Vereker E, Nolan Y, Queenan P, Whittaker E, O’Neill LA, Lynch MA. The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. The Journal of biological chemistry. 2001;276:45564–45572. doi: 10.1074/jbc.M108757200. [DOI] [PubMed] [Google Scholar]
- 76.Vereker E, O’Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Loane DJ, Deighan BF, Clarke RM, Griffin RJ, Lynch AM, Lynch MA. Interleukin-4 mediates the neuroprotective effects of rosiglitazone in the aged brain. Neurobiol Aging. 2009;30:920–931. doi: 10.1016/j.neurobiolaging.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 78.Cowley TR, O’Sullivan J, Blau C, Deighan BF, Jones R, Kerskens C, Richardson JC, Virley D, Upton N, Lynch MA. Rosiglitazone attenuates the age-related changes in astrocytosis and the deficit in LTP. Neurobiol Aging. 2012;33:162–175. doi: 10.1016/j.neurobiolaging.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Progress in neurobiology. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 80.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 81.Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chapman TR, Barrientos RM, Ahrendsen JT, Hoover JM, Maier SF, Patterson SL. Aging and infection reduce expression of specific brain-derived neurotrophic factor mRNAs in hippocampus. Neurobiol Aging. 2012;33:832 e831–814. doi: 10.1016/j.neurobiolaging.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. J Neurosci. 2011;31:4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33:1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 86.Blackmore DG, Golmohammadi MG, Large B, Waters MJ, Rietze RL. Exercise increases neural stem cell number in a growth hormone-dependent manner, augmenting the regenerative response in aged mice. Stem Cells. 2009;27:2044–2052. doi: 10.1002/stem.120. [DOI] [PubMed] [Google Scholar]
- 87.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci. 2012;32:6435–6443. doi: 10.1523/JNEUROSCI.5925-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kohman RA, Deyoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol Clin. 2006;24:521–538. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 92.Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Experimental neurology. 2010 doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corona AW, Fenn AM, Godbout JP. Cognitive and Behavioral Consequences of Impaired Immunoregulation in Aging. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- 94.Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burton MD, Johnson RW. Interleukin-6 trans-signaling in the senescent mouse brain is involved in infection-related deficits in contextual fear conditioning. Brain Behav Immun. 2012;26:732–738. doi: 10.1016/j.bbi.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, JOC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He Y, Appel S, Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain research. 2001;909:187–193. doi: 10.1016/s0006-8993(01)02681-6. [DOI] [PubMed] [Google Scholar]
- 100.Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. The Journal of biological chemistry. 2007;282:15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- 101.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33:195 e191–112. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX(3)CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buchanan JB, Sparkman NL, Chen J, Johnson RW. Cognitive and neuroinflammatory consequences of mild repeated stress are exacerbated in aged mice. Psychoneuroendocrinology. 2008;33:755–765. doi: 10.1016/j.psyneuen.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DeArmond SJ. Alzheimer’s disease and Creutzfeldt-Jakob disease: overlap of pathogenic mechanisms. Current opinion in neurology. 1993;6:872–881. doi: 10.1097/00019052-199312000-00008. [DOI] [PubMed] [Google Scholar]
- 107.Price DL, Borchelt DR, Sisodia SS. Alzheimer disease and the prion disorders amyloid beta-protein and prion protein amyloidoses. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6381–6384. doi: 10.1073/pnas.90.14.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nature reviews Neuroscience. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 109.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sly LM, Krzesicki RF, Brashler JR, Buhl AE, McKinley DD, Carter DB, Chin JE. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain research bulletin. 2001;56:581–588. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- 111.Lee J, Chan SL, Mattson MP. Adverse effect of a presenilin-1 mutation in microglia results in enhanced nitric oxide and inflammatory cytokine responses to immune challenge in the brain. Neuromolecular medicine. 2002;2:29–45. doi: 10.1385/NMM:2:1:29. [DOI] [PubMed] [Google Scholar]
- 112.Streit WJ, Xue QS. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- 113.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. The American journal of pathology. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao C, Li WW, Franklin RJ. Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiol Aging. 2006;27:1298–1307. doi: 10.1016/j.neurobiolaging.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 116.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barrientos RM, Watkins LR, Rudy JW, Maier SF. Characterization of the sickness response in young and aging rats following E. coli infection. Brain Behav Immun. 2009;23:450–454. doi: 10.1016/j.bbi.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Godbout JP, Johnson RW. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Immunology and allergy clinics of North America. 2009;29:321–337. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 120.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Godbout JP, Johnson RW. Interleukin-6 in the aging brain. J Neuroimmunol. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 125.Rachal Pugh C, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neuroscience and biobehavioral reviews. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 126.Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 127.Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 128.Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation research. 2009;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci. 2010;30:7598–7603. doi: 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lynch AM, Loane DJ, Minogue AM, Clarke RM, Kilroy D, Nally RE, Roche OJ, O’Connell F, Lynch MA. Eicosapentaenoic acid confers neuroprotection in the amyloid-beta challenged aged hippocampus. Neurobiol Aging. 2007;28:845–855. doi: 10.1016/j.neurobiolaging.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 134.Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Paglinawan R, Malipiero U, Schlapbach R, Frei K, Reith W, Fontana A. TGFbeta directs gene expression of activated microglia to an anti-inflammatory phenotype strongly focusing on chemokine genes and cell migratory genes. Glia. 2003;44:219–231. doi: 10.1002/glia.10286. [DOI] [PubMed] [Google Scholar]
- 136.Clarke RM, Lyons A, O’Connell F, Deighan BF, Barry CE, Anyakoha NG, Nicolaou A, Lynch MA. A pivotal role for interleukin-4 in atorvastatin-associated neuroprotection in rat brain. The Journal of biological chemistry. 2008;283:1808–1817. doi: 10.1074/jbc.M707442200. [DOI] [PubMed] [Google Scholar]
- 137.Lyons A, Griffin RJ, Costelloe CE, Clarke RM, Lynch MA. IL-4 attenuates the neuroinflammation induced by amyloid-beta in vivo and in vitro. J Neurochem. 2007;101:771–781. doi: 10.1111/j.1471-4159.2006.04370.x. [DOI] [PubMed] [Google Scholar]
- 138.Derecki NC, Quinnies KM, Kipnis J. Alternatively activated myeloid (M2) cells enhance cognitive function in immune compromised mice. Brain Behav Immun. 2011;25:379–385. doi: 10.1016/j.bbi.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain research. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- 141.Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. [PubMed] [Google Scholar]
- 142.Lyons A, Lynch AM, Downer EJ, Hanley R, O’Sullivan JB, Smith A, Lynch MA. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem. 2009;110:1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- 143.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 144.Corona AW, Huang Y, O’Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J Neuroinflammation. 2010;7:93. doi: 10.1186/1742-2094-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Corona AW, Norden DM, Skendelas JP, Huang Y, O’Connor JC, Lawson M, Dantzer R, Kelley KW, Godbout JP. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX3CR1)-deficient mice. Brain Behavior and Immunity. 2012 doi: 10.1016/j.bbi.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 148.Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Broderick C, Hoek RM, Forrester JV, Liversidge J, Sedgwick JD, Dick AD. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am J Pathol. 2002;161:1669–1677. doi: 10.1016/S0002-9440(10)64444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Masocha W. Systemic lipopolysaccharide (LPS)-induced microglial activation results in different temporal reduction of CD200 and CD200 receptor gene expression in the brain. J Neuroimmunol. 2009;214:78–82. doi: 10.1016/j.jneuroim.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 151.Cox FF, Carney D, Miller AM, Lynch MA. CD200 fusion protein decreases microglial activation in the hippocampus of aged rats. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 152.Downer EJ, Cowley TR, Lyons A, Mills KH, Berezin V, Bock E, Lynch MA. A novel anti-inflammatory role of NCAM-derived mimetic peptide, FGL. Neurobiol Aging. 2010;31:118–128. doi: 10.1016/j.neurobiolaging.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 153.Ojo B, Rezaie P, Gabbott PL, Davies H, Colyer F, Cowley TR, Lynch M, Stewart MG. Age-related changes in the hippocampus (loss of synaptophysin and glial-synaptic interaction) are modified by systemic treatment with an NCAM-derived peptide, FGL. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.09.013. [DOI] [PubMed] [Google Scholar]


