Abstract
Aim
To evaluate the usefulness of ultrasound (US) using contrast agent and elastosonography in the characterization of thyroid nodules.
Materials and methods
From November 2006 to July 2007, 23 patients with single thyroid nodules underwent B-mode US and power Doppler, US examination using contrast agent, elastosonography and fine needle aspiration cytology (FNAC). Sixteen patients underwent thyroidectomy.
Results
The 23 nodules included 14 benign and 9 malignant lesions. Analysis of time/intensity curves showed that wash-in (8.8 ± 1.3 vs 12.1 ± 2.6 s; p = 0.002, t-test) and peak enhancement (15.3 ± 4.6 vs 22.2 ± 3.9 s; p = 0.001, t-test) occurred significantly earlier in the malignant nodules than in the benign nodules. Wash-out was monophasic in 70% of benign nodules, but in none of the malignant nodules; polyphasic in 30% of benign nodules and in 100% of malignant nodules. Polyphasic wash-out showed a statistically significant association with malignancy (p = 0.0007, χ2). Polyphasic wash-out yielded a sensitivity of 100%, specificity of 71%, positive predictive value (PPV) of 69%, negative predictive value (NPV) of 100% and diagnostic accuracy of 83%. In 78% of the benign nodules (11/14) elastosonographic patterns was 1–2 (elevated elasticity); in 88% of the malignant nodules (8/9) elastosonographic patterns was 3–4 (reduced elasticity). Elastosonography yielded a sensitivity of 88%, specificity of 78%, PPV of 72%, NPV of 91% and diagnostic accuracy of 82%. Elastosonographic patterns 3–4 is associated with malignancy (p = 0.001, χ2).
Conclusion
US using contrast agent and elastosonography can be a useful diagnostic tool in the evaluation of single thyroid nodules, particularly when FNAC result is non-diagnostic or suggests a follicular lesion, and in nodules <1 cm.
Keywords: Thyroid, Ultrasound, Elastosonography, Contrast agent
Sommario
Scopo
Valutare l'utilità dell'ecografia con ecoamplificatore e dell'elastosonografia nella caratterizzazione del nodulo tiroideo.
Materiali e metodi
Periodo novembre 2006–luglio 2007, studiati 23 pazienti con nodulo solitario tiroideo, sottoposti a ecografia B-mode e power Doppler, ecografia con mezzo di contrasto, elastosonografia e FNAC (Fine Needle Aspiration Citology). Sedici pazienti sono stati sottoposti a tiroidectomia.
Risultati
I 23 noduli studiati comprendevano 14 lesioni benigne e 9 maligne. Alla valutazione delle curve intensità/tempo le lesioni maligne presentavano un tempo di wash-in (8,8 ± 1,3 vs 12,1 ± 2,6 secondi; p = 0,002, t-test) e un tempo di picco (15,3 ± 4,6 vs 22,2 ± 3,9 secondi; p = 0,001, t-test) significativamente più precoci rispetto alle lesioni benigne. La fase di wash-out presentava andamento monofasico nel 70% dei noduli benigni e in nessuno dei maligni; polifasico nel 30% dei noduli benigni e nel 100% dei maligni. L'andamento polifasico è associato in maniera statisticamente significativa (p = 0,0007, χ2) alla malignità. L'andamento polifasico ha mostrato sensibilità del 100%, specificità del 71%, VPP (valore predittivo positivo) del 69%, VPN (valore predittivo negativo) del 100% e accuratezza diagnostica dell'83%. Nel 78% (11/14) dei noduli benigni sono stati rilevati i pattern elastosonografici 1–2 (maggiore elasticità), nel 88% (8/9) dei noduli maligni i pattern 3–4 (minore elasticità). L'elastosonografia ha mostrato sensibilità dell'88%, specificità del 78%, VPP dell'72%, VPN del 91% e accuratezza diagnostica del 82%. Il pattern elastosonografico 3–4 è associato (p = 0,001, χ2) alla malignità.
Conclusioni
L'ecografia con ecoamplificatore e l'elastosonografia possono rappresentare ausili diagnostici nella valutazione del nodulo tiroideo solitario, soprattutto quando la FNAC è non diagnostica, quando indica lesione follicolare e nei noduli <1 cm.
Introduction
Thyroid nodules are frequently detected at ultrasound (US) screenings, and they occur in 50% of the elderly population [1]. In 80% of cases single nodules are benign (colloid nodules, simple cysts and thyroiditis), 10–15% are follicular adenomas and 5% are carcinomas [2]. Nodules requiring surgery should be selected using diagnostic tools with an elevated NPV. US is the most sensitive diagnostic imaging technique in the diagnosis of thyroid nodules. However, US is not sufficiently sensitive and specific in the diagnosis of malignancy; and the predictive value is not high or low enough to exclude the necessity to perform fine needle aspiration cytology (FNAC) [3–5].
FNAC provides information concerning the nature of the thyroid nodule with a diagnostic accuracy of 92–95% [6,7]. However, there are 2 important shortcomings to FNAC, such as non-diagnostic results (due to inadequate sample quality) and in follicular thyroid neoplasms. These diagnostic limitations can be overcome by employing new diagnostic methods of elevated accuracy which can differentiate benign from malignant nodules, such as US contrast agents and elastosonography. Intravascular US contrast agents permit detection of neoplastic growth characterized by tortuous blood vessels and arteriovenous shunts [8,9].
Elastosonography examines the mechanical and elastic properties of the soft tissue which rely on the composition and structural organization of the macromolecules. One of the mechanical properties is the elasticity which determines the deformation or distortion of the tissues in response to external compression. Some pathological conditions induce considerable changes in the soft tissue structure modifying the elastic properties and leading to an increased firmness and a reduced mobility of the involved tissue.
The aim of this study was to evaluate the usefulness of US using contrast agent and elastosonography in the characterization of single thyroid nodules.
Materials and methods
Patient population
From November 2006 to July 2007, 23 patients (5 males and 18 females), age range 20–82 years (mean age 44 years) underwent US examination which revealed a single thyroid nodule (8–53 mm in diameter).
Clinical evaluation
Clinical history was obtained and all patients underwent clinical examination. Thyroid-stimulating hormone (THS), thyroid peroxidase antibodies (anti-TPO), thyroglobulin antibodies (anti-Tg), and calcitonin levels were determined.
B-mode US and power Doppler
All patients underwent B-mode US to evaluate dimensions of the nodule, echogenicity, presence of perinodular halo, and presence and types of calcifications. Vascularization of the nodule was studied using power Doppler (PD) and classified as follows: no blood flow, mainly perinodular blood flow, intranodular and perinodular blood flow. US examinations were performed using Hitachi EUB 8500 Logos equipment (Hitachi Medical Systems, Tokyo, Japan) and a linear 13 MHz probe.
US using contrast agent
US examination was performed, baseline and after bolus injection of contrast agent (SonoVue, Bracco, Milan, Italy) 2.4 ml, 1 ml/s, followed by 10 ml physiological solution, using a peripheral intravenous cannula of 20 Gauge. The examination was performed using Technos MPX equipment (Esaote Biomedica, Genoa, Italy) in continuous imaging with low mechanical index (MI = 0.05–0.08) and contrast-specific algorithm contrast tuned imaging (CnTI). The examination lasted 3 min from the start of contrast agent injection. Dedicated software elaborated all obtained data during the 3 min of scanning providing time/intensity curves. We evaluated arrival of the contrast agent (wash-in), the time required to reach peak enhancement and the wash-out phase (monophasic/polyphasic). Wash-in was defined as the first point on the curve showing signal intensity corresponding to 10% of the basic value of the signal recorded at the start of the scanning. Peak time or duration of the wash-in phase was considered as the time elapsed between the start of contrast agent injection and the peak enhancement.
Elastosonography
Elastosonographic examinations were performed using EUB 8500 Logos equipment (Hitachi Medical Systems, Tokyo, Japan) and a 13 MHz linear probe to which a flat base had been applied to achieve optimal adherence to the tissue as well as uniform compression. For the elaboration of the data, we used dedicated software CAM (Combined Autocorrelation Method) with an algorithm able to process in a very short time all radio-frequency impulses coming from the lesion while minimizing the artifacts caused by lateral dislocation. An indicator showed in real-time the correct performance of the examination through a scale of values from 1 to 5 which appeared on the side of the elastosonographic image. The signal coming from the examined tissue was immediately processed (“real-time”) and the elastogram was displayed over the B-mode image in a color scale: red (soft tissue), blue (inelastic tissue) and green (intermediate). During the procedure, the quality of the examination was evaluated and considered acceptable only if the elastosonographic image was continuously displayed over the B-mode with the illuminated indicator showing a value between 3 and 5. The elastosonographic images thus obtained were classified in 4 patterns according to the elasticity (Fig. 1).
Fig. 1.
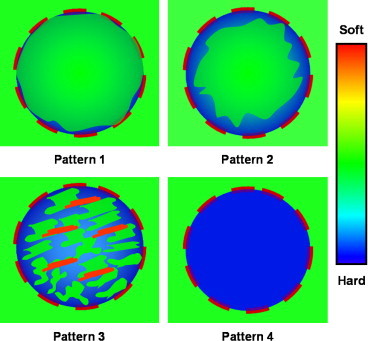
Elastosonographic patterns.
FNAC
All nodules underwent cytological analysis after US guided FNAC using 23 Gauge needles and 5 ml syringes. FNAC was performed after the study of the vascular pattern, the examination using contrast agent and elastosonography in order to avoid post biopsy alterations. Sixteen patients underwent thyroidectomy with histological analysis of the surgical specimen. Outcome of cytological and histological examination was compared with outcome of US examination using contrast agent and elastosonography.
Statistical analysis
Statistical analysis was carried out using the Student t-test unpaired and χ2 test, considering for both a statistically significant value of 5%. Sensitivity, specificity, PPV, NPV were calculated as well as diagnostic accuracy of the monophasic/polyphasic wash-out curve and elastosonography in the identification of malignant nodules.
Ethical approval for this study was granted by the Medical Research Ethics Committee of our university, and informed consent was obtained from all patients.
Results
Cytological analysis yielded the following result: 9 benign lesions (8 colloid/hyperplastic nodules and 1 affected by chronic inflammation), 11 suspicious lesions (6 follicular lesions, 3 Hürtle cell lesions, 2 suspected carcinomas) and 3 malignant lesions. Sixteen patients underwent surgery (all patients whose FNAC outcome was suspicious or positive for carcinoma, and 2 due to the size of the nodules although FNAC outcome was benign).
Histological examination of the 16 nodules evidenced 7 benign lesions (1 struma, 2 Hürtle cell adenomas, 4 micro/macrofollicular adenomas) and 9 malignant lesions (7 papillary carcinomas, 1 Hürtle cell carcinoma, 1 undifferentiated carcinoma). Histological and cytological analysis diagnosed 14 benign and 9 malignant lesions among the 23 studied nodules.
TSH was normal (0.27–4.2 mUI/l) in 19 patients, increased in 2 and reduced in 2; anti-Tg was normal (<20 UI/ml) in 18 patients and increased in 5 patients; anti-TPO was <10 UI/ml in 14 patients and >10 UI/ml in 9 patients. Calcitonin levels were normal in all patients.
At B-mode US examination, 9 nodules (2 malignant and 7 benign) were isoechoic, 14 (7 malignant and 7 benign) were hypoechoic (p = 0.182, χ2). Microcalcifications were found in 4 malignant nodules and in none of the benign nodules (p = 0.006, χ2); macrocalcifications were found in 4 benign nodules and in none of the malignant nodules. A perinodular halo was visible in 10 nodules (3 malignant and 7 benign) but not in 13 nodules (6 malignant and 7 benign) (p = 0.431, χ2).
PD examination revealed no blood flow in 1 benign nodule, mainly perinodular blood flow in 14 nodules (5 malignant and 9 benign) and intranodular and perinodular blood flow in 8 nodules (4 malignant and 4 benign) (p = 0.574, χ2).
Evaluation of time/intensity curves showed that wash-in occurred significantly earlier in malignant lesions than in benign lesions (8.8 ± 1.3 vs 12.1 ± 2.6 s; p = 0.002, t-test) (Fig. 2a, b).
Fig. 2.
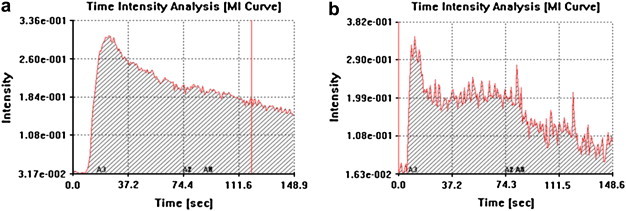
Time/intensity curves of a benign nodule (a) and a malignant nodule (b).
Peak enhancement (peak time) occurred significantly earlier in malignant nodules than in benign nodules (15.3 ± 4.6 vs 22.2 ± 3.9 s; p = 0.001, t-test).
Wash-out phase was regular and monophasic in 10/14 benign nodules (70%) and in none of the malignant nodules; irregular and polyphasic in 4/14 benign nodules (30%) and in 9/9 malignant nodules (100%) (Fig. 2 a,b; Table 1). Irregular and polyphasic wash-out curves showed a statistically significant association with malignancy (p = 0.0007, χ2). Analysis of wash-out curves showed a sensitivity of 100%, specificity of 71%, PPV of 69%, NPV of 100% and diagnostic accuracy of 83%.
Table 1.
Characteristics of the wash-out phase
| Wash-out monophasic | Wash-out polyphasic | Total | |
|---|---|---|---|
| Malignant nodules | 0 | 9 | 9 |
| Benign nodules | 10 | 4 | 14 |
| Total | 10 | 13 | 23 |
Elastosonographic pattern 1 (Fig. 3) was found in 2/14 benign nodules (14%) and in none of the malignant nodules; pattern 2 (Fig. 4) in 9/14 (64%) benign nodules and in 1/9 (12%) malignant nodules; pattern 3 (Fig. 5) in 3/14 (22%) benign nodules and in 4/9 (44%) malignant nodules; pattern 4 (Fig. 6) in none of the benign nodules and in 4/9 (44%) malignant nodules (Table 2). In 11/14 benign nodules (78%) elastosonographic patterns was 1–2, and in 8/9 malignant nodules (88%) elastosonographic patterns was 3–4. Elastosonography yielded a sensitivity of 88%, specificity of 78%, PPV of 72%, NPV of 91% and diagnostic accuracy of 82%. Elastosonographic patterns 3–4 showed a significant association with malignancy (p = 0.001, χ2).
Fig. 3.
Elastosonographic pattern 1.
Fig. 4.
Elastosonographic pattern 2.
Fig. 5.
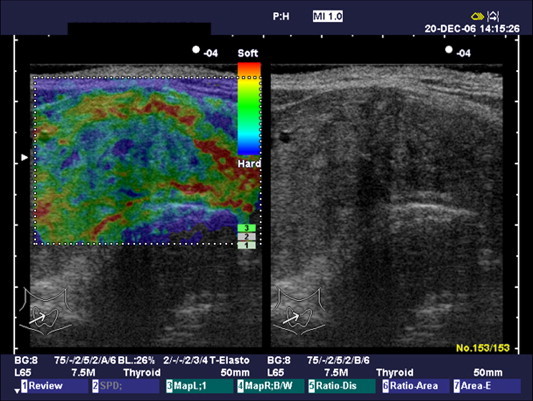
Elastosonographic pattern 3.
Fig. 6.
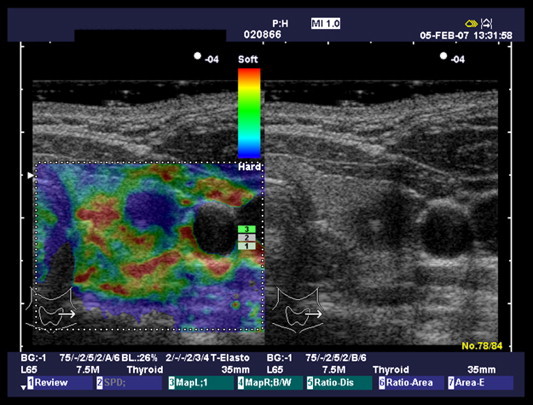
Elastosonographic pattern 4.
Table 2.
Elastosonographic patterns
| Pattern 1 | Pattern 2 | Pattern 3 | Pattern 4 | Total | |
|---|---|---|---|---|---|
| Malignant nodules | 0 | 1 | 4 | 4 | 9 |
| Benign nodules | 2 | 9 | 3 | 0 | 14 |
| Total | 2 | 10 | 7 | 4 | 23 |
Discussion
High resolution US has made it possible to identify nonpalpable thyroid nodules [10,11] which are frequently found in the general population (19–46%) [12]. Numerous studies have analyzed the relationship between US findings, blood flow and the risk of malignancy in thyroid nodules [13–15] but so far with discordant results. US can reach a diagnostic accuracy of more than 90% in the diagnosis of carcinoma, particularly papillary carcinoma, but neither US nor FNAC are sufficiently accurate in the diagnosis of follicular carcinoma [16].
FNAC is considered the most accurate diagnostic method. However, there are 2 important shortcomings to FNAC, such as non-diagnostic results due to inadequate sample quality and in follicular thyroid neoplasm or Hürtle cell lesions. Inadequacy of the sample quality is reported in 7–29.5% of FNAC [6,17,18]. Inadequate FNAC, performed at least twice, poses the problem concerning the most appropriate management of the patient. Some authors recommend surgical treatment for all nodules when FNAC result is non-diagnostic, whereas others recommend surgery only in nodules which are considered suspicious on the basis of clinical and laboratory results. FNAC is furthermore recommended only in nodules presenting a maximum diameter >10 mm, but not in micronodules unless they present suspicious US and blood flow characteristics. In the presence of follicular proliferation or Hürtle cells, cytological analysis cannot differentiate adenoma from carcinoma, as the differential diagnosis is based on the presence of capsular or vascular involvement. Histological examination is therefore recommended after thyroidectomy, even though only 10–20% of the excised nodules are malignant. Neoplastic proliferation is characterized by anarchic neoangiogenesis with numerous tortuous vessels and arteriovenous shunts [8,9]. Intranodular vessels are small with a slow blood flow resulting in a weak PD flow signal. Intravascular contrast agents can depict newly formed tumor vascularity. Analysis of the transit of contrast agent through the neoplastic lesions using time/intensity curves provides quantitative and objective information.
Calliada et al. [19] evaluated time/intensity curves (morphology of the curve, peak value and time, mean duration of enhancement and wash-out characteristics) after injection of first generation contrast agent in a study of 29 solid focal lesions of the thyroid (2 malignant and 27 benign). Evaluation of intensimetric data showed slightly different results with frequent overlaps; however, peak enhancement occurred significantly earlier in carcinomas and peak enhancement was more intense than in benign lesions. Spiezia et al. [20] studied 54 thyroid nodules using first generation contrast agent and analysis of time/intensity curves. Wash-in occurred significantly earlier in carcinomas than in hyperplastic nodules and adenomas (8.1 ± 1.41 vs 19.6 ± 2.2 and 16.1 ± 2.8 s; p < 0.0001) while peak enhancement occurred earlier in carcinomas and adenomas than in benign hyperplastic nodules (14.6 ± 1.2 and 23.1 ± 3.8 vs 33.0 ± 3.0 s; p < 0.0001). No significant difference was revealed in peak intensity, final signal intensity and variation in intensity signal from hyperplastic nodules, adenomas and carcinomas. Appetecchia et al. [21] used a second generation contrast agent in 20 patients with thyroid nodules and evaluated wash-in time, intranodular blow flow and wash-out time. The neoplastic lesions (4 follicular adenomas and 6 carcinomas) showed early wash-in, a peripheral homogeneous enhancement pattern, rapid centripetal progression and persistence of contrast agent in the nodule. A rapid wash-out was observed in 6 cases. The non-neoplastic lesions (uninodular goiter) showed a different perfusion as compared to the surrounding healthy parenchyma.
In our study, time/intensity curves obtained after administration of US contrast agent were evaluated particularly for wash-in time, peak time and the characteristics of the wash-out curve. Wash-in occurred significantly earlier in malignant lesions as compared to benign lesions (8.8 ± 1.3 vs 12.1 ± 2.6 s; p = 0.002, t-test). These results are in agreement with the results obtained by Calliada et al. [19], Spiezia et al. [20] and Appetecchia et al. [21]. The early wash-in in neoplastic lesions could be explained by the rapid blood flow through the arteriovenous shunts. Peak enhancement occurred significantly earlier in the malignant nodules as compared to the benign lesions (15.3 ± 4.6 vs 22.2 ± 3.9 s; p = 0.001, t-test). This observation is in agreement with the results obtained by Calliada et al. [19], Spiezia et al. [20]. Wash-out phase was regular and monophasic in 10/14 (70%) benign nodules and in none of the malignant nodules; irregular and polyphasic in 4/14 (30%) benign nodules and in 9/9 (100%) malignant nodules. Irregular and polyphasic wash-out curves showed a statistically significant association with malignancy (p = 0.0007, χ2). Analysis of wash-out curves showed a sensitivity of 100%, specificity of 71%, PPV of 69%, NPV of 100% and diagnostic accuracy of 83%. Argalia et al. [22] examined 61 single thyroid nodules (43 benign and 18 malignant) using first generation contrast agent and time/intensity curves. The curves were regular and monophasic in 93% of benign lesions and in 11% of malignant lesions, whereas, they were irregular and polyphasic in 89% of malignant lesions and in 7% of benign lesions (sensitivity 88%, specificity 93%).
Bartolotta et al. [23] examined contrast enhancement characteristics in 18 patients with single thyroid nodules (13 malignant and 5 benign lesions) using US after administration of SonoVue. Three enhancement patterns were identified: absent, dot-like and diffused (homogeneous or inhomogeneous). None of the enhancement patterns were found to be specific of malignancy, but malignant nodules tended to have no or dot-like vascularization, whereas benign lesions had homogeneous or inhomogeneous vascularization.
The mechanical properties of the soft tissues rely on the composition and structural organization of the macromolecules. One of the mechanical properties is the elasticity which determines the deformation or distortion of the tissues in response to external compression (Young's modulus of elasticity). Some pathological conditions induce considerable changes in the soft tissue structure modifying the elastic properties and leading to an increased firmness and a reduced mobility of the involved tissue.
Elastosonography analyses the changes in radio-frequency (RF) coming from a structure before and after application of external compression. There are basically two different elastosonographic techniques which are related to the type of stimulus employed to compress the examined structures: manual compression and mechanical compression which is obtained by emitting low-frequency RF impulses. The most frequently employed and studied elastosonographic technique is currently real-time free-hand US elastosonography using gradual manual compression and a US transducer which permits immediate visualization of the obtained elastogram displayed over the B-mode image. The result of the elastosonographic examination can be influenced by a series of factors: large calcifications and thyroid cysts, carotid artery pulsations and out-of-plane displacement of the lesion due to the compression, as the thyroid is surrounded by anatomical structures which can be displaced, such as the windpipe and the jugular vein, because there is no bone structure in the area which can serve as a basis for the soft tissues being compressed. The examination can be difficult to perform if the nodule is situated in the lower portion of the gland in patients with a short, thick neck.
Lyshchik et al. [24] evaluated sensitivity and specificity of elastosonography in the differentiation of benign and malignant thyroid nodules (52 lesions: 22 malignant and 30 benign). Two different imaging methods were used: real-time elastosonography and off-line processing of strain image reconstruction using RF data obtained during US examination. Thyroid gland tumor strains were measured, and the strain index (normal thyroid gland-to-tumor strain ratio) was calculated. A strain index value greater than 4 proved to be a significant predictor of malignancy (p < 0.01) with a specificity of 96% and sensitivity of 82%.
Tanaka et al. [25] evaluated the ability of elastosonography to differentiate benign from malignant thyroid tumors by studying 290 nodules (140 benign, 150 malignant) and identifying 4 elastosonographic patterns of decreasing elasticity. Malignant lesions were classified as pattern 4 in 62.6%, pattern 3 in 13.6%, pattern 2 in 12.9% and pattern 1 in 10.9% of cases. Benign lesions were classified as pattern 4 in 19.6%, pattern 3 in 4.4%, pattern 2 in 16.7% and pattern 1 in 59.4% of cases. A statistically significant difference between benign and malignant lesions was found. Elastosonography yielded a sensitivity of 89.1%, specificity of 59.4% and diagnostic accuracy of 74.7%. Sensitivity was higher (91.9%) when the elastosonographic pattern was associated with B-mode US findings suspicious for malignancy.
Rago et al. [26] studied 92 patients who had surgical treatment of single thyroid nodules due to compression symptoms or because FNAC outcome was suspicious. Tissue elasticity was evaluated and 5 grades of decreasing elasticity were identified: 49 nodules (all benign) were grades 1 and 2; 13 were grade 3 (1 carcinoma and 12 benign nodules); 30 nodules (all carcinomas) were grades 4 and 5. Grades 4 and 5 showed an elevated predictive value for malignancy (p < 0.0001), with a sensitivity of 97%, specificity of 100%, PPV of 100% and NPV of 98%.
In our series, elastosonographic pattern 1 was found in 2/14 benign nodules (14%) and in none of the malignant nodules; pattern 2 in 9/14 (64%) benign nodules and in 1/9 (12%) malignant nodules; pattern 3 in 3/14 (22%) benign nodules and in 4/9 (44%) malignant nodules; pattern 4 in none of the benign and in 4/9 (44%) malignant nodules. Patterns 1–2 were found in 11/14 (78%) benign nodules, while patterns 3–4 were found in 8/9 (88%) malignant nodules. Elastosonography yielded a sensitivity of 88%, specificity of 78%, PPV of 72%, NPV of 91% and diagnostic accuracy of 82%. Elastosonographic patterns 3–4 showed a significant association with malignancy (p = 0.001, χ2).
Conclusions
The results of this study show that analysis of time/intensity curves provide useful quantitative data in addition to data obtained using B-mode US and PD. This information is particularly useful when FNAC outcome is non-diagnostic or suggests a follicular lesion, and in the evaluation of small nodules (<1 cm). Elastosonography is a useful complementary tool in the diagnosis of thyroid carcinoma.
Conflict of interest statement
None declared.
References
- 1.Giuffrida D., Gharib H. Controversies in the management of cold, hot, and occult thyroid nodules. Am J Med. 1995;99(6):642–650. doi: 10.1016/s0002-9343(99)80252-6. [DOI] [PubMed] [Google Scholar]
- 2.Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351(17):1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 3.Wienke J.R., Chong W.K., Fielding J.R., Zou K.H., Mittelstaedt C.A. Sonographic features of benign thyroid nodules. J Ultrasound Med. 2003;22(10):1027–1031. doi: 10.7863/jum.2003.22.10.1027. [DOI] [PubMed] [Google Scholar]
- 4.Iannuccilli J.D., Cronan J.J., Monchik J.M. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004;23(11):1455–1464. doi: 10.7863/jum.2004.23.11.1455. [DOI] [PubMed] [Google Scholar]
- 5.Frates M.C., Benson C.B., Charboneau J.W. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237(3):794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 6.Chehade J.M., Silverberg A.B., Kim J., Case C., Mooradian A.D. Role of repeated fine needle aspiration of thyroid nodules with benign cytologic features. Endocr Pract. 2001;7(4):237–243. doi: 10.4158/EP.7.4.237. [DOI] [PubMed] [Google Scholar]
- 7.Sabel M.S., Staren E.D., Gianakakis L.M., Dwarakanathan S., Prinz R.A. Use of fine-needle aspiration biopsy and frozen section in the management of the solitary thyroid nodule. Surgery. 1997;122(6):1021–1026. doi: 10.1016/s0039-6060(97)90204-x. [DOI] [PubMed] [Google Scholar]
- 8.Taylor K.J., Ramos I., Carter D. Correlation of Doppler US tumor signals with neovascular morphologic features. Radiology. 1988;166(1):57–62. doi: 10.1148/radiology.166.1.2447604. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove D. Ultrasound contrast enhancement of tumours. Clin Radiol. 1996;51:44–49. [PubMed] [Google Scholar]
- 10.Ezzat S., Sarti D.A., Cain D.R., Braunstein G.D. Thyroid incidentalomas: prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154(16):1838–1840. doi: 10.1001/archinte.154.16.1838. [DOI] [PubMed] [Google Scholar]
- 11.Watters D.A., Ahuja A.T., Evans R.M. Role of ultrasound in the management of thyroid nodules. Am J Surg. 1992;164(6):654–657. doi: 10.1016/s0002-9610(05)80728-7. [DOI] [PubMed] [Google Scholar]
- 12.Burguera B., Gharib H. Thyroid incidentalomas: prevalence, diagnosis, significance, and management. Endocrinol Metab Clin North Am. 2000;29(1):187–203. doi: 10.1016/s0889-8529(05)70123-7. [DOI] [PubMed] [Google Scholar]
- 13.Papini E., Guglielmi R., Bianchini A. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and colour Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 14.Solbiati L. Thyroid gland. In: James E.M., editor. Diagnostic ultrasound. Mosby; St. Louis: 1998. pp. 703–729. [Google Scholar]
- 15.Rago T., Vitti P., Chiovato L. Role of conventional ultrasonography and color flow-doppler sonography in predicting malignancy in “cold” thyroid nodules. Eur J Endocrinol. 1998;138(1):41–46. doi: 10.1530/eje.0.1380041. [DOI] [PubMed] [Google Scholar]
- 16.Fukunari N. Thyroid ultrasonography B-mode and color-Doppler. Biomed Pharmacother. 2002;56(1):55s–59s. doi: 10.1016/s0753-3322(02)00213-5. [DOI] [PubMed] [Google Scholar]
- 17.Belfiore A., La Rosa G.L. Fine needle aspiration biopsy of the thyroid. Endocrinol Metab Clin North Am. 2001;30(2):361–400. doi: 10.1016/s0889-8529(05)70191-2. [DOI] [PubMed] [Google Scholar]
- 18.Amrikachi M., Ramzy I., Rubenfeld S., Wheeler T.M. Accuracy of fine needle aspiration of thyroid: a review of 6226 cases and correlation with surgical or clinical outcome. Arch Pathol Lab Med. 2001;125(4):484–488. doi: 10.5858/2001-125-0484-AOFNAO. [DOI] [PubMed] [Google Scholar]
- 19.Calliada F., Pallavicini D., Pasamonti M. Topical role and future perspectives of sonographic contrast agents in the differential diagnosis of solid thyroid lesions. Rays. 2000;25(2):191–197. [PubMed] [Google Scholar]
- 20.Spiezia S., Farina R., Cerbone G. Analysis of color Doppler signal intensity variation after levovist injection: a new approach to the diagnosis of thyroid nodules. J Ultrasound Med. 2001;20(3):223–231. doi: 10.7863/jum.2001.20.3.223. [DOI] [PubMed] [Google Scholar]
- 21.Appetecchia M., Bacaro D., Brigida R. Second generation ultrasonographic contrast agents in the diagnosis of neoplastic thyroid nodules. J Exp Clin Cancer Res. 2006;25(3):325–330. [PubMed] [Google Scholar]
- 22.Argalia G., De Bernardis S., Mariani D. Ultrasonographic contrast agent: evaluation of time-intensity curves in the characterisation of solitary thyroid nodules. Radiol Med (Torino) 2002;103(4):407–413. [PubMed] [Google Scholar]
- 23.Bartolotta T.V., Midiri M., Galia M. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: initial results. Eur Radiol. 2006;16(10):2234–2241. doi: 10.1007/s00330-006-0229-y. [DOI] [PubMed] [Google Scholar]
- 24.Lyshchik A., Higashi T., Asato R. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202–211. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K., Fukunari N., Igarashi Y. Evaluation of thyroid malignancy using real-time tissue elastography. Eur Radiol. 2006;16(Suppl. 1):547. ECR 2006-D – Satellite Symposia. [Google Scholar]
- 26.Rago T., Santini F., Scutari M., Pinchera A., Vitti P. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92(4):2917–2922. doi: 10.1210/jc.2007-0641. [DOI] [PubMed] [Google Scholar]


