Abstract
Reports that ataxia telangiectasia mutated (ATM) is required for full activation of Akt raise the hypothesis that ATM plays a role in IGF-1 signaling through the Akt/mTOR pathway. Differentiated C2C12 cells harboring either ATM-targeting shRNA or non-targeting shRNA and myotubes from a C2C12 lineage previously exposed to empty vector lentivirus were incubated in the presence or absence of 10 nM IGF-1 followed by western blot analysis. Parallel experiments were performed in isolated soleus muscles from mice expressing only one functional ATM allele (ATM+/−) compared to muscles from wild-type (ATM+/+) mice. IGF-1 increased phosphorylation of Akt S473, Akt T308, and p70 S6 kinase (S6K) in myotubes expressing non-targeting shRNA and in empty vector controls, but the IGF-1 effects were significantly reduced in myotubes with shRNA-mediated ATM knockdown. Likewise, IGF-1-stimulated phosphorylation of Akt S473, Akt T308, mTOR, and S6K was lower in isolated soleus muscles from ATM+/− mice compared to muscles from ATM+/+ mice. The ATM inhibitor KU55933 prevented stimulation of S6K phosphorylation in C2C12 myotubes exposed to IGF-1, suggesting that decreased IGF-1 action is not limited to chronic conditions of decreased ATM function. Stimulation of IRS-1 tyrosine 612 phosphorylation by IGF-1 was unaffected by ATM deficiency, though IGF-1 phosphatidylinositol 3-kinase activity tended to be lower in muscle from ATM haploinsufficient mice compared to wild type muscle. The data suggest that ATM is a modulator of IGF-1 signaling downstream of IRS-1 in skeletal muscle.
Keywords: Akt, IGF-1, mTOR
INTRODUCTION
Ataxia telangiectasia mutated (ATM), a serine/threonine protein kinase that belongs to the PI3K-related protein kinase (PIKK) family, is known for its role in cellular processes including cell cycle control, DNA repair, and apoptosis (Abraham, 2001; Shiloh, 2003; Bhatti et al., 2011). However, recent studies have elucidated roles for ATM in regulation of cytosolic signaling (Yang et al., 2011). For example, ATM participates in insulin stimulated phosphorylation of the eukaryotic initiation factor 4E binding protein 1 (4EBP1) (Yang & Kastan, 2000). Furthermore, it has demonstrated that ATM is necessary for full activation of Akt in response to insulin in Cos cells (Viniegra et al., 2005), and a role of ATM in insulin-stimulated activation of Akt in cultured L6 myoblasts has been described (Halaby et al., 2008).
Akt is a serine/threonine kinase that is central to a pathway shared by both insulin and IGF-1 signaling (reviewed in (Siddle, 2011)). The effect of IGF-1 on this pathway begins with its binding to the IGF-1 receptor (IGF1R), resulting in IGF1R autophosphorylation and the recruitment and tyrosine phosphorylation of insulin receptor substrate-1 (IRS-1). IRS-1 serves as an adaptor protein between IGF1R and PI3K and leads to Akt activation through generation of phosphatidylinositol 3,4,5 trisphosphates by PI3K and subsequent recruitment of Akt. Activation of Akt leads to activation of mammalian target of rapamycin (mTOR) and its downstream target S6K, stimulating protein synthesis.
Despite the findings described above, whether or not ATM plays a role in activation of Akt is not a settled issue. For example, insulin-stimulated phosphorylation of Akt was normal in L6 myotubes and in mouse soleus muscle in the presence of the ATM inhibitor KU55933 (Jeong et al., 2010). Likewise, siRNA-mediated decrease in ATM expression did not affect insulin-stimulated phosphorylation of Akt in adipocytes (Hresko & Mueckler, 2005). Furthermore, it has even been reported that thymocytes from mice expressing nonfunctional ATM have increased levels of phosphorylated Akt compared to wild type thymocytes (Kuang et al., 2009).
This study was undertaken to determine whether ATM is required for IGF-1 signaling from IRS-1 through S6K. Because it has been reported that IGF1R expression is decreased by ATM deficiency (Peretz et al., 2001), we hypothesized that soleus muscle and cultured myotubes with decreased ATM expression would have impaired signaling responses to IGF-1.
METHODS
Ethical approval
All animal procedures approved by the Saint Louis University Institutional Animal Care and Use Committee.
Materials
Dulbeco’s modified Eagle’s medium (DMEM), phosphate buffered saline (PBS), and trypsin/EDTA were purchased from Washington University School of Medicine Tissue Culture Center (Saint Louis, MO). Fetalplex Animal Serum Complex and antibiotic/antimycotic solution (10,000 U penicillin/ml, 10 mg streptomycin/ml, 0.025 mg fungizone/ml) were purchased from Gemini Bio-products (Woodland, CA). IGF-1 was obtained from PEPROTECH INC. (Rocky Hill, NJ). Cell culture dishes were obtained from Becton Dickinson Labware (Franklin Lakes, NJ). Reagents for enhanced chemiluminescence (ECL) were obtained from PerkinElmer Life Sciences (Boston, MA). Pre cast 4–20 %Tris-HEPES polyacrylamide gels were obtained from NuSep Inc. (Austell, GA). Pre-cast 3–8 % NuPAGE Tris acetate gels were obtained from Invitrogen (Calsbad, CA). Antibodies recognizing IRS-1, Akt, phospho-Akt S473, phospho-Akt T308, phospho-S6K T389, S6K, phospho-mTOR S2481, ATM, and phospho-ATM S1981 were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Agarose-LE™ was purchased from Ambion, Applied Biosystems Inc. (Foster City, CA). Antibodies against IRS-1 phospho-Y612 were obtained from Novus Biologicals LLC(Littleton, CO) and Cell Signaling Technology, Inc. Y612 is the residue phosphorylated on human IRS-1, while the corresponding amino acid for mouse and rat IRS-1 is Y608. The REDExtract-N-Amp™ Tissue PCR kit was obtained from Sigma (Saint Louis, MO).
KU55933 (Hickson et al., 2004), an ATM inhibitor, was generously provided by Kudos Pharmaceuticals, Ltd. (Cambridge, UK).
Cell Culture
C2C12 myoblasts harboring either ATM-targeting shRNA (KD) or non-targeting shRNA (NT) or previously exposed to empty vector lentivirus (EV) have been previously described (Jeong et al., 2010). Myoblasts were cultured in 10 cm-diameter dishes in DMEM containing 10% FetalPlex (Gemini Bio-Products, Woodland, CA) and 1% antibiotic/antimycotic solution. The cells were incubated at 37 °C with an atmosphere of room air with 5% CO2 and were passaged every 48 hours by trypsinization using 0.05% trypsin with 0.02% EDTA. To induce differentiation of myoblasts into myotubes, cells were seeded in 6-well plates and, after overnight growth, they were supplied with fresh DMEM containing 2% horse serum and 1% antibiotic/antimycotic mixture every 48 hours for 3–4 days.
Prior to experiments, differentiated C2C12 myotubes (EV, NT, and KD) in 6-well plates were serum starved for 3 hours, followed by incubation in the absence or presence 10 nM IGF-1 for 20 minutes. To determine the impact of acute inhibition of ATM on IGF-1 signaling, differentiated wild-type C2C12 myotubes were incubated in the presence of 10 μM KU55933 (KU) or 0.1% DMSO (vehicle) for 1 hour followed by incubation for 20 min in the absence or presence of 10 nM IGF-1, with KU or DMSO still present.
Animals
ATM transgenic mice heterozygous for an ATM truncation that results in non-functional ATM (Barlow et al., 1996) were obtained from Jackson Laboratory (Bar Harbor, ME) and housed at the Saint Louis University animal facility where they had free access to food and water. Mice obtained from a breeding colony of heterozygous mice (ATM +/−) were genotyped from tail snips using the REDExtract-N-AMP™ Tissue kit (Sigma, St. Louis, MO) as previously described (Jeong et al., 2010).
Muscle collection
Mice were anesthetized with pentobarbital sodium (50 mg/kg) intraperitoneally. Soleus muscles were harvested and allowed to recover for 1 hour in oxygenated (95% O2 and 5% CO2) Krebs-Henseleit buffer (KHB) supplemented with 8 mM glucose, 32 mM mannitol, and 0.1% bovine serum albumin at 35 °C. Muscles were then incubated in the presence or absence of 30 nM IGF-1 for 30 minutes at 35°C and were immediately clamp-frozen with tongs cooled in liquid nitrogen and stored at −80°C until use. After removal of muscles, animals were killed by thoracotomy while still under pentobarbital anesthesia.
Cell and tissue preparation
After incubations, cells were washed twice with ice-cold PBS and then scraped in buffer containing 50 mM HEPES, pH 7.4, with 150 mm NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 10 mM Na3PO4, 100 mM NaF, 2 mm Na3VO4, 10 μg/mL leupeptin, 0.5 μg/mL pepstatin, 10 μg/mL aprotinin, and 1 mM phenylmethylsulfonyl fluoride. Lysates were centrifuged at 4 °C for 10 min at 14,000 × g. Supernatants were assayed for protein concentration by the bicinchoninic acid method (BCA, Thermo Scientific) and subjected to western blot analysis.
Muscle samples were homogenized in the cell lysis buffer described above. Homogenates were centrifuged and assayed for protein concentration as described above.
Western blot analysis
Samples were solubilized in Laemmli sample buffer and separated by SDS-PAGE using 4–20% pre-cast Tris-HEPES gels (Pierce) for analysis of most proteins. For analysis of ATM (~350 kDa), samples were run on 3–8% pre-cast NuPAGE Tris-acetate gels (Invitrogen) suitable for separation of high molecular weight proteins. For western blots of ATM, a high range molecular weight marker set (up to 460 kDa; HiMark, Invitrogen) was used. Proteins were transferred to nitrocellulose membranes and blocked with 5% nonfat milk in Tris-buffered saline. Membranes were exposed to primary antibodies for 1 hour at room temperature or overnight at 4 °C, washed, and probed with the appropriate horseradish peroxidase-conjugated secondary antibodies. Bound antibodies were detected using enhanced chemiluminescence. Band intensities were quantified with TotalLab software (Nonlinear Dyanmics Limited, Newcastle upon Tyne, UK) and expressed in units relative to the control values for each data set.
Statistics
Data were analyzed using ANOVA, followed by Fisher’s least significant difference posthoc comparisons when p<0.05 was obtained. All values were expressed as means ± standard errors.
RESULTS
ATM and phosphorylated ATM
As shown in figure 1A, C2C12 myotubes expressing shRNA against ATM had only about 22% the amount of ATM protein that was present in the control (empty vector and nontargeting shRNA) cells (p<0.005). Fortuitously, this low level of ATM protein is similar to the low level of ATM protein present in skeletal muscle of fat-fed rats compared to chow-fed animals (Halaby et al., 2008). Thus, the use of the shRNA causes a decrease in ATM protein that is in a relevant, physiological range.
Figure 1. ATM protein levels.
A: ATM protein levels in C2C12 myotubes from a line previously exposed to lentiviral empty vector (EV), myotubes expressing non-targeting shRNA (NT), and myotubes with ATM knocked down (KD) by expression of shRNA against ATM (n=8/group). B and C: ATM protein (B, n=3/group) and phosphorylation of ATM at an autophosphorylation site (C, n=9/group) in isolated soleus muscles from wild-type (ATM +/+) and ATM +/− mice after 30 min of exposure to 30 nM IGF-1. Values are means with standard errors. *p<0.005 for lower values in KD compared to EV and NT. †p<0.05 for lower levels in ATM+/− compared to the corresponding ATM+/+ groups. ‡p<0.05 for greater value than in ATM+/+ control without IGF-1. §p<0.05, lower than value for ATM+/+ with IGF-1.
We chose to use heterozygous mice that expressed one functional allele of ATM and one nonfunctional allele (ATM +/−), because we wanted to avoid a non-physiological condition of total absence of ATM. While complete loss of ATM function exists in the rare human disease ataxia telangiectasia, we were more interested in the role of attenuated ATM function that would have more physiological implications, such as in the aforementioned effect of fat feeding to decrease, but not eliminate, ATM protein content of muscle (Halaby et al., 2008). An additional advantage of studying ATM +/− mice is that their body weights are similar to body weights of wild-type mice (ATM +/+ 24.1±2.0 g, ATM +/− 25.6±2.0 g; mean ± SD). In comparison, body weights of ATM −/− mice are only about 70% of body weights in wild-type mice (ATM +/+ 23.7±2.2 g, ATM −/− 16.7±1.2 g; mean ± SD). Thus, using ATM +/− mice instead of ATM −/− mice avoids a potentially confounding phenotype of small body size.
As shown in figure 1B, ATM protein levels were about half in soleus muscles of ATM+/− mice compared to wild type (p<0.05). In response to IGF-1, ATM phosphorylation at S1981 increased in wild-type mice but not in ATM +/− mice (figure 1C). S1981 is an autophosphorylation site of ATM; hence, phosphorylation of S1981 is a marker of ATM activation. Thus, IGF-1 appears to activate ATM in wild-type soleus, consistent with a previous report that ATM is activated by insulin (Yang & Kastan, 2000).
IGF-1-stimulated Akt phosphorylation
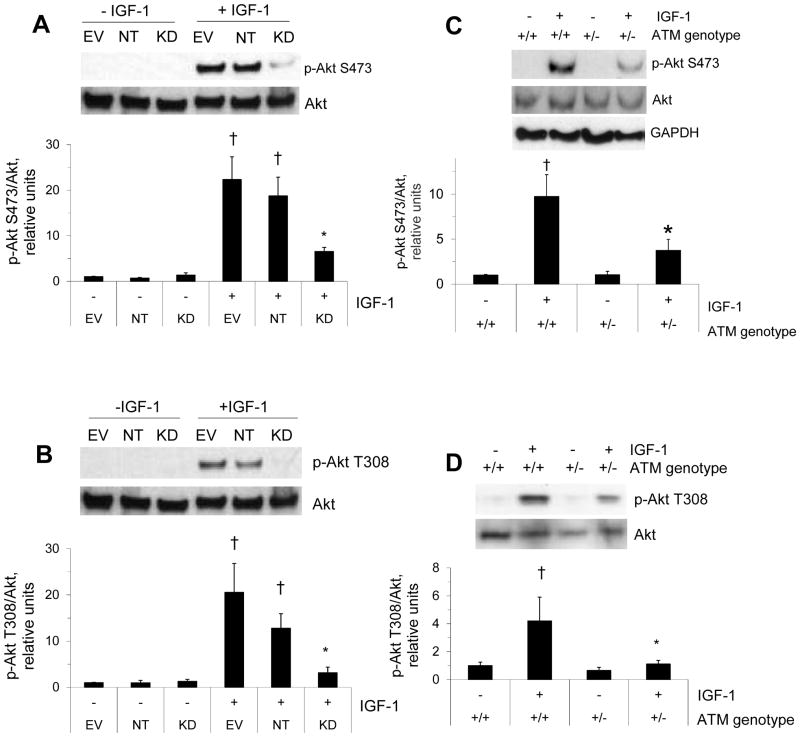
Because there are contrasting reports in the literature regarding whether or not ATM plays a role upstream of Akt, we began by determining whether IGF-1-stimulated Akt phosphorylation was downregulated by ATM deficiency. C2C12 myotubes expressing shRNA against ATM had markedly reduced IGF-1-stimulated Akt phosphorylation compared to myotubes from a line previously infected with empty vector lentivirus or expressing non-targeting shRNA (p<0.05, fig. 2A and B). Likewise, as shown in figure 2C–D, IGF-1-stimulated phosphorylation of Akt was found to be significantly blunted (p<0.05) in mice lacking one functional ATM allele (+/−) compared to wild type mice (+/+). Akt/GAPDH was not different between wild-type muscle and muscle from animals that were haploinsufficient for ATM (+/+ 1.0±0.1, +/− 1.0±0.2, relative units). Likewise, while there was some variability in Akt levels for figure 2D, on average Akt levels did not differ between groups (+/+ 1.00±0.04, +/− 0.93±0.17). Thus, it appears that Akt levels are normal in muscle from +/− mice.
Figure 2. IGF-1-stimulated Akt phosphorylation.
A, B: C2C12 myotubes as described in the figure 1A legend were incubated in the absence or presence of 10 nM IGF-1 for 20 min before western blots for Akt and phosphorylated Akt. C, D: soleus muscles were incubated as described in the figure 1B–C legend before western blots for Akt, phosphorylated Akt, and GAPDH. Data represent means ±SE (n=4). †represents a significant effect of IGF-1 compared to the corresponding control without IGF-1 (p<0.05). * represents significantly lower IGF-1-stimulated Akt phosphorylation compared to EV and KD (A, B) or wild-type (C and D), p<0.05.
IGF-1-stimulated IRS-1 phosphorylation and phosphatidylinositol 3-kinase (PI3K) activity
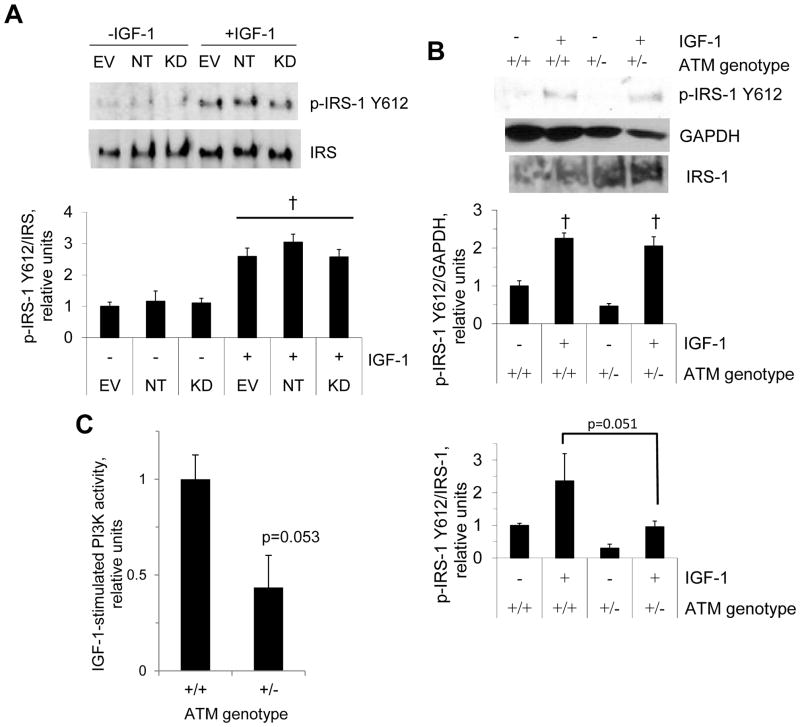
IRS-1 is a target of IGF1R and functions as an adaptor protein between IGF1R and PI3K. Given that ATM deficiency is reportedly linked to decreased IGF1R expression (Peretz et al., 2001), we next determined whether IGF-1-stimulated IRS-1 phosphorylation is affected by ATM deficiency. We assessed phosphorylation of tyrosine 612, as phosphorylation of this site is essential to full activation of PI3K (Esposito et al., 2001). For skeletal muscle samples, P-IRS and total IRS were probed from the same samples but on separate blots. As shown in figure 3A, increased tyrosine 612 phosphorylation of IRS-1 following IGF-1 treatment was not impaired in C2C12 cells in which ATM was knocked-down with ATM-targeting shRNA. However, there was a nearly-significant trend (p=0.052 from ANOVA) for IGF-1-stimulated P-IRS-1/IRS-1 (figure 3B) to be greater for wild-type muscle than for muscle from transgenic mice (p=0.051). On the other hand, there was also a trend toward greater IRS-1 levels in the muscles from ATM +/− mice (IRS-1/GAPDH: +/+ 1.00±0.17, +/−1.86±0.46, p=0.11, n=6/group). While the ratio of phosphorylated to non-phosphorylated IRS-1 tended to be lower for the heterozygous mice, the trend toward higher total IRS-1 levels maintained the P-IRS signal (i.e. P-IRS-1/GAPDH). This suggests that IGF-1-stimulated levels of tyrosine-phosphorylated IRS-1 are normal in mice lacking one functional allele of ATM.
Figure 3. IGF-1-stimulated IRS-1 phosphorylation.
A: C2C12 myotubes were incubated in the absence or presence of IGF-1, as described for figure 2A– B, and myotube lysates were probed for IRS-1 and phosphorylation of IRS-1 Y612, a key phosphorylation site for activation of PI3K (Esposito et al., 2001). B: soleus muscles from ATM +/+ and ATM +/− mice were incubated in the absence or presence of IGF-1 as described for figure 1B–C, and muscle homogenates were probed for IRS-1 Y612 phosphorylation and IRS-1. C: IGF-1-stimulated PI3K activity (n=6). Data represent means ±SE (n=4 for all except n=3 for p-IRS-1/IRS-1). † represents a significant main effect of IGF-1 (p<0.05).
We next performed assays for PI3K activity using a kinase reaction kit (Echelon Biosciences Inc., Salt Lake City, UT) with competitive ELISA detection of PI3K product. As shown in figure 3C, IGF-1-stimulated PI3K activity tended to be lower in muscle from ATM haploinsufficient mice than muscle from wild-type mice (p=0.053).
Phosphorylation of mTOR and S6K following IGF-1 treatment
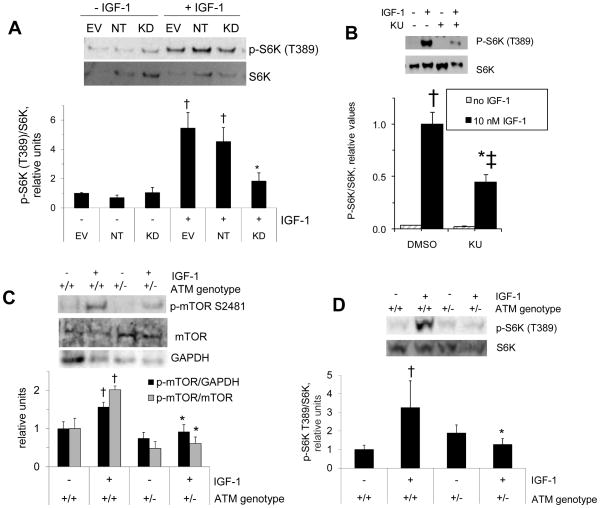
mTOR can be found in distinct protein complexes, mTOR complex 1 (mTORC1) and mTORC2 (reviewed in (Watanabe et al., 2011)). mTORC1 is sensitive to rapamycin and holds S6K among its substrates. As shown in figure 4A, IGF-1-stimulated S6K phosphorylation at T389 (an mTORC1 target site) was significantly reduced in C2C12 cells expressing ATM-targeting shRNA (p<0.05). Furthermore, C2C12 cells incubated with 10 μM KU55933 (KU), a pharmacological inhibitor of ATM, also displayed decreased IGF-1-stimulated S6K phosphorylation (figure 4B). As shown in figure 4C, IGF-1-stimulated mTOR phosphorylation (p-mTOR/GAPDH) at an mTOR autophosphorylation site was significantly lower in soleus from ATM+/− mice than in soleus from ATM+/+ mice (p<0.05). Total mTOR was probed from the same samples on separate blots. mTOR/GAPDH was not different between wild-type and transgenic muscles (wild type 1.0±0.1, ATM +/− 1.2±0.2, relative units, n=6/group). Accordingly, p-mTOR/mTOR was qualitatively similar to P-mTOR/GAPDH. Likewise, S6K phosphorylation following incubation of muscles with IGF-1 was significantly impaired (p<0.05) in mice lacking one functional ATM allele (fig. 4D). In summary, decreases in S6K phosphorylation at the mTOR target site suggest that mTOR activity was impaired in muscle or myotubes with decreased ATM protein or in myotubes in the presence of an ATM inhibitor.
Figure 4. IGF-1-stimulated phosphorylation S6K and mTOR.
A: myotubes were incubated in the absence or presence of IGF-1 as described for figure 2A–B, and myotube lysates were probed for S6K and phosphorylation of S6K at an mTOR target site (n=4). B: C2C12 myotubes (n=3) were incubated in the absence or presence of an ATM inhibitor (KU) and then further incubated for 20 min in the absence or presence of 10 nM IGF-1 before western blots for phosphorylated S6K. C, D: soleus muscles, as described in figure 1B–C, were incubated in the absence or presence of IGF-1 before determination of mTOR phosphorylation at an autophosphorylation site, GAPDH, and total mTOR (C, n=8–9 for p-mTOR/GAPDH; n=3 for p-mTOR/mTOR) and S6K phosphorylation (D, n=6). Data represent means ±SE. †greater than the corresponding control (p<0.05). *represents significant reduction in IGF-1 action compared to the corresponding controls (p<0.05). ‡IGF-1 effect still significant (though lower than the IGF-1 effect in vehicle-treated cells) in the presence of KU55933 (p<0.05).
DISCUSSION
In this study, we assessed the effects of ATM deficiency on IGF-1 signaling through the Akt pathway. By employing parallel models--cultured C2C12 myotubes expressing ATM-targeting shRNA and transgenic mice decreased expression of functional ATM--we have shown that ATM deficiency results in reduced IGF-1 signaling through the Akt/mTOR pathway.
The current findings of a role for ATM in IGF-1-stimulated Akt phosphorylation in mouse soleus stands in contrast to previous findings that insulin-stimulated Akt phosphorylation is normal in mouse soleus in the presence of an ATM inhibitor (Jeong et al., 2010). Additionally, we have found that insulin-stimulated Akt phosphorylation is normal in soleus muscle from transgenic mice that are haploinsufficient for ATM or completely lacking functional ATM (unpublished data, under review elsewhere). Thus, in mouse soleus muscle, it appears that a role of ATM in phosphorylation of Akt might be dependent upon whether the insulin receptor (IR) or IGF-1 receptor (IGF1R) has activated the signaling cascade. This idea would be consistent with a model by which IR and IGF1R signal strength toward metabolic outcomes or other pathways varies in amplitude by the receptor that is activated (Boucher et al., 2010; Siddle, 2011; Urso et al., 1999). An elegant demonstration of this was done by Ursø et al, who constructed chimeric receptors consisting of the extracellular domain of the neurotrophin receptor TrkC and the cytoplasmic domains of either the IR or the IGF1R (Urso et al., 1999). Expression of these chimeric receptors in adipocytes allowed signaling to be initiated by a single ligand, neurotrophin, with differences in outcomes attributable to the form of the cytosolic receptor domain. In these chimeras, the neurotrophin signal to glucose transport was activated in the IGF1R chimera but higher in the IR chimera. In contrast, signaling through the mitogenic pathway of Shc phosphorylation, Grb2 binding to Shc, and phosphorylation of mitogen activated protein kinase was higher for the IGF1R chimera than the IR chimera.
Our working hypothesis is that given sufficient signal, such as might be the case for insulin-stimulated Akt phosphorylation in soleus, ATM is not required for full phosphorylation of Akt (Jeong et al., 2010). On the other hand, a relatively weaker metabolic signal emanating from IGF1R activation might require additional signaling pathways upstream of Akt, such as ATM. If signal strength indeed determines a requirement for ATM in phosphorylation of Akt, that could explain tissue and cell-type differences in the role of ATM in Akt phosphorylation, possibly depending on the abundances and molar ratios of IR and IGF1R. For example, ATM is not required for insulin-stimulated phosphorylation of Akt in cells or tissues that respond well to insulin in terms of stimulation of glucose transport, including mouse soleus, L6 myotubes, and adipocytes (Jeong et al., 2010; Hresko & Mueckler, 2005). In contrast, ATM does play a role in insulin-stimulated phosphorylation of Akt in L6 myoblasts, C2C12 myotubes, rhabdomyosarcoma cells, Cos cells, 293T cells, mouse embryonic fibroblasts, and human fibroblasts (Viniegra et al., 2005; Halaby et al., 2008; Jeong et al., 2010).
The fact that ATM deficiency had no effect on IGF-1-stimulated phosphorylation of IRS-1 Y612, an immediate downstream target of IGF-1R, while phosphorylation of Akt Ser473 and Akt T308 following IGF-1 treatment was significantly decreased, suggests that ATM intersects IGF-1 signaling downstream of IRS-1. Thus, while others have shown that ATM deficiency causes decreased IGF1R expression (Peretz et al., 2001), we have found no effect of ATM haploinsufficiency on IGF-1-stimulated phosphorylation of IRS-1, a direct target of IGF-1R tyrosine kinase activity. Our finding of a tendency toward lower PI3K activity in muscle from ATM haploinsuffient mice compared to wild type muscle suggests that tyrosine-phosphorylated IRS-1 is prevented from activating PI3K in the transgenic muscle. This finding is consistent with a previous report that serine phosphorylation of IRS-1 secondary to Jun N-terminal Kinase activation in ATM-deficient cells is related to decreased insulin signaling downstream of IRS-1 (Schneider et al., 2006).
Although the mechanism for activation of ATM by insulin is not known, it has been reported that activation of PI3K is crucial to activation of ATM by ionizing radiation (IR) or exposure to high NaCl concentrations (Irarrazabal et al., 2006). For example, activation of ATM by IR or NaCl in Jurkat cells was prevented by expression of a dominant negative form of the p85 regulatory subunit of PI3K or siRNA-mediated knockdown of expression of the p110α catalytic subunit of PI3K (Irarrazabal et al., 2006). Thus, ATM is likely to lie downstream of PI3K in insulin and IGF-1 signaling.
Though the mechanism for activation of ATM by insulin or IGF-1 has not been elucidated, it is instructive that oxidation of ATM by reactive oxygen species (ROS) directly activates ATM (Guo et al., 2010). Intriguingly, it has been established that insulin and IGF-1 stimulate production of reactive oxygen species, probably through activation of the NADPH oxidase complex containing the catalytic subunit Nox4 and several regulatory proteins, including p47phox and rac (Goldstein et al., 2005; Handayaningsih et al., 2011). Given that recruitment of p47phox and rac to the plasma membrane by 3-phosphoinositide products of PI3K activates ROS production by Nox4 (Baumer et al., 2008), it seems possible that PI3K-dependent H2O2 production by Nox4 could lead to local oxidation and activation of ATM.
Our data suggest that the effect of ATM on IGF-1 signaling lies between IRS-1 and Akt, but it is unlikely that ATM directly phosphorylates Akt on S473 or T308. For example, while a direct association between ATM and Akt has been demonstrated, ATM was not found to be an in vivo kinase for Akt (Viniegra et al., 2005). Notably, S473 and T308 sites of Akt do not have consensus serine-glutamine (SQ) or threonine-glutamine (TQ) motifs, which are present in known ATM target sites (O’Neill et al., 2000). A model suggested by Viniegra et al proposes that ATM regulates an Akt S473 kinase (Viniegra et al., 2005). The insulin-responsive Akt S473 kinase appears to be mTOR complex 2 (mTORC2), that is an association of mTOR, rictor, and several other proteins (Hresko & Mueckler, 2005; Sarbassov et al., 2005). Thus, it seems possible that ATM might regulate mTORC2 activity through influence on a component of mTORC2. Intriguingly, it has been reported that phosphorylation of S2481 of mTOR is not inhibitable by rapamycin, is specific for intact mTORC2, does not occur in mTORC1, and is considered to be a biomarker for intact mTORC2 (Copp et al., 2009). If this report regarding mTORS2481 generalizes to skeletal muscle, it is possible that haploinsufficiency of ATM leads to decreased mTORC2 abundance or IGF-1-stimulated mTORC2 phosphorylation of mTOR, as IGF-1-stimulated S2481 phosphorylation was decreased by ATM haploinsufficiency. On the other hand, another group has reported rapamycin sensitivity of phosphorylation of mTOR S2481 (Soliman et al., 2010), so the issue of complex-specific mTOR S2481 phosphorylation remains unresolved.
Mice that do not express functional ATM grow more slowly and weigh less than their wild-type littermates (Barlow et al., 1996). Patients with ataxia telangiectasia (A–T), a condition resulting from ATM deficiency, also display retarded growth and small stature (Boder & Sedgwick, 1958). For example, children over the age of 12 with A–T had body mass index values in the 3rd percentile despite adequate nutritional intake (Schubert et al., 2005). Furthermore, adult-onset A–T patients with mild symptoms exhibited leg weakness and atrophy accompanied by presence of atrophic quadriceps muscle fibers (Hiel et al., 2006). Although the mechanism for decreased growth and muscle atrophy in ATM deficiency is not known, it seems possible that the blunted IGF-1 signaling currently described could play a role. It is tempting to speculate that activation of ATM could be a means of stimulating components of IGF-1 signaling, such as PI3K and Akt, thereby promoting protein synthesis in conditions of muscle atrophy or muscle wasting.
In summary, we have described a new role for ATM in regulation of IGF-1 signaling in skeletal muscle and cultured myotubes, in which ATM appears to be necessary for full effects of IGF-1 on phosphorylation of Akt, mTOR, and S6K. This ATM effect on IGF-1 action occurs downstream of IRS-1 but upstream of Akt, perhaps at the level of PI3K. These findings suggest that future work should be done to determine whether activation of ATM through genetic or pharmacological approaches could be a means for increasing signaling through Akt, mTOR, and S6K in skeletal muscle.
What is the current scientific knowledge on this topic? In some cultured cells, ataxia telangiectasia mutated (ATM) is required for activation of Akt by insulin. However, this is not the case in other cell or tissue types, including skeletal muscle. Furthermore, it is not known whether ATM plays a role in skeletal muscle IGF-1 signaling.
What new finding does this research study add to this field? We found that IGF-1 caused autophosphorylation of ATM in skeletal muscle. However, IGF-1-stimulated phosphorylation of Akt, S6K, and mTOR (but not IRS-1) was impaired in C2C12 myotubes with reduced ATM expression and/or muscle from ATM-haploinsufficient mice. These demonstrate activation of ATM by IGF-1 and a role for ATM in IGF-1 signaling downstream of IRS-1.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development (NICHD) through a Pilot Project (subcontract to R24HD050837, PI: Richard Lieber) award from the National Skeletal Muscle Research Center and by Grant Number R15DK080437 from the National Institute Of Diabetes And Digestive And Kidney Diseases (NIDDK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, the NIDDK or the National Institutes of Health. Additional support was provided by the Saint Louis University Beaumont Faculty Department Fund, the Pfizer Students and Teachers as Research Scientists (STARS) Program (University of Missouri, St. Louis), and The Saint Louis University Monsanto Scholars Program.
References
- Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Baumer AT, Ten Freyhaus H, Sauer H, Wartenberg M, Kappert K, Schnabel P, Konkol C, Hescheler J, Vantler M, Rosenkranz S. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem. 2008;283:7864–7876. doi: 10.1074/jbc.M704997200. [DOI] [PubMed] [Google Scholar]
- Bhatti S, Kozlov S, Farooqi AA, Naqi A, Lavin M, Khanna KK. ATM protein kinase: the linchpin of cellular defenses to stress. Cell Mol Life Sci. 2011;68:2977–3006. doi: 10.1007/s00018-011-0683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boder E, Sedgwick R. Ataxia-telangiectasia; a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics. 1958;21:526–554. [PubMed] [Google Scholar]
- Boucher J, Tseng YH, Kahn CR. Insulin and insulin-like growth factor-1 receptors act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. J Biol Chem. 2010;285:17235–17245. doi: 10.1074/jbc.M110.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–1827. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito DL, Li Y, Cama A, Quon MJ. Tyr(612) and Tyr(632) in human insulin receptor substrate-1 are important for full activation of insulin-stimulated phosphatidylinositol 3-kinase activity and translocation of GLUT4 in adipose cells. Endocrinology. 2001;142:2833–2840. doi: 10.1210/endo.142.7.8283. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- Halaby MJ, Hibma JC, He J, Yang DQ. ATM protein kinase mediates full activation of Akt and regulates glucose transporter 4 translocation by insulin in muscle cells. Cell Signal. 2008;20:1555–1563. doi: 10.1016/j.cellsig.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Handayaningsih AE, Iguchi G, Fukuoka H, Nishizawa H, Takahashi M, Yamamoto M, Herningtyas EH, Okimura Y, Kaji H, Chihara K, Seino S, Takahashi Y. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology. 2011;152:912–921. doi: 10.1210/en.2010-0981. [DOI] [PubMed] [Google Scholar]
- Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- Hiel JA, van Engelen BG, Weemaes CM, Broeks A, Verrips A, ter Laak H, Vingerhoets HM, van den Heuvel LP, Lammens M, Gabreels FJ, Last JI, Taylor AM. Distal spinal muscular atrophy as a major feature in adult-onset ataxia telangiectasia. Neurology. 2006;67:346–349. doi: 10.1212/01.wnl.0000224878.22821.23. [DOI] [PubMed] [Google Scholar]
- Hresko RC, Mueckler M. mTOR/RICTOR is the Ser473 kinase for Akt/PKB in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- Irarrazabal CE, Burg MB, Ward SG, Ferraris JD. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: Role in osmoprotective transcriptional regulation. Proc Natl Acad Sci U S A. 2006;103:8882–8887. doi: 10.1073/pnas.0602911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong I, Patel AY, Zhang Z, Patil PB, Nadella ST, Nair S, Ralston L, Hoormann JK, Fisher JS. Role of ataxia telangiectasia mutated in insulin signaling of muscle-derived cell lines and mouse soleus. Acta Physiologica. 2010;198:465–475. doi: 10.1111/j.1748-1716.2009.02069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang X, Shen J, Wong PK, Yan M. Deregulation of mTOR signaling is involved in thymic lymphoma development in Atm−/− mice. Biochem Biophys Res Commun. 2009;383:368–372. doi: 10.1016/j.bbrc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- O’Neill T, Dwyer AJ, Ziv Y, Chan DW, Lees-Miller SP, Abraham RH, Lai JH, Hill D, Shiloh Y, Cantley LC, Rathbun GA. Utilization of oriented peptide libraries to identify substrate motifs selected by ATM. J Biol Chem. 2000;275:22719–22727. doi: 10.1074/jbc.M001002200. [DOI] [PubMed] [Google Scholar]
- Peretz S, Jensen R, Baserga R, Glazer PM. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci U S A. 2001;98:1676–1681. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Schubert R, Reichenbach J, Zielen S. Growth factor deficiency in patients with ataxia telangiectasia. Clin Exp Immunol. 2005;140:517–519. doi: 10.1111/j.1365-2249.2005.02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol. 2011;47:R1–10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem. 2010;285:7866–7879. doi: 10.1074/jbc.M109.096222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso B, Cope DL, Kalloo-Hosein HE, Hayward AC, Whitehead JP, O’Rahilly S, Siddle K. Differences in signaling properties of the cytoplasmic domains of the insulin receptor and insulin-like growth factor receptor in 3T3-L1 adipocytes. J Biol Chem. 1999;274:30864–30873. doi: 10.1074/jbc.274.43.30864. [DOI] [PubMed] [Google Scholar]
- Viniegra JG, Martinez N, Modirassari P, Losa JH, Parada CC, Lobo VJ, Luquero CI, Alvarez-Vallina L, Cajal S, Rojas JM, Sanchez-Prieto R. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280:4029–4036. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Wei L, Huang J. mTOR signaling, function, novel inhibitors, and therapeutic targets. J Nucl Med. 2011;52:497–500. doi: 10.2967/jnumed.111.089623. [DOI] [PubMed] [Google Scholar]
- Yang DQ, Halaby MJ, Li Y, Hibma JC, Burn P. Cytoplasmic ATM protein kinase: an emerging therapeutic target for diabetes, cancer and neuronal degeneration. Drug Discov Today. 2011;16:332–338. doi: 10.1016/j.drudis.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–898. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]