Abstract
Background
Histone deacetylases (HDACs) regulate multiple developmental processes and cellular functions. However, their roles in blood development have not been determined, and in Xenopus laevis, a specific function for HDACs has yet to be identified. Here, we employed the class I selective HDAC inhibitor, valproic acid (VPA), to show that HDAC activity is required for primitive hematopoiesis.
Results
VPA treatment during gastrulation resulted in a complete absence of red blood cells (RBCs) in Xenopus tadpoles, but did not affect development of other mesodermal tissues, including myeloid and endothelial lineages. These effects of VPA were mimicked by Trichostatin A (TSA), a well-established pan-HDAC inhibitor, but not by valpromide, which is structurally similar to VPA but does not inhibit HDACs. VPA also caused a marked, dose-dependent loss of primitive erythroid progenitors in mouse yolk sac explants at clinically relevant concentrations. In addition, VPA treatment inhibited erythropoietic development downstream of bmp4 and gata1 in Xenopus ectodermal explants.
Conclusions
These findings suggest an important role for class I HDACs in primitive hematopoiesis. Our work also demonstrates that specific developmental defects associated with exposure to VPA, a significant teratogen in humans, arise through inhibition of class I HDACs.
INTRODUCTION
During vertebrate development a primitive form of hematopoiesis emerges from mesoderm and is subsequently superseded by definitive hematopoiesis. In Xenopus laevis, primitive hematopoiesis begins in the ventral blood islands (VBI), analogous to the mammalian yolk sac and the zebrafish intermediate cell mass (Paik and Zon; Ciau-Uitz et al., 2000). The VBI is derived from two regions of presumptive mesoderm that contribute to anterior and posterior domains (aVBI and pVBI) (Ciau-Uitz et al., 2000). The aVBI originates from mesodermal cells of the dorsal marginal zone that migrate during gastrulation to a ventral-anterior position. The aVBI gives rise predominantly to myeloid cells, but also erythroid and endothelial lineages (Smith et al., 2002; Walmsley et al., 2002). Precursors of the aVBI are detected during late neurulation and co-express several hematopoietic and angiogenic markers (Walmsley et al., 2002). The pVBI arises from ventral marginal zone mesoderm and is the primary source for primitive erythrocytes, although it also gives rise to a small population of leukocytes and lymphoid cells (Ciau-Uitz et al.; Ciau-Uitz et al., 2000). Both VBI compartments are specified during gastrulation, although markers of hematopoietic differentiation are not detectable until early tailbud stages (Maéno et al., 1992; Maéno et al., 1994; Kumano et al., 1999; Walmsley et al., 2008).
In Xenopus laevis embryos, the definitive phase of blood development originates from the dorsal lateral plate (DLP) in tailbud stage embryos (Turpen, 1998). The DLP is analogous to the AGM (aorta-gonad-mesonephros) region in mammals, and the dorsal aorta in zebrafish. Cells from the DLP give rise to the adult hematopoietic stem cells (HSCs) that populate the Xenopus dorsal aorta (Turpen, 1998).
Primitive hematopoiesis in mammals is not well understood, and much of our understanding of this process comes from non-amniote vertebrate embryos such as Xenopus and zebrafish, in which early development is more readily observed. Studies in these systems have revealed that bone morphogenetic proteins (BMPs) are essential for primitive hematopoiesis; overexpression of bmp4 in Xenopus embryos results in expansion of the VBI, whereas inhibition of BMP signaling by dominant negative BMP receptors prevents blood formation (Paik and Zon; Maéno et al., 1996; Kumano et al., 1999; Walmsley et al., 2002). However, the mechanisms downstream of BMPs that drive hematopoiesis are not well understood. Gata1 is a transcription factor required for primitive erythropoiesis that is thought to function downstream of bmp4 (Zhang and Evans, 1996). Runx1 is also required for primitive hematopoiesis in Xenopus, but whether bmp4 regulates runx1 has not been addressed (Tracey et al., 1998).
Histone deacetylases (HDACs) comprise a family of multiple, highly conserved genes (reviewed in (Haberland et al., 2009b)). Among the zinc-dependent HDACs, HDACs 1, 2, 3, and 8 compose the group labeled as “class I”, and function mostly within the nucleus to regulate multiple target proteins, including acetylated histones. Class II (HDAC 4-7, 9, 10) and class IV (HDAC 11) HDACs are structurally more diverse, and function both in the cytoplasm and nucleus (Haberland et al., 2009b). Loss of function studies in mice have shown that global depletion of any of the class I hdac genes results in embryonic or perinatal lethality (Lagger et al., 2002; Montgomery et al., 2007; Montgomery et al., 2008; Haberland et al., 2009a). Although each of these enzymes is required for embryogenesis, conditional knockout studies in mice demonstrate that during later development, class I HDACs have more defined roles in organogenesis. In zebrafish, some recent studies have begun to address the role of individual HDACs in development (Cunliffe, 2004; Pillai et al., 2004; Yamaguchi et al., 2005; Farooq et al., 2008), but a specific role for HDAC function has not yet been reported in Xenopus. Moreover, what role, if any, HDACs play in Xenopus or mouse blood development has yet to be determined.
We have previously found that prolonged exposure to HDAC inhibitors causes multiple developmental defects in Xenopus and zebrafish (Gurvich et al., 2005) and proposed that HDACs are key targets of the human teratogen valproic acid (VPA), a widely prescribed antiepileptic and mood stabilizing drug that is also a potent HDAC inhibitor (Gottlicher et al., 2001; Phiel et al., 2001). However, the prolonged exposure to inhibitors and the pleiomorphic developmental defects observed in these prior studies made it difficult to distinguish primary from secondary mechanisms of teratogenesis.
Therefore, in the present work, we narrowed the window of exposure to the gastrula stage of development, and found that HDAC inhibitors markedly and specifically block primitive hematopoiesis, resulting in a complete absence of red blood cells from Xenopus embryos. This suggests an essential role for HDACs in the first wave of blood development. Furthermore, our pharmacological studies suggest that HDACs function downstream of bmp4 and gata1 signaling and are specifically required for primitive erythropoiesis. We also report that runx1 is activated by bmp4 in an HDAC-dependent manner. Consistent with this, restoring runx1 expression partially rescues VPA mediated hematopoietic defects. These findings demonstrate for the first time a critical role for class I HDACs in primitive hematopoiesis and also support the hypothesis that HDACs are key targets in VPA-mediated teratogenesis.
RESULTS
HDAC inhibition blocks primitive erythropoiesis
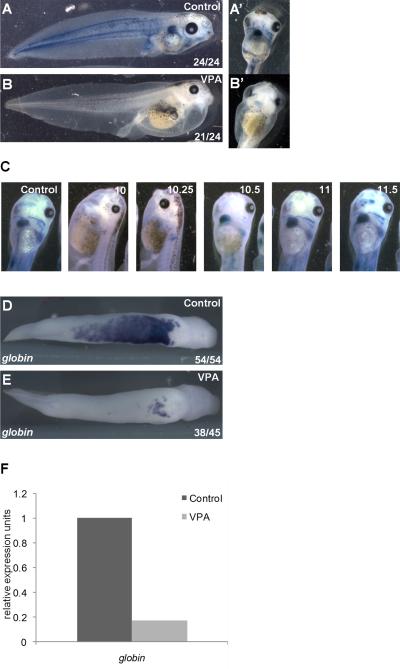
Prior studies using VPA and VPA analogs have suggested that the multiple developmental defects caused by VPA arise through inhibition of HDACs (Phiel et al., 2001; Gurvich et al., 2005). However, these development defects were pleiotropic, most likely because embryos were exposed to VPA through multiple developmental stages. To focus on a specific developmental process, exposure of Xenopus embryos to VPA was limited to the gastrulation stage (Nieuwkoop and Faber stage 10 to stage 12), when primitive blood is specified (Maéno et al., 1992; Maéno et al., 1994; Kumano et al., 1999). Visual inspection of stage 42 tadpoles, 72 hours after exposure to VPA, revealed that this pulse treatment of VPA blocked development of circulating erythrocytes despite normal establishment of the embryonic anterior-posterior and dorsal-ventral axes (Figure 1A, B). The lack of red blood cells (RBCs) was confirmed by staining embryos with benzidine, which marks hemoglobin (Figure 1A, B). As reported previously, VPA treated embryos displayed mild edema, likely due to heart looping defects caused by VPA-mediated HDAC inhibition (Kook et al., 2003; Gurvich et al., 2005). VPA-inhibition of RBC development was sensitive to developmental stage; initiation of VPA treatment an hour later at mid-gastrula (stage 10.5) was insufficient to block RBC development (Figure 1C). To confirm that VPA treatment resulted in a loss of erythrocytes, and not simply a loss of circulation, whole-mount in-situ hybridization (WISH) was performed to examine the expression of alpha T1 hemoglobin (globin) in VPA treated embryos. At late tailbud stages (stage 30), globin is normally expressed throughout the VBI in a characteristic V-shaped pattern in the ventral mesoderm (Figure 1D). Embryos exposed to VPA during gastrulation exhibited a pronounced reduction in globin expression (Figure 1E). These findings were confirmed by qRT-PCR, which revealed a five-fold reduction in globin expression (Figure 1F).
Figure 1. HDAC inhibition blocks primitive erythropoiesis in Xenopus laevis.
A) Circulating RBCs visualized in tadpoles (stage 43) by benzidine staining (blue) for hemoglobin. RBCs are also present in pronephros (arrow) and in heart (A’, ventral view). B) Treatment of gastrulating embryos (stage 10-12) with the class I HDAC inhibitor VPA blocks blood formation, indicated by benzidine staining (B’, ventral view). C) Numbers indicate stages during gastrulation at which VPA was administered; in all conditions, VPA was removed at the end of gastrulation (stage 12). Benzidine positive RBCs are readily visible within the heart and great vessels in controls and when VPA was added at stage 10.5 or later. D,E) Expression of the erythrocyte marker alpha-T1 hemoglobin in the VBI detected by WISH in tailbud embryos (stage 31). In VPA treated embryos (E), globin expression is dramatically reduced, with residual staining restricted to the aVBI. F) Embryos were treated with control buffer or VPA during the gastrula stage and globin expression in stage 28 embryos was assessed by quantitative RT-PCR.
HDAC activity contributes to the development of primitive erythrocyte progenitors
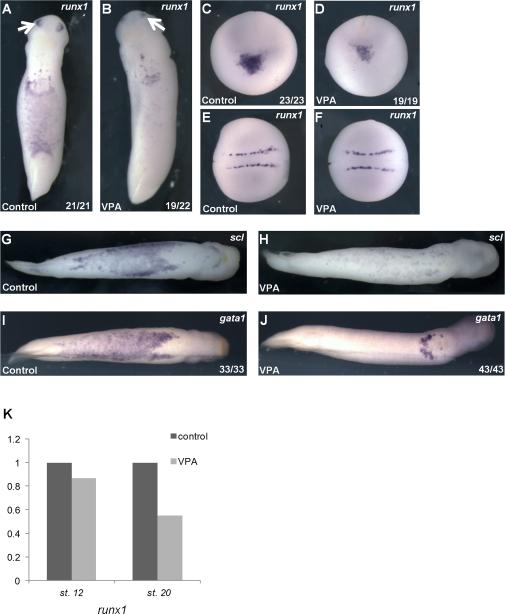
To determine whether loss of globin was due to aberrant differentiation or an earlier step in blood development, we examined the expression of runx1, scl, and gata1, markers of primitive erythroid progenitor cells at early tailbud stages (Tracey et al., 1998; Walmsley et al., 2008). VPA treatment reduced expression of all three markers in the VBI at early tailbud stages (stage 25), similar to the effect of VPA on globin expression at later tailbud stages (Figures 2 A-J, and 1D-F). These data suggest that loss of blood in VPA treated embryos may arise due to a defect in the development of erythroid progenitor cells or in the earlier specification of erythroid lineages. We therefore examined earlier expression of runx1. Late neurula stage embryos express runx1 in a ventral-anterior population of cells that co-express markers for erythroid, endothelial, and myeloid lineages (Walmsley et al., 2002). VPA treatment reduced runx1 expression in this mixed progenitor population (Figures 2C, D). Interestingly, VPA treatment did not affect runx1 expression in non-hematopoietic tissues such as neuronal Rohan-Beard cells and the olfactory placode (Figures 2A-F), suggesting a distinct role for HDACs in primitive hematopoiesis. Based on qRT-PCR analysis of intact embryos, which detects expression in non-hematopoietic as well as presumptive hematopoietic lineages, VPA treatment reduced Runx1 expression only slightly in late gastrulae (stage 12) but reduced Runx1 expression approximately 40% in neurula stage embryos (stage 18, Figure 2K). Because runx1 was only partially diminished, and cells within the aVBI that express runx1 are a mixed population consisting of progenitors for erythroid, myeloid and endothelial lineages, we speculated that VPA only affects a subset of these cells fated to become erythrocytes whereas the remaining runx1 positive cells may differentiate into other VBI derived tissues. This was supported by the observation that expression of the endothelial marker aplnr and the myeloid marker mpo were unchanged with VPA treatment, suggesting that HDAC inhibition interferes uniquely with development of the erythroid lineage (Figure 3Y-BB).
Figure 2. VPA inhibits the development of erythroid progenitors.
A) Primitive erythroid marker runx1 is expressed within the VBI and olfactory placode (arrows) in control early tailbud (stage 26) embryos. B) VPA reduced runx1 expression in the VBI. C) In control late neurulae (stage 19, anterior view, with dorsal oriented to the top), runx1 is expressed in precursors of the aVBI, which lie in a ventral-anterior domain at this stage. D) VPA markedly reduces runx1 expression within presumptive hematopoietic cells in neurula stage embryos (same orientation as panel C). E) and F) Dorsal view (anterior to the left)- Expression of runx1 in Rohan Beard cells in the same embryos as (C) and (D) is not affected by VPA. G-J) Ventral views, anterior to the right. VPA treatment (H) and (J) reduces expression of erythroid markers scl (G) and (H) and gata1 (I) and (J) in the VBI of tailbud embryos (stage 31). K) runx1 expression in whole embryos is reduced with VPA treatment at late neurula stage (stage 18), as determined by qRT-PCR (data represent the mean of 4 independent experiments, normalized to ODC).
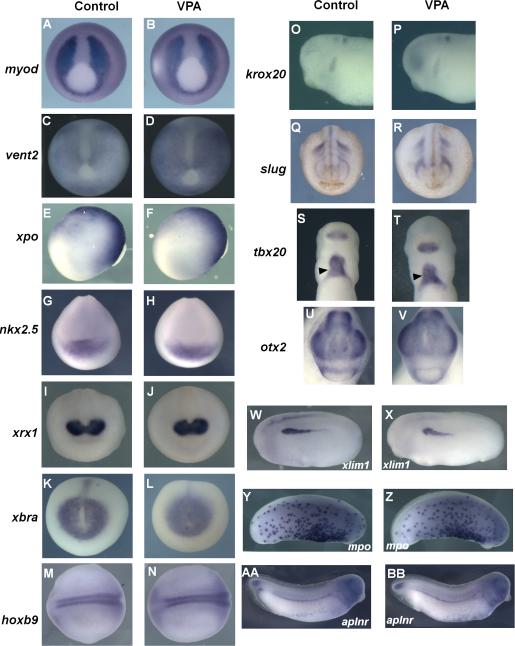
Figure 3. Marker analysis with VPA treatment.
Embryos were treated with buffer control (A,C,E,G, I, K, M,O,Q,S,U,W, Y, AA) or VPA during stage 10-12 (B,D,F,H,J,L,N,P,R,T,V,X, Z, BB) . Whole mount in situ hybridization performed for the indicated markers shows no change in expression with VPA treatment. myod (A,B), vent2 (C,D), nkx2.5 (G,H), xrx1 (I,J), xbra (K,L), slug (Q,R), tbx20 (S,T), otx2 (U,V)- ventral view. xpo (E,F),lim (W,X), krox20 (O,P)- lateral view, anterior to the left, hoxb9 (M,N); dorsal view, anterior to the left. Also unaffected by VPA treatment were VBI derived myeloid marker mpo (Y, Z) and vascular marker aplnr (AA, BB)-lateral view, anterior to the right. myod, vent2, xpo, xbra- late gastrula. nkx2.5, xrx1, slug, hoxb9- neurula stages. lim1, krox20, mpo, aplnr, tbx20, otx2- tailbud stages.
To test whether presumptive hematopoietic cells were re-specified to other mesodermal tissue types, expression of multiple mesodermal and ectodermal markers was examined, including xbra (pan-mesoderm), vent2 (ventral mesoderm), myod1 (dorsal mesoderm), xlim1 (lateral mesoderm), xpo (ventral- posterior), nkx2.5 and tbx20 (cardiac mesoderm), otx2, xrx, and krox20 (anterior neuro-ectoderm), hoxb9 (posterior neural), and slug (neural crest) (Figure 3). VPA treatment did not alter expression patterns for any of the markers examined, consistent with apparently normal tadpole morphology at later stages, indicating that exposure to VPA during the gastrula stage does not alter global anterior-posterior or dorsal-ventral patterning. Therefore, HDAC activity during gastrulation plays an important role in the development of primitive hematopoietic progenitors.
To determine whether VPA disrupts primitive erythropoiesis by either increasing apoptosis or reducing proliferation of hematopoietic progenitor cells, TUNEL and phospho-H3 staining were performed, respectively. No differences in cell death or proliferation were observed in the VBI between VPA-treated and untreated embryos at either late gastrula (stage 12) or tailbud (stage 30) stages (data not shown).
VPA inhibits hematopoietic differentiation downstream of BMP4 signaling
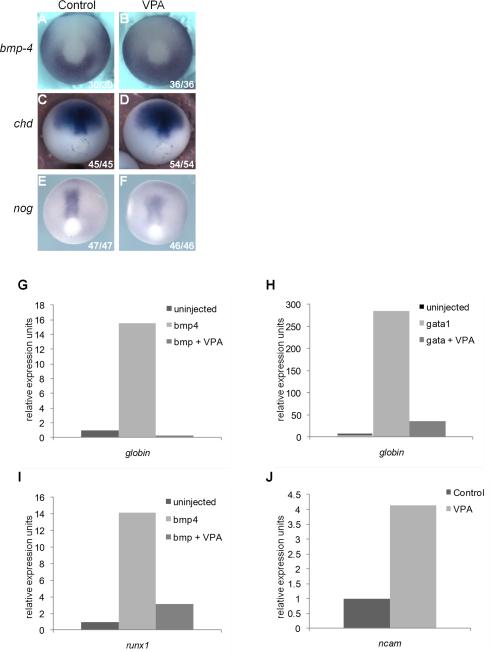
Primitive hematopoiesis in vertebrates requires BMP signaling, particularly Bmp4 (Hemmati-Brivanlou and Thomsen, 1995; Sadlon et al., 2004; Snyder et al., 2004). Thus VPA could impair hematopoiesis by either reducing bmp4 expression or enhancing expression of BMP antagonists, including noggin (nog) and chordin (chd), which are expressed in the gastrula and play critical roles in regulating dorsal-ventral patterning (Kumano et al., 1999). We therefore used WISH to examine expression of bmp4, nog, and chd in gastrula stage embryos. VPA treatment did not significantly alter expression of these genes, indicating that loss of RBCs does not arise through misregulated expression of bmp4 or its inhibitors (Figure 4A-F).
Figure 4. HDAC inhibition prevents hematopoietic induction in ectodermal explants downstream of bmp4 and gata1.
A-F) Expression of bmp-4 (A, B), chordin (C,D), and noggin (E,F) in late gastrula stage embryos is unaffected by VPA (B, D, F); orientation: posterior view, dorsal to the top. G-I) Quantitative RT-PCR of ectodermal explants harvested at tailbud stages (stage 28-31). G) Injection of bmp4 mRNA (2 ng) induced globin expression and VPA inhibited this effect. H) Injection gata1 mRNA (200pg) induced globin expression and VPA treatment inhibited this effect. I) Injection of bmp4 induced expression of runx1 and VPA blocked this effect. J) VPA upregulated endogenous expression of ncam in uninjected explants.
To test whether HDACs regulate blood development downstream of BMP signaling, we examined globin induction in ectodermal explants in response to bmp4. As reported previously, over-expression of bmp4 induces blood development in ectodermal explants, as assessed by induction of globin (Hemmati-Brivanlou and Thomsen, 1995) (Figure 4G). However, when explants expressing bmp4 were also exposed to VPA, globin expression was markedly reduced (Figure 4G). This suggests that HDAC activity is required downstream of bmp4 to induce blood formation. Bmp4 also induced runx1 expression in ectodermal explants, and this too was blocked by VPA treatment (Figure 4I). Interestingly, expression of another BMP induced mesodermal marker, xbra, was not affected by VPA treatment (data not shown), indicating that VPA is not a direct or proximal inhibitor of BMP signaling, but rather acts at a downstream step in BMP-dependent blood development.
Expression of the transcription factor gata1 is regulated by BMPs in Xenopus and zebrafish (Zhang and Evans, 1996; Huber et al., 1998; Lieschke et al., 2002) and is required for hematopoiesis in many vertebrates. Over-expression of gata1 induces blood development in Xenopus ectodermal explants (Xu et al., 1997) (Figure 4H). To determine whether HDACs function downstream of gata1 in blood development, gata1 RNA was injected into ectodermal explants and globin expression was assessed by qRT-PCR. Over-expression of gata1 induced globin expression, as expected, and this was blocked by VPA treatment (Figure 4H). We observe that gata1 expression is reduced in the VBI upon VPA treatment (Figure 2I, J). This is likely an indirect effect of VPA on gata1, due to a global reduction of erythroid progenitors in the tailbud embryo. In contrast, VPA treatment of neurula stage (stage 20) embryos showed minimal effect on gata1 expression (data not shown), implying that VPA functions downstream of gata1. However, further investigation is necessary to fully understand the relationship between VPA and gata1, and we cannot yet eliminate the possibility that VPA may also act upstream of gata1.
Endogenous BMP signaling maintains epidermal fates and suppresses neural induction in ectodermal cells in Xenopus, whereas inhibition of endogenous BMP signaling induces neural markers in ectodermal explants. Furthermore, overexpression of either bmp4 or gata1 can block induction of the neural marker ncam (Xu et al., 1997; Shibata et al., 1998). Consistent with its ability to inhibit bmp4 and gata1-induced hematopoiesis, VPA induced expression of ncam in ectodermal explants, indicating that HDAC inhibition disrupts endogenous signaling downstream of bmp4 and gata1 (Figure 4J). However, VPA did not neuralize explants, as indicated by failure to induce other neural markers such as sox2 and n-tubulin (data not shown). Together, these results suggest that HDAC activity is required for bmp4 signaling downstream of gata1.
VPA inhibits primitive hematopoiesis in mouse embryos
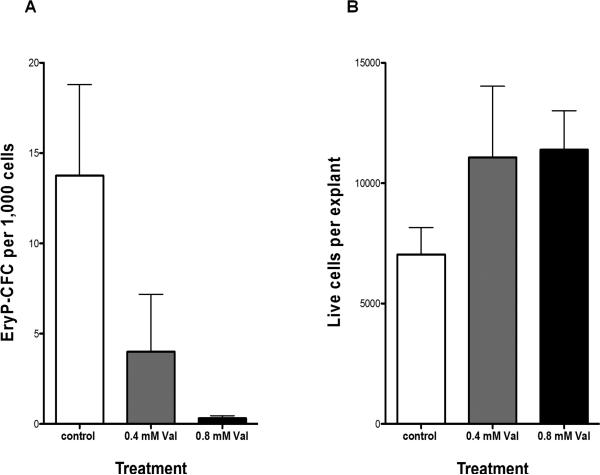
To test whether HDACs contribute to primitive erythropoiesis in mammals, mouse embryos were harvested at the neural plate stage (E7.5), which corresponds to mid-gastrulation, and isolated yolk sacs were cultured as explants in control medium or medium with VPA. After 48 hours, the explants were trypsinized and single cells were plated in methylcellulose to quantify the number of primitive erythroid progenitors (EryP-CFC) (Palis et al., 1995). VPA (at clinically relevant concentrations of 0.4 and 0.8mM) causes a dose-dependent loss of EryP-CFC (Figure 5) without affecting cell viability. Thus, VPA impairs primitive erythropoiesis in mammals as well as in amphibians.
Figure 5. VPA inhibits primitive erythroid progenitor emergence in mouse yolk sac explants.
A) EryP-CFC (mean ± SEM) per 1000 yolk sac explant cells cultured in vitro for 48 hours with 0, 0.4, and 0.8 mM VPA. B) Number of live cells (mean ± SEM) per yolk sac explant with 0, 0.4, and 0.8 mM VPA.
Class I HDACs are required for primitive hematopoiesis
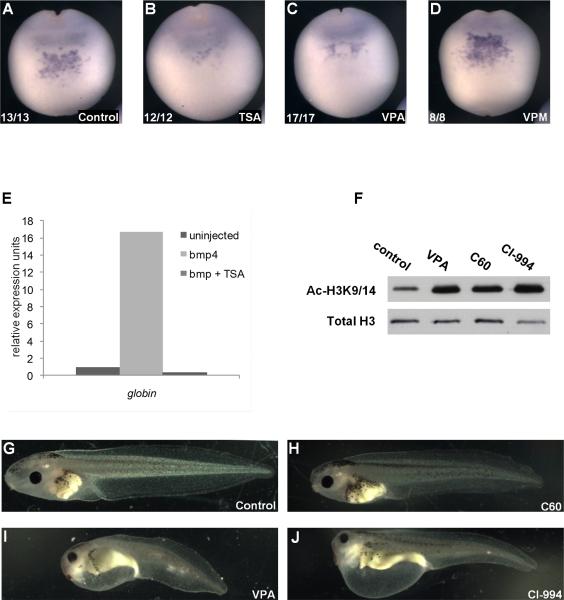
VPA is an established HDAC inhibitor, but also has activities that are independent of HDACs, based on pharmacological analyses with VPA structural analogs (Nau et al., 1991; Gurvich et al., 2004). To confirm that the hematopoietic effects observed with VPA were indeed a result of HDAC inhibition, a structurally unrelated pan-HDAC inhibitor, trichostatin A (TSA) was investigated for its potential to disrupt blood development. TSA reduced runx1 expression in the VBI in a similar pattern to VPA and inhibited globin expression in bmp4 expressing ectodermal explants (Figure 6A-C, E). Alternatively, Valpromide (VPM), an amide derivative of VPA that does not inhibit class I or class II HDACs (Gurvich et al., 2004), did not affect runx1 expression (Figure 6D). This is in agreement with previous work demonstrating that global developmental defects caused by long term exposure to VPA can be recapitulated with TSA, but not VPM (Gurvich et al., 2005). We previously reported that VPA inhibits class I and IIa HDACs, based on overexpression/immunoprecipitation assays (Gurvich et al., 2004); however, the class IIa assays may have been confounded by coimmunoprecipitation with class I HDACs. More recent work using recombinant HDACs and optimized substrates has revealed that VPA potently and selectively inhibits class I HDACs and spares class II HDACs (Fass et al., 2011), suggesting that the effect of VPA on blood development is primarily due to inhibition of one or more class I HDACs. Furthermore, the class II selective inhibitor valpropylhydroxamic acid (VAHA), a hydroxamic acid derivative of VPA (inhibition of HDAC6 > HDAC5, 7, 9 > HDAC4 > HDAC1,2,3), does not interfere with blood development and has no apparent effect on overall development (Fass et al., 2011). These pharmacological data provide strong support for the hypothesis that class I HDACs play an important role in primitive hematopoiesis.
Figure 6. VPA mediated developmental defects are due to inhibition of class I HDACs.
A) runx1 expression in the anterior ventral region of late neurula stage control embryos (stage 18). B, C) TSA (B) and VPA (C) suppress runx1 expression. D) The inactive VPA analog VPM does not inhibit runx1 expression. E) bmp4 mRNA (2ng) induction of globin expression in ectodermal explants is blocked by TSA (bmp+TSA). F) VPA (class I HDAC inhibitor), CI-994 (selective inhibitor of HDACs 1, 2, and 3), and C60 (IC50 for HDACs 1 and 2>>HDAC3) increase global acetylation of histone H3 at lysine 9/14 (H3K9/14Ac) at 24 hours post treatment, as demonstrated by western blotting. Bottom panel: total H3 (loading control). G-J) Lateral view of stage 45 tadpoles treated with vehicle (DMSO) control (G), C60 (H), VPA (I), and CI-994 (J) from late neurula (stage 19) to tailbud (stage 31) stages.
To test whether inhibition of a specific class I HDAC could account for the developmental phenotypes observed with VPA, we utilized two class I selective HDAC inhibitors that differ in their activities against HDAC3. CI-994 inhibits HDACs 1, 2, and 3 with similar IC50s whereas Compound-60 (C60) preferentially inhibits HDAC1 and 2, and is significantly less potent against HDAC3 (Methot et al., 2008). C60 had no apparent effect on development, whereas CI-994 closely mimicked the developmental effects of VPA (Figure 6G-J). Both compounds inhibited overall HDAC activity in vivo, as assessed by increased global histone acetylation in the embryo (Figure 6F). In contrast to VPA, which causes global acetylation changes after only 1.5 hours of exposure, CI-994 and C60 require approximately 4.5 hours of exposure before showing any effect on global histone acetylation (data not shown). However, RBC specification occurs during gastrulation, and is completed in up to four hours. Therefore, it was not possible to assay the effects of CI-994 and C60 on primitive hematopoiesis. However, the ability of CI-994, but not C60, to induce similar developmental defects to VPA suggests that HDAC3 may be a major target responsible for VPA-induced developmental defects.
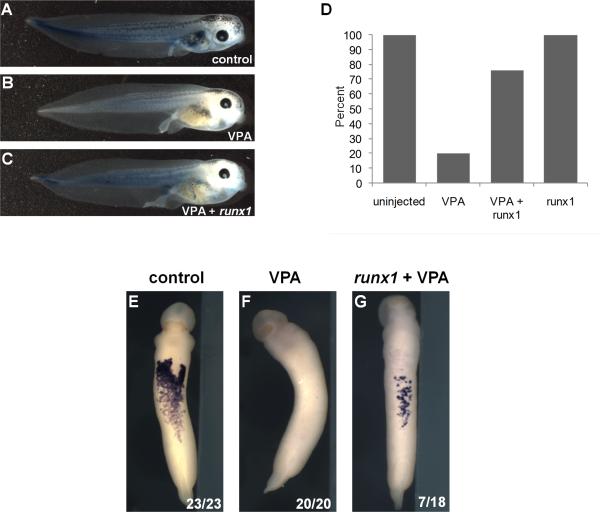
Because runx1 is required for primitive erythropoiesis in Xenopus (Tracey et al., 1998) and is suppressed by VPA, we tested whether restoring runx1 expression could rescue blood development in VPA-treated embryos. Overexpression of runx1 partially rescues erythropoiesis in VPA-treated tadpoles, as assessed by benzidine staining (Figure 7C,D) and partial recovery of globin expression in the ventral blood islands by WISH (Figure 7E-G). Together, these data suggest that class I HDACs function downstream of bmp4, between gata1 and runx1, to activate erythropoiesis.
Figure 7. Runx1 expression rescues VPA-mediated hematopoietic defects.
A) Benzidine staining of hemoglobin (blue) in untreated stage 43 tadpole. B) Treatment with VPA inhibits RBC development. C) Injection of runx1 mRNA partially recovered erythrocyte development in VPA treated embryos. D) Percentage of benzidine positive tadpoles for uninjected (n=20), VPA-treated (n=25), VPA-treated + runx1 (n=21), and runx1 expression alone (n=21). This experiment was repeated with 4 separate clutches of embryos and the table shows data from all 4 experiments. Intensity of benzidine stain was reduced in VPA and VPA+runx1 groups (as shown in panel C). E-G) Expression of globin was detected by WISH in all (23/23) control tadpoles, 0/20 VPA-treated tadpoles, and 7/18 VPA-treated, runx1-expressing tadpoles.
DISCUSSION
In the present study, we show that valproic acid, a potent human teratogen, markedly impairs primitive hematopoiesis in both Xenopus laevis and mouse embryos. We further identify class I HDACs as primary molecular targets that mediate this effect of VPA. Remarkably, restricting HDAC inhibition to the gastrula stage blocks primitive erythropoiesis despite normal germ layer specification, axis formation, and morphogenesis of other embryonic structures. Furthermore, inhibition of HDACs during gastrulation blocks primitive erythropoiesis in Xenopus without disrupting endothelial or myeloid lineages, which are also derived from the VBI. Consistent with these observations in Xenopus, VPA exposure blocked the expansion of primitive erythroid progenitors in yolk sac explants from mice without affecting overall cell viability. Finally, we identify an important regulatory role for class I HDACs in the development of hematopoietic progenitors through interaction with the BMP signaling pathway. While it is known that BMP signaling is responsible for primitive hematopoiesis through the activation of transcription factors such as gata1 and gata2, the targets and precise function of these transcription factors in early hematopoietic development is complex and poorly understood. We show here for the first time that class I HDACs are involved in the initiation of the hematopoietic program by bmp4 and gata1 and are therefore required for blood formation.
Recent work in zebrafish has confirmed the importance of HDAC1/2 for primitive hematopoiesis, demonstrating that nucleosome remodeling and deacetylase (NuRD) complexes, which include HDACs 1 and 2, are essential for the initiation of erythropoiesis (Li et al., 2009). The authors concluded that NuRD complexes act upstream of scl and gata1. Our work, however, demonstrates that HDAC activity is required downstream of gata1. These two studies are complementary, and suggest a regulatory network that requires multiple HDACs at different stages during hematopoiesis.
Our studies demonstrate a requirement for class I HDACs downstream of bmp4 and gata1 in primitive hematopoiesis, as inhibition of HDACs prevents erythroid development induced by bmp4 and gata1 expression in ectodermal explants. Furthermore, while the relationship between runx1 and bmp4 has been unclear, our experiments demonstrate that bmp4 can induce runx1 expression, and this requires HDAC activity, as runx1 induction is blocked by VPA treatment. In addition, reintroduction of runx1 mRNA partially rescues erythropoiesis in VPA treated embryos, suggesting a requirement for class I HDACs upstream of runx1. Taken together, these observations suggest that HDACs function downstream of bmp4 and gata1 to regulate both primitive erythropoiesis and expression of runx1, although it is likely that additional factors are regulated by HDACs in this context.
Several mechanisms could account for HDAC function during erythropoiesis. HDACs generally function in repressor complexes, and HDAC inhibition could de-repress unknown factors that repress erythroid development. Specifically, GATA1 functions as a repressor as well as an activator of gene expression in definitive hematopoiesis [51], and class I HDACs may therefore contribute to an essential repressive function of GATA1 in primitive hematopoiesis. Alternatively, HDACs also play activating as well as repressive roles in gene expression (Nusinzon and Horvath, 2005; Haberland et al., 2009b) and could thus function either with or downstream of GATA1 to regulate chromatin structure and expression of target genes required for hematopoiesis. A third possibility is that HDACs could modulate the acetylation state of GATA1 protein or other transcription factors involved in hematopoiesis. Acetylation of human GATA1 has been proposed to regulate chromatin binding and GATA1-dependent transcription (Boyes et al., 1998; Lamonica et al., 2006), and HDAC inhibition in hematopoietic cell lines causes degradation of GATA1 protein due to hyperacetylation (Hernandez-Hernandez et al., 2006). Thus inhibition of class I HDACs could lead to persistent acetylation and subsequent degradation of GATA1 protein or other modulation of GATA1 function, preventing hematopoietic development.
VPA is also associated with multiple, potentially devastating birth defects in humans, including spina bifida and other neural tube closure defects, craniofacial defects including cleft lip and palate, and defects in limb, cardiac, and genitourinary development (Jentink et al., 2010); VPA has thus been extensively studied as a model teratogen in rodents, Xenopus, and zebrafish (Narotsky et al., 1994; Menegola et al., 1996; Phiel et al., 2001; Gurvich et al., 2005). Prolonged exposure to VPA causes multiple developmental defects in Xenopus, including defects in anterior development, shortened anterior-posterior axis, cardiac looping defects, and pigment defects. Class I HDACs, including hdac1, 2, and 3, are expressed in early Xenopus embryos (hdac1 and hdac3 are readily detected from blastula to neurula stages, whereas hdac2 drops precipitously during gastrulation and remains low during neurula stages; data not shown) and thus are highly plausible targets of VPA. Additional studies in zebrafish with hdac1 mutants and hdac3 knockdown reveal that loss of function of either class I hdac replicates many of the effects observed with long term VPA exposure, including disruption of heart looping, hematopoiesis, craniofacial development, anterior-posterior axis formation, and liver and pancreas development (Cunliffe, 2004; Pillai et al., 2004; Gurvich et al., 2005; Yamaguchi et al., 2005; Farooq et al., 2008). The similar morphological defects observed in Xenopus and zebrafish with hdac loss of function, VPA, and pharmacologically distinct HDAC inhibitors provide compelling support for the hypothesis that inhibition of HDACs is a primary mechanism for VPA-mediated teratogenesis. Our findings in mouse embryos suggest that exposure to VPA during the first trimester could also affect primitive hematopoiesis in mammals, including humans. Reduced HDAC function could also explain spontaneous birth defects that morphologically resemble VPA-induced developmental defects in humans.
EXPERIMENTAL PROCEDURES
Embryo manipulations
Xenopus husbandry and egg procurement was carried out in accordance with the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC approved protocols #260500 (approved 5/9/2007-5/9/2010) and #800878 (approved 2/28/2008-2/18/2011). Embryos were obtained, cultured, and microinjected as described previously (Sive et al., 2000). Embryos were staged according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1994). Capped mRNAs were produced by in vitro transcription using the mMessage mMachine Kit (Ambion). Each injected blastomere received 10 nl of RNA. Embryos were injected either equatorially (for whole embryo experiments) or in the animal pole (for explant experiments) in each blastomere of 2-cell stage embryos. Ectodermal explant assays were performed as described previously (Sive et al., 2000). Explants were excised using a Gastromaster tip at st. 8, cultured until tailbud stages (st. 28-31), and collected for quantitative RT-PCR. Drug treatments were performed from stage 10 to stage 12 unless otherwise noted. Final concentrations used in each treatment were as follows: 2mM valproate (Sigma); 100 nM trichostatin A (Sigma); 2mM valpromide (gift from Katwijk Chemie B.V.); 2mM VAHA; 250 uM for each Compound60 and CI995.
Mice were used in accordance with the guidelines of the University of Rochester School of Medicine and Dentistry Institutional Animal Care and Use Committee (IACUC approved protocol # 2007-027R 100636 (approved 5/9/2007-5/9/2012). Timed pregnant ICR mice (Taconic Farms) were killed by CO2 inhalation at day 7.5 of gestation, and neural plate stage embryos were dissected into yolk sac and embryo proper fractions as previously described (Palis et al., 1995). Individual yolk sacs were cultured in 100 μL of Iscove modified Dulbecco medium supplemented with 15% serum replacement (Invitrogen), 5% plasma-derived serum (Antech), 16% BIT (Stem Cell Technologies), 50ug/ml ascorbic acid (Sigma), 0.5% Protein Free Hybridoma Medium II, (Invitrogen), 2mM glutamine (Invitrogen), 0.1mM monothioglycerol, 2 U/mL erythropoietin, and 100 ng/mL stem cell factor in a 96-well plate for 48 hours in 5% CO2, room air, 37°C. VPA at 0, 0.4, and 0.8 mM was added to similarly staged individual explants at the start of the culture. At the end of the culture period, each yolk sac explant was trypsinized into single cells, and total cell number and cell viability were quantified. Eighty percent of the explanted cells were plated in methylcellulose supplemented with 10% plasma-derived serum, 5% protein-free hybridoma media II (Gibco Invitrogen), 2mM glutamine, 2 U/mL erythropoietin, 100 ng/mL stem cell factor, 10 ng/mL interleukin-3 (IL-3), 10 ng/mL IL-6, and 5 ng/mL granulocyte-macrophage colony-stimulating factor, 0.1mM monothioglycerol and were cultured in 5% CO2, room air, 37°C. Primitive erythroid and macrophage colonies were quantified at 5 and 7 days of culture, respectively, as previously described (Palis and Koniski, 2005).
In-situ hybridization, benzidine staining, and immunoblotting
Anti-sense probes using Digoxygenin-UTP (Roche) were synthesized using the Megascript Kit (Ambion). Plasmids used in these studies were kind gifts from the following colleagues: we thank Jean-Pierre Saint-Jeannet for Xlim1 and Xpo, Paul Krieg for aplnr and nkx2.5, Richard Harland for noggin and Hoxb9, Paul Mead for gata1, Eddy DeRobertis for chd, Frank Conlon for Tbx20, Christoph Niehrs for Vent2, and Monica Vetter for Xrx. Whole-mount in situ hybridization was performed as described previously (Harland, 1991). Benzidine staining was performed as described on http://www.xenbase.org/xenwiki/index.php/Protocols. Color development was allowed to proceed for 3 minutes. Histone isolation was performed as described previously, with little modification (Gurvich et al., 2004). Briefly, 20 embryos were lysed, acid extracted overnight, and precipitated with trichloroacetic acid. 0.5 embryo equivalent was used for immunodetection by anti-H3acK9/14 antibody (Millipore) and anti-H3 antibody (Cell Signaling). Immunoblotting was performed as described previously (Fass et al., 2011). 10 embryos were lysed; 0.5 or 1 embryo equivalent was detected with anti-HDAC3 (Cell Signaling) or anti-HDAC1/2 (Millipore). Ponceau S (Sigma) staining was performed to ensure equal protein loading.
RNA quantitation
Quantitative RT-PCR (qRT-PCR) was performed as described previously (Blythe et al.). Primers for these studies included: Alpha globin T3: forward: CATGCCTACAACCTGAGAGTG: reverse:GAATTTGTCCCAAGCAGCAT. Ornithine decarboxylase (ODC): forward:GATCATGCACATGTCAAGCC; reverse:TCTACGATACGATCCAGCCC. Runx1: forward:CATATCTCCAGGAAGGGCAA; reverse:AATGCCAACTCGAGGATCAC. Ncam: forward:GCTGTGGCTAGTGGGAAAGC; reverse:TGTTTCCTCTGGCTTAGAGTTTT. For each experiment, individual samples were analyzed in triplicate. Data were analyzed by first normalizing to the house-keeping gene ODC as loading control, and then calculating fold change compared to the indicated control condition using the ΔΔC(t) method (reviewed in (Taneyhill and Adams, 2008)). Embryonic cDNA was synthesized using oligo(dT)15mer primers as described (Yang et al., 2002).
Supplementary Material
Bullet points.
Class I Histone Deacetylase (HDAC) activity contributes to primitive erythropoiesis in Xenopus and mouse.
Exposure to valproic acid or other class I HDAC inhibitors specifically during gastrulation blocks primitive erythropoiesis.
HDAC activity contributes to an early step in primitive erythropoiesis.
Class I HDACs function downstream of BMP and Gata1
ACKNOWLEDGMENTS
We thank Nancy Speck, Daniel Kessler, Michael May, Judy Meinkoth, and the members of the Klein Lab for their insights and encouragement. We also thank Mitch Weiss, Gerd Blobel, and Wei Tong for insightful comments. This work was supported by the following NIH grants: 1R01GM076621 (Klein), 1R01HL110806-01 (Klein); and R01DK09361 (Palis). In addition, RS was supported by the Cancer Pharmacology Training Grant R25 CA101871-07.
Funding: NIH: 1R01GM076621 (Klein), 1R01HL110806-01 (Klein); R25 CA101871-07 (Shah); R01DK09361 (Palis)
REFERENCES
- Blythe SA, Cha SW, Tadjuidje E, Heasman J, Klein PS. beta-Catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev Cell. 19:220–231. doi: 10.1016/j.devcel.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A, Liu F, Patient R. Genetic control of hematopoietic development in Xenopus and zebrafish. Int J Dev Biol. 54:1139–1149. doi: 10.1387/ijdb.093055ac. [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Cunliffe VT. Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development. 2004;131:2983–2995. doi: 10.1242/dev.01166. [DOI] [PubMed] [Google Scholar]
- Farooq M, Sulochana KN, Pan X, To J, Sheng D, Gong Z, Ge R. Histone deacetylase 3 (hdac3) is specifically required for liver development in zebrafish. Dev Biol. 2008;317:336–353. doi: 10.1016/j.ydbio.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Fass DM, Shah R, Ghosh B, Hennig K, Norton S, Zhao WN, Reis SA, Klein PS, Mazitschek R, Maglathlin RL, Lewis TA, Haggarty SJ. Effect of Inhibiting Histone Deacetylase with Short-Chain Carboxylic Acids and Their Hydroxamic Acid Analogs on Vertebrate Development and Neuronal Chromatin. ACS Med Chem Lett. 2011;2:39–42. doi: 10.1021/ml1001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich N, Berman MG, Wittner BS, Gentleman RC, Klein PS, Green JB. Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 2005;19:1166–1168. doi: 10.1096/fj.04-3425fje. [DOI] [PubMed] [Google Scholar]
- Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64:1079–1086. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- Haberland M, Mokalled MH, Montgomery RL, Olson EN. Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev. 2009a;23:1625–1630. doi: 10.1101/gad.1809209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009b;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Thomsen GH. Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4. Dev Genet. 1995;17:78–89. doi: 10.1002/dvg.1020170109. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hernandez A, Ray P, Litos G, Ciro M, Ottolenghi S, Beug H, Boyes J. Acetylation and MAPK phosphorylation cooperate to regulate the degradation of active GATA-1. EMBO J. 2006;25:3264–3274. doi: 10.1038/sj.emboj.7601228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TL, Zhou Y, Mead PE, Zon LI. Cooperative effects of growth factors involved in the induction of hematopoietic mesoderm. Blood. 1998;92:4128–4137. [PubMed] [Google Scholar]
- Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, de Jong-van den Berg LT. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362:2185–2193. doi: 10.1056/NEJMoa0907328. [DOI] [PubMed] [Google Scholar]
- Kook H, Lepore JJ, Gitler AD, Lu MM, Wing-Man Yung W, Mackay J, Zhou R, Ferrari V, Gruber P, Epstein JA. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J Clin Invest. 2003;112:863–871. doi: 10.1172/JCI19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano G, Belluzzi L, Smith WC. Spatial and temporal properties of ventral blood island induction in Xenopus laevis. Development. 1999;126:5327–5337. doi: 10.1242/dev.126.23.5327. [DOI] [PubMed] [Google Scholar]
- Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 2002;21:2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamonica JM, Vakoc CR, Blobel GA. Acetylation of GATA-1 is required for chromatin occupancy. Blood. 2006;108:3736–3738. doi: 10.1182/blood-2006-07-032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jia S, Wang S, Wang Y, Meng A. Mta3-NuRD complex is a master regulator for initiation of primitive hematopoiesis in vertebrate embryos. Blood. 2009;114:5464–5472. doi: 10.1182/blood-2009-06-227777. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Oates AC, Paw BH, Thompson MA, Hall NE, Ward AC, Ho RK, Zon LI, Layton JE. Zebrafish SPI-1 (PU.1) marks a site of myeloid development independent of primitive erythropoiesis: implications for axial patterning. Dev Biol. 2002;246:274–295. doi: 10.1006/dbio.2002.0657. [DOI] [PubMed] [Google Scholar]
- Maéno M, Ong RC, Kung H-f. Positive and Negative Regulation of the Differentiation of Ventral Mesoderm for Erythrocytes in Xenopus laevis. Development, Growth & Differentiation. 1992;34:567–577. doi: 10.1111/j.1440-169X.1992.00567.x. [DOI] [PubMed] [Google Scholar]
- Maéno M, Ong RC, Xue Y, Nishimatsu S, Ueno N, Kung HF. Regulation of primary erythropoiesis in the ventral mesoderm of Xenopus gastrula embryo: evidence for the expression of a stimulatory factor(s) in animal pole tissue. Dev Biol. 1994;161:522–529. doi: 10.1006/dbio.1994.1050. [DOI] [PubMed] [Google Scholar]
- Maéno M, Mead PE, Kelley C, Xu RH, Kung HF, Suzuki A, Ueno N, Zon LI. The role of BMP-4 and GATA-2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis. Blood. 1996;88:1965–1972. [PubMed] [Google Scholar]
- Menegola E, Broccia ML, Nau H, Prati M, Ricolfi R, Giavini E. Teratogenic effects of sodium valproate in mice and rats at midgestation and at term. Teratog Carcinog Mutagen. 1996;16:97–108. doi: 10.1002/(SICI)1520-6866(1996)16:2<97::AID-TCM4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Methot JL, Chakravarty PK, Chenard M, Close J, Cruz JC, Dahlberg WK, Fleming J, Hamblett CL, Hamill JE, Harrington P, Harsch A, Heidebrecht R, Hughes B, Jung J, Kenific CM, Kral AM, Meinke PT, Middleton RE, Ozerova N, Sloman DL, Stanton MG, Szewczak AA, Tyagarajan S, Witter DJ, Secrist JP, Miller TA. Exploration of the internal cavity of histone deacetylase (HDAC) with selective HDAC1/HDAC2 inhibitors (SHI-1:2). Bioorg Med Chem Lett. 2008;18:973–978. doi: 10.1016/j.bmcl.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Montgomery RL, Davis CA, Potthoff MJ, Haberland M, Fielitz J, Qi X, Hill JA, Richardson JA, Olson EN. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21:1790–1802. doi: 10.1101/gad.1563807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM, Richardson JA, Bassel-Duby R, Olson EN. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest. 2008;118:3588–3597. doi: 10.1172/JCI35847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narotsky MG, Francis EZ, Kavlock RJ. Developmental toxicity and structure-activity relationships of aliphatic acids, including dose-response assessment of valproic acid in mice and rats. Fundam Appl Toxicol. 1994;22:251–265. doi: 10.1006/faat.1994.1029. [DOI] [PubMed] [Google Scholar]
- Nau H, Hauck RS, Ehlers K. Valproic acid-induced neural tube defects in mouse and human: aspects of chirality, alternative drug development, pharmacokinetics and possible mechanisms. Pharmacol Toxicol. 1991;69:310–321. doi: 10.1111/j.1600-0773.1991.tb01303.x. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop Faber. Normal Table of Xenopus laevis. Garland Publishing Inc.; New York, NY: 1994. [Google Scholar]
- Nusinzon I, Horvath CM. Histone deacetylases as transcriptional activators? Role reversal in inducible gene regulation. Sci STKE. 2005;2005:re11. doi: 10.1126/stke.2962005re11. [DOI] [PubMed] [Google Scholar]
- Paik EJ, Zon LI. Hematopoietic development in the zebrafish. Int J Dev Biol. 54:1127–1137. doi: 10.1387/ijdb.093042ep. [DOI] [PubMed] [Google Scholar]
- Palis J, Koniski A. Analysis of hematopoietic progenitors in the mouse embryo. Methods Mol Med. 2005;105:289–302. doi: 10.1385/1-59259-826-9:289. [DOI] [PubMed] [Google Scholar]
- Palis J, McGrath KE, Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Pillai R, Coverdale LE, Dubey G, Martin CC. Histone deacetylase 1 (HDAC-1) required for the normal formation of craniofacial cartilage and pectoral fins of the zebrafish. Dev Dyn. 2004;231:647–654. doi: 10.1002/dvdy.20168. [DOI] [PubMed] [Google Scholar]
- Sadlon TJ, Lewis ID, D'Andrea RJ. BMP4: its role in development of the hematopoietic system and potential as a hematopoietic growth factor. Stem Cells. 2004;22:457–474. doi: 10.1634/stemcells.22-4-457. [DOI] [PubMed] [Google Scholar]
- Shibata K, Ishimura A, Maéno M. GATA-1 inhibits the formation of notochord and neural tissue in Xenopus embryo. Biochem Biophys Res Commun. 1998;252:241–248. doi: 10.1006/bbrc.1998.9490. [DOI] [PubMed] [Google Scholar]
- Sive HL, Granger RM, Harland RM. Early development of Xenopus laevis: a laboratory manual. Cold Springs Harbor Laboratory Press; Cold Springs Harbor, NY: 2000. [Google Scholar]
- Smith SJ, Kotecha S, Towers N, Latinkic BV, Mohun TJ. XPOX2-peroxidase expression and the XLURP-1 promoter reveal the site of embryonic myeloid cell development in Xenopus. Mech Dev. 2002;117:173–186. doi: 10.1016/s0925-4773(02)00200-9. [DOI] [PubMed] [Google Scholar]
- Snyder A, Fraser ST, Baron MH. Bone morphogenetic proteins in vertebrate hematopoietic development. J Cell Biochem. 2004;93:224–232. doi: 10.1002/jcb.20191. [DOI] [PubMed] [Google Scholar]
- Taneyhill LA, Adams MS. Investigating regulatory factors and their DNA binding affinities through real time quantitative PCR (RT-QPCR) and chromatin immunoprecipitation (ChIP) assays. Methods Cell Biol. 2008;87:367–389. doi: 10.1016/S0091-679X(08)00219-7. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr., Pepling ME, Horb ME, Thomsen GH, Gergen JP. A Xenopus homologue of aml-1 reveals unexpected patterning mechanisms leading to the formation of embryonic blood. Development. 1998;125:1371–1380. doi: 10.1242/dev.125.8.1371. [DOI] [PubMed] [Google Scholar]
- Turpen JB. Induction and early development of the hematopoietic and immune systems in Xenopus. Dev Comp Immunol. 1998;22:265–278. doi: 10.1016/s0145-305x(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Walmsley M, Ciau-Uitz A, Patient R. Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development. 2002;129:5683–5695. doi: 10.1242/dev.00169. [DOI] [PubMed] [Google Scholar]
- Walmsley M, Cleaver D, Patient R. Fibroblast growth factor controls the timing of Scl, Lmo2, and Runx1 expression during embryonic blood development. Blood. 2008;111:1157–1166. doi: 10.1182/blood-2007-03-081323. [DOI] [PubMed] [Google Scholar]
- Xu RH, Kim J, Taira M, Lin JJ, Zhang CH, Sredni D, Evans T, Kung HF. Differential regulation of neurogenesis by the two Xenopus GATA-1 genes. Mol Cell Biol. 1997;17:436–443. doi: 10.1128/mcb.17.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Tonou-Fujimori N, Komori A, Maeda R, Nojima Y, Li H, Okamoto H, Masai I. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development. 2005;132:3027–3043. doi: 10.1242/dev.01881. [DOI] [PubMed] [Google Scholar]
- Yang J, Tan C, Darken RS, Wilson PA, Klein PS. Beta-catenin/Tcf-regulated transcription prior to the midblastula transition. Development. 2002;129:5743–5752. doi: 10.1242/dev.00150. [DOI] [PubMed] [Google Scholar]
- Zhang C, Evans T. BMP-like signals are required after the midblastula transition for blood cell development. Dev Genet. 1996;18:267–278. doi: 10.1002/(SICI)1520-6408(1996)18:3<267::AID-DVG7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.