Abstract
Recent studies demonstrate an increased risk of second primary malignancies (SPM) in multiple myeloma (MM) patients on maintenance lenalidomide following autologous stem cell transplantation (ASCT). There may be other risk factors driving SPM post-ASCT in MM, so we explored this possibility through analysis of our large transplant database in conjunction with our long-term followup program. We conducted a retrospective cohort study of 841 consecutive MM patients who underwent ASCT from 1989–2009 at City of Hope, as well as a nested case-control analysis evaluating the role of all therapeutic exposures before, during and after ASCT. Median length of follow-up for the entire cohort was 3.4 years (range 0.3–19.9). Sixty cases with seventy SPM were identified. The overall cumulative incidence of SPM was 7.4% at five years and 15.9% at ten years if non-melanoma skin cancers (NMSCs) were included, and 5.3% at 5 years and 11.2% at 10 years if NMSCs were excluded. Multivariate analysis of the entire cohort revealed association of both older age (≥55yrs) (RR 2.3 p<0.004) and race (non-Hispanic white) (RR 2.4 p=0.01) with an increased risk of SPM. Furthermore, thalidomide exposure demonstrated a trend towards increased risk (OR=3.5, p=0.15); however, not enough patients were treated with lenalidomide to accurately assess the risk of this agent. Exclusion of NMSCs retained the association with these variables, but was accompanied with loss of statistical significance. This large single-institution analysis identified race and older age to be associated with increased risk of developing SPM. The trend toward increased risk with thalidomide exposure suggests a class effect from immunomodulatory drugs that may not be restricted to lenalidomide.
Keywords: second primary malignancy, transplantation, myeloma
INTRODUCTION
High dose therapy with autologous stem cell transplant (ASCT) has been considered a standard of care for patients with multiple myeloma (MM) since the IFM2004 trial demonstrated an improved survival for patients treated with high-dose chemotherapy compared with conventional chemotherapy [1, 2]. Over the ensuing years, myeloma has become one of the leading indications for ASCT in the United States. The combination of high dose therapy in conjunction with induction and maintenance therapy with novel agents such as thalidomide, lenalidomide, and bortezomib has further improved survival for patients with myeloma. Ten-year survival in younger patients is approaching fifty percent [3].
As myeloma survival improves, the long-term impacts of novel therapies and ASCT are under investigation. Potential long-term adverse effects have led to a paradigm of intensive treatment for cytogenetically-defined high-risk patients and toxicity-minimizing treatment in lower-risk patients. This approach may involve the choice of induction regimens and, or, the optimization of consolidation and maintenance therapy post-ASCT. Thalidomide maintenance has demonstrated improved progression-free survival (PFS) in several randomized trials as well as improved overall survival in some of the studies [4, 5]. Lenalidomide, which has a more favorable toxicity profile with respect to peripheral neuropathy and sedation, has been used for maintenance therapy in more recent trials. Three randomized trials have reported a PFS advantage to lenalidomide maintenance either with or without ASCT, and one group also reported an overall survival benefit [6–8]. However, in both the IFM2005 trial [6] and the CALGB100104 trial [7] of maintenance lenalidomide versus observation post-ASCT, there was an increased incidence of second primary malignancies (SPM) in the maintenance arm (8% versus 3–4% SPM in the control arms).
It remains unclear, however, what factors are driving SPM in myeloma patients post ASCT, and lenalidomide may not be the only putative contributor. The causes are likely to be multi-factorial, involving host factors in addition to treatment. In fact, it has been demonstrated in large epidemiological studies that patients with monoclonal gammopathy of undetermined significance (MGUS) are at increased risk for additional malignancies [9]. Previous studies from our institution and others, have shown an increased risk of secondary malignancies in patients post-ASCT for lymphoid neoplasms, thereby suggesting that treatment-related factors independent of immunomodulatory drugs (IMiDs) may also drive therapy-related malignancy risk [10–12]. In myeloma, the risk of post-ASCT SPM may in part be mediated by the alkylator therapies commonly used for treatment of myeloma prior to the advent of novel agents. A retrospective study evaluating exposure to alkylators prior to ASCT for myeloma did demonstrate an increased incidence of MDS in the group with prolonged chemotherapy exposure [13]. To better address the relationship of SPM to treatment and demographic variables, we conducted an analysis of patients undergoing ASCT for myeloma at City of Hope over the past twenty years.
PATIENTS AND METHODS
The Long-Term Follow-Up Program supports complete follow-up of all patients receiving ASCT at City of Hope. This protocol was approved by the Institutional Review Board and conforms to the standards provided in the Declaration of Helsinki. A total of 869 individuals underwent ASCT for MM at City of Hope between 1989 and 2009. Of these, 28 patients refused participation in the Long-Term Follow-up Program. This report includes the remaining 841 individuals, resulting in a participation rate of 96.8%.
Demographic and Clinical Characteristics
Demographic information (date of birth, gender, and race/ethnicity) and clinical characteristics (primary diagnosis, date of diagnosis, date of ASCT, disease status at ASCT, conditioning regimens and stem cell source) were obtained from the ASCT database.
Second Primary Malignancies (SPM) and Vital Status Information
Information regarding SPM and vital status of the cohort was ascertained as of December 31, 2010. To ensure complete ascertainment of SPMs, institutional long-term follow-up data was combined with data from the California Cancer Registry and National Death Index Plus (NDI Plus) Program. For institutional long-term follow-up, medical records served as the primary source of data. If the date of last medical visit at COH was not recent, or if there were gaps in patients’ history within the window of interest, a standard protocol was used to contact physicians outside COH to obtain relevant details for the period of interest. If the physician was not available or unable to provide recent information, the patient was contacted to obtain this information. Patient vital status was obtained through the following resources: NDI Plus, Social Security Death Index (SSDI), medical records, and institutional long-term follow-up efforts.
Cohort analysis
Cumulative incidence of SPM was estimated by taking into consideration death from other causes as a competing risk [14]. Person-years at risk were computed from the date of ASCT, to the date of death, date of SPM, or the date of censoring (December 31, 2010 – for those still alive without SPM), whichever occurred first. For multiple occurrences of non-melanoma skin cancers [NMSCs: basal or squamous cell carcinoma (BCC or SCC)], the date of first occurrence was considered as onset date. The proportional hazard regression method was used to examine the associations between demographic (sex and race) and clinical characteristics (age at diagnosis of multiple myeloma and year of ASCT), and the development of SPMs, measured by hazard ratios and their corresponding 95% confidence intervals and p values [15]. The analyses were conducted with inclusion of the entire cohort, as well as with exclusion of NMSCs.
Nested Case-Control analysis
A nested case-control study was also conducted to examine the role of pre-ASCT, ASCT-related and post-ASCT therapeutic exposures associated with SPM. Controls were MM patients post-ASCT but with no SPM, matched to index case by year of ASCT (±5 years); additionally, each control was required to have longer post-ASCT follow-up than its associated case. The conditional logistic regression method was used for the case-control analysis; odds ratios (OR) and their corresponding 95% confidence intervals and p values are presented.
Statistical computing was conducted using SAS 9.2 (SAS institute Inc, Cary, NC, USA). All quoted p values are two-sided.
RESULTS
Cohort Study
Patient demographic and clinical characteristics are detailed in Table 1. The median length of follow-up was 3.4 years (range 0.3–19.9). Median age at diagnosis of MM was 55 years (18–76) and at ASCT was 56 years (18–77 years). Sixty-one percent of the patients were males: 61% were non-Hispanic white, 18% were Hispanic and 13% were African American. Sixty-two percent of patients had received a single ASCT, 27% had received tandem autologous ASCT, while the remaining 11% had received multiple ASCTs (72 of these patients received an allogeneic transplantion after an ASCT).
Table 1.
Demographic and clinical characteristics of the cohort
| Characteristics | Entire Cohort N (%) |
SPM Patients* including NMSC N (%) |
SPM Patients** excluding NMSC N (%) |
|---|---|---|---|
| Number of patients | 841 | 60 | 42 |
| Gender | |||
| Female | 330 (39%) | 17 (28%) | 12 (29%) |
| Male | 511 (61%) | 43 (72%) | 30 (71%) |
| Race/ethnicity | |||
| Non-Hispanic Caucasian | 511 (61%) | 49 (82%) | 31 (74%) |
| Hispanic | 151 (18%) | 5 (8%) | 5 (12%) |
| African American | 107 (13%) | 4 (7%) | 4 (9%) |
| Others | 72 (9%) | 2 (3%) | 2 (5%) |
| Age at ASCT (years) | |||
| Median (range) | 56 (18–77) | 59 (32–77) | 56 (32–69) |
| Mean (SD) | 55 (8.9) | 57 (9.4) | 55 (9.5) |
| Age at MM diagnosis (years) | |||
| Median (range) | 55 (18–76) | 57 (31–76) | 54 (31–68) |
| Mean (SD) | 54 (8.9) | 55 (9.4) | 53 (9.3) |
| Year of MM diagnosis | |||
| 1983–1999 | 206 (24%) | 30 (50%) | 25 (60%) |
| 2000–2004 | 301 (36%) | 20 (33%) | 11 (26%) |
| 2005–2009 | 334 (40%) | 10 (17%) | 6 (14%) |
| Year of ASCT | |||
| 1989–1999 | 133 (16%) | 22 (37%) | 18 (43%) |
| 2000–2004 | 278 (33%) | 22 (37%) | 15 (36%) |
| 2005–2009 | 430 (51%) | 16 (27%) | 9 (21%) |
| Source of stem cell | |||
| Peripheral blood | 841 (100%) | 60 (100%) | 42 (100%) |
| Disease status at ASCT | |||
| Partial remission | 578 (69%) | 44 (73%) | 33 (79%) |
| Stable disease | 115 (14%) | 9 (15%) | 4 (9%) |
| Complete remission | 111 (13%) | 7 (12%) | 5 12%) |
| Progressive disease | 31 (4%) | 0 (0%) | 0 (0%) |
| Unknown | 6 (0.7%) | 0 (0%) | 0 (0%) |
| Transplantation type | |||
| Single autologous | 527 (63%) | 32 (53%) | 22 (55%) |
| Tandem autologous | 229 (27%) | 18 (30%) | 12 (29%) |
| Multiple | 85 (10%) | 10 (17%) | 7 (17%) |
Including non-melanoma skin cancer
Excluding non-melanoma skin cancer
NMSC=non-melanoma skin cancer, ASCT=autologous stem cell transplantation, SD=standard deviation, SPM=second primary malignancy
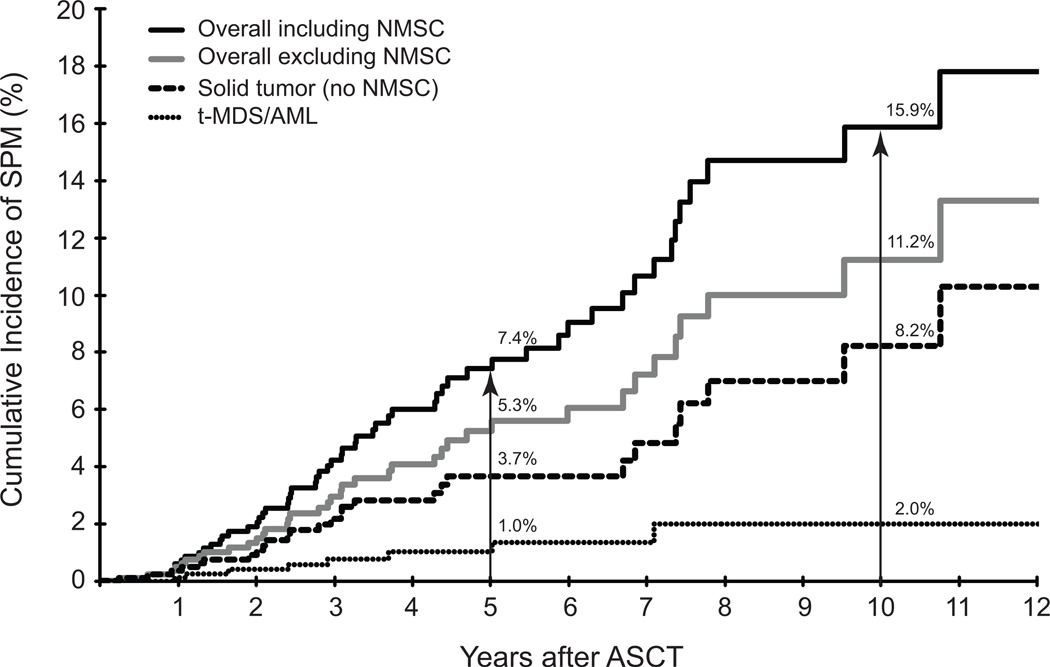
As of December 31, 2010, sixty patients had developed seventy SPMs. The SPMs included non-melanoma skin cancer [NMSC: BCC (n=13), SCC (n=14)]; melanoma (n=4); therapy-related myelodysplasia/acute myeloid leukemia (t-MDS/AML, n=9); prostate (n=5); colorectal cancer (n=4); oropharyngeal (n=4); breast (n=3); ALL (n=2); bladder (n=2); and one each of adrenocortical carcinoma, esophageal carcinoma, gastric carcinoma, germ cell tumor, non-Hodgkin lymphoma, pancreatic carcinoma, renal cell carcinoma, thyroid cancer, uterine cancer, and site unknown. The overall cumulative incidence was 7.4% at 5 years and 15.9% at 10 years (Figure 1). After excluding NMSCs, the overall cumulative incidence was 5.3% at 5 years and 11.2% at 10 years (Figure 1). The cumulative incidence of t-MDS/AML was 1.0% at 5 years and 2.0% at 10 years, while for patients with solid tumors (excluding NMSCs) it was 3.7% at 5 years and 8.2% at 10 years (Figure 1).
Figure 1. Cumulative incidence of second malignancy in the cohort.
Cumulative incidence of second malignancy (%) from date of ASCT (years) was calculated using death from other causes as a competing risk. N at risk was 841 at ASCT (time 0), 378 at 5 years and 82 at 10 years. The solid black line represents overall cumulative incidence (all types of SPM), the grey line represents overall cumulative incidence (SPM excluding non-myeloma skin cancers), the dashed line represents solid tumors (SPM excluding non-myeloma skin cancers), and the dotted line represents t-MDS/AML.
Cohort risk factor analysis
Table 2 presents the results for the multivariable analysis both including and excluding NMSCs. With the inclusion of NMSCs, multivariable Cox regression analysis revealed that non-Hispanic white race (RR=2.4, 95% CI, 1.2–4.6, p=0.01) and older age (≥55) at diagnosis of MM (RR=2.3, 95% CI, 1.3–4.1, p=0.004) were associated with an increased relative risk of developing SPMs after adjustment for gender and year of ASCT. However, after excluding non-melanoma skin cancer, the magnitude of these associations was mitigated and the associations became non-significant.
Table 2.
Cohort multivariate analysis of risk factors associated with 2nd malignancy
| Including non-melanoma skin cancer |
Excluding non-melanoma skin cancer |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Cohort/SPM | HR (95%CI) | P value |
Cohort/SPM | HR (95%CI) | P value |
| Number of patients | 841/60 | 841/42 | ||||
| Gender | ||||||
| Female | 330/17 | 1.0 | 330/12 | 1.0 | ||
| Male | 511/43 | 1.44 (0.82–2.53) | 0.21 | 511/30 | 1.42 (0.72–.78) | 0.31 |
| Race/ethnicity | ||||||
| Others | 1.0 | 330/11 | 1.0 | |||
| Non-Hispanic Caucasian | 511/49 | 2.37 (1.22–4.61) | 0.01 | 511/31 | 1.53 (0.76–.09) | 0.16 |
| Age at MM (years) | ||||||
| <55 | 408/24 | 1.0 | 408/21 | 1.0 | ||
| ≥55 | 433/36 | 2.32 (1.30–4.14) | 0.004 | 433/21 | 1.64 (0.84–.23) | 0.15 |
| Year of ASCT | ||||||
| 1989–1999 | 133/22 | 1.0 | 133/18 | 1.0 | ||
| 2000–2004 | 278/22 | 65 (0.33–1.25) | 0.20 | 278/15 | 0.58 (0.27–.25) | 0.17 |
| 2005–2009 | 430/16 | 0.56 (0.26–1.22) | 0.14 | 430/9 | 0.42 (0.17–.07) | 0.07 |
ASCT=autologous stem cell transplantation, SPM=second primary malignancy, HR=hazard ratio, CI=confidence interval
Case-control study
The demographic characteristics and therapeutic exposures for the cases (including and excluding NMSCs) and their matched controls are presented in Table 3. Table 4 shows results for the variables that were retained in the multivariable analysis of the case-control study. Similar to the cohort analysis results, older age at MM diagnosis and non-Hispanic white ethnicity were associated with increased risk of developing SPM after ASCT when NMSCs were included. Individually, none of the therapeutic agents used in the pre-ASCT, peri-ASCT, or post-ASCT period was associated with development of SPM after ASCT (Table 3). However, exposure to thalidomide either pre-ASCT or post-ASCT demonstrated a trend toward positive association when NMSCs were included in the analysis (OR=3.5, 95% CI, 0.6–19.4, p=0.15) (Table 4). Only six patients (3 cases and 3 controls) were exposed to lenalidomide prior to development of SPM, and exposure to this agent was not associated with an increased risk of SPM (OR=1.0, 95% CI, 0.14–7.10). Among the 60 cases and 60 controls, 107 had information regarding total CD34 yields. The mean CD34 yield for the 55 cases was slightly lower than that for the 52 controls (7.8 × 106 CD34+ cells versus 9.5 × 106, p=0.08). The median for cases was 7.4 × 106 CD34+ cells (range 1.8–24.5 × 106) and the median for controls was 8.6 × 106 CD34+ cells (range 1.7–32.4 × 106). Five of the cases had poor-risk cytogenetics (deletion 13 by metaphase karytoyping or deletion 17p by FISH), as did five controls (deletion 13 by metaphase karyotyping, deletion 17p, t[14;16]). Similar to the cohort analysis results, exclusion of the SPMs resulted in some mitigation of the magnitude of risk with these variables; the associations were no longer statistically significant.
Table 3.
Demographic and therapeutic exposure among cases and controls
| Including non-melanoma skin cancer |
Excluding non-melanoma skin cancer |
|||||
|---|---|---|---|---|---|---|
| Characteristics | Cases N (%) |
Controls N (%) |
P value | Cases N (%) |
Controls N (%) |
P value |
| Number of patients | 60 | 60 | 42 | 42 | ||
| Gender | ||||||
| Female | 17 (28%) | 26 (43%) | 12 (29%) | 18 (43%) | ||
| Male | 43 (72%) | 34 (57%) | 0.09 | 30 (71%) | 24 (57%) | 0.19 |
| Race/ethnicity | ||||||
| Others | 11 (18%) | 22 (37%) | 11 (26%) | 16 (38%) | ||
| Non-Hispanic Caucasian | 49 (82%) | 38 (63%) | 0.02 | 31 (74%) | 26 (62%) | 0.15 |
| Age at MM diagnosis | ||||||
| <55 | 24 (40%) | 38 (63%) | 21 (50%) | 27 (64%) | ||
| ≥55 | 36 (60%) | 22 (37%) | 0.01 | 21 (50%) | 15 (36%) | 0.14 |
| Pre-ASCT exposure | ||||||
| Pre-HCT radiation | 20 (33%) | 17 (28%) | 0.51 | 14 (33%) | 12 (29%) | 0.59 |
| Cyclophosphamide | 7 (12%) | 12 (20%) | 0.23 | 6 (14%) | 9 (21%) | 0.41 |
| Doxorubicin | 42 (70%) | 47 (78%) | 0.18 | 32 (76%) | 34 (81%) | 0.53 |
| Etoposide | 7 (12%) | 5 (8%) | 0.48 | 6 (14%) | 3 (7%) | 0.27 |
| Prednisone | 19 (32%) | 15 (25%) | 0.40 | 15 (36%) | 14 (33%) | 0.81 |
| Thalidomide | 22 (37%) | 19 (32%) | 0.29 | 14 (33%) | 11 (26%) | 0.27 |
| Vincristine | 40 (67%) | 48 (80%) | 0.04 | 30 (71%) | 35 (83%) | 0.12 |
| Priming agents* | ||||||
| Cyclophosphamide | 40 (70%) | 38 (69%) | 1.00 | 23 (59%) | 25 (66%) | 0.18 |
| Paclitaxel | 9 (16%) | 12 (22%) | 0.37 | 6 (15%) | 10 (26%) | 0.14 |
| Conditioning agents** | ||||||
| Total Body Irradiation | 9 (16%) | 10 (18%) | 0.74 | 7 (18%) | 9 (24%) | 0.42 |
| Busulfan | 16 (29%) | 18 (33%) | 0.53 | 10 (26%) | 11 (29%) | 0.71 |
| Cyclophosphamide | 19 (35%) | 22 (40%) | 0.37 | 13 (34%) | 15 (39%) | 0.48 |
| Etoposide | 3 (5%) | 4 (7%) | 0.57 | 3 (8%) | 4 (11%) | 0.57 |
| Melphalan | 50 (90%) | 49 (89%) | 0.57 | 34 (89%) | 33 (87%) | 0.57 |
| Post-ASCT exposure | ||||||
| Dexamethazone | 19 (35%) | 14 (25%) | 0.28 | 12 (35%) | 10 (25%) | 0.59 |
| Interferon α | 18 (33%) | 16 (29%) | 0.59 | 12 (33%) | 12 (29%) | 1.00 |
| Prednisone | 5 (10%) | 3 (5%) | 0.42 | 5 (10%) | 3 (5%) | 0.42 |
| Thalidomide | 30 (50%) | 26 (47%) | 0.29 | 19 (50%) | 18 (47%) | 0.74 |
| Thalidomide exposure timing | ||||||
| None | 21 (35%) | 26 (44%) | 16 (38%) | 19 (45%) | ||
| Pre-ASCT | 9 (15%) | 8 (13%) | 0.19 | 7 (17%) | 5 (12%) | 0.17 |
| Post-ASCT | 17 (28%) | 15 (25%) | 0.15 | 12 (28%) | 12 (29%) | 0.42 |
| Both pre- and post-ASCT | 13 (22%) | 11 (18%) | 0.12 | 7 (17%) | 6 (14%) | 0.26 |
| Either pre- or post-ASCT | 39 (65%) | 34 (56%) | 0.12 | 26 (62%) | 23 (55%) | 0.27 |
3 cases and 5 controls without information about priming agents.
5 cases and 5 controls without conditioning information.
ASCT=autologous stem cell transplantation, MM=multiple myeloma
Table 4.
Case-control multivariate analysis of risk factors associated with 2nd malignancy
| Including non-melanoma skin cancer |
Excluding non-melanoma skin cancer |
|||||
|---|---|---|---|---|---|---|
| Ca/Co | OR (95% CI) | P value | Ca/Co | OR (95% CI) | P value | |
| Number of patients | 60/60 | 42/42 | ||||
| Gender* | ||||||
| Female | 17/26 | 1.0 | 12/18 | 1.0 | ||
| Male | 43/34 | 1.55 (0.63–3.84) | 0.34 | 30/24 | 1.76 (0.60–5.12) | 0.30 |
| Race/ethnicity* | ||||||
| Others | 11/22 | 1.0 | 11/16 | 1.0 | ||
| Non-Hispanic Caucasian | 49/38 | 2.90 (0.76–11.13) | 0.12 | 21/26 | 1.82 0.41–8.13) | 0.44 |
| Age at MM diagnosis* | ||||||
| <55 | 24/38 | 1.0 | 21/27 | 1.0 | ||
| ≥55 | 36/22 | 3.14 (1.15–8.57) | 0.03 | 21/15 | 2.43 (0.75–7.85) | 0.14 |
| Thalidomide exposure timing* | ||||||
| None | 21/26 | 1.0 | 16/19 | 1.0 | ||
| Pre-ASCT | 9/8 | 4.70 (0.50–44.30) | 0.18 | 7/5 | 8.55 (0.38–190) | 0.18 |
| Post-ASCT | 17/15 | 3.31 (0.54–20.30) | 0.20 | 12/12 | 2.29 (0.34–15.5) | 0.39 |
| Both pre- and post-ASCT | 13/11 | 2.63 (0.30–22.75) | 0.38 | 7/6 | 1.71 (0.12–24.2) | 0.69 |
| Any Thalidomide exposure§ | ||||||
| None | 21/26 | 1.0 | 16/19 | 1.0 | ||
| Either pre- or post-ASCT | 39/34 | 3.48 (0.62–19.40) | 0.15 | 26/23 | 2.59 (0.42–15.9) | 0.30 |
All the variables were included in the multivariate conditional logistic regression model.
OR obtained from multivariate conditional logistic regression model with gender, race/ethnicity, and age at MM diagnosis in the model.
ASCT=autologous stem cell transplantation, MM=multiple myeloma, Ca=Cases, Co=Controls, OR=odds ratio, CI=confidence interval
DISCUSSION
Patients with myeloma are surviving longer with the use of novel agents and ASCT. Methods to further decrease post-ASCT relapse rates include maintenance therapy with combinations of novel agents. Much controversy has ensued regarding the optimal maintenance regimen, and the duration of maintenance therapy. This debate intensified with the initial reports of increased SPM in patients on lenalidomide maintenance in the CALGB100104 and IFM2005 trials [16, 17]. In addition, a pooled analysis of nine European trials demonstrated an increased incidence of SPM in patients on lenalidomide maintenance after melphalan, and also for patients on thalidomide after melphalan [18]. In all series the SPM were a combination of solid tumors and hematological malignancies. The increased SPM incidence in myeloma patients is a complex story and likely represents an interplay of host and genetic factors in addition to treatment-related risks. We have analyzed the effects of multiple host demographic, disease and treament variables to determine their relevance to later development of SPM in this cohort of 841 patients.
Our cohort analysis confirms that second malignancy remains an issue in MM patients, especially the older (≥55yr) subgroup. Our overall cumulative incidence of 15.9% is consistent with findings in the German group who reported cumulative incidence of 15.7% at ten years in a retrospective study of SPM incidence (including NMSCs) post-ASCT for myeloma [19]. The Arkansas group also identified older age (≥65yrs) as associated with SPM onset [20]. This high SPM incidence in our patient cohort likely reflects the combined expected increase in malignancy in older adults in the general population, as well as the increased risk of malignancy in patients with myeloma. However, this data must be considered in the context of the increased survival of patients with myeloma due to more effective therapies. Indeed, the OS of our entire cohort was 60% at five years, with an OS of 58% in patients 55 years of age or older.
Host characteristics beyond age may also play a role in the risk of developing SPM. Analysis of our cohort revealed ethnicity as a risk factor with a higher incidence in non-Hispanic white patients. Fifty-two percent of the SPM in this cohort were skin cancers, and these are typically more prevalent in non-Hispanic whites. We analyzed the incidence of SPM both including and excluding NMSCs, since of four major studies in 2012 analyzing secondary malignancy outcomes following lenalidomide or thalidomide maintenance therapy, NMSCs are included in two analyses [6, 20] and excluded in two analyses [7, 8]. As our institution is located in Southern California, where the population has relatively high sun exposure, we felt it important to include all skin cancers. We also examined prior chemotherapy exposures since several drug classes have been implicated in t-MDS/AML. Neither cyclophosphamide and busulfan as part of pre-ASCT therapy, or cyclophosphamide-mobilizing therapy attained statistical significance as risk factors in our analysis, nor did the epipodophyllotoxins; however, investigators have found such associations with alkylator therapy in myeloma patients in past studies [21]. The relative risks are likely due in part to dose, as well as duration of exposure [22] and lack of association in this study may be due in part to the shorter-term and lower-dose alkylators used in more modern regimens.
Immunomodulatory (IMiD) agents in modern regimens have either been combined with alkylators as the backbone of therapy or supplanted alkylators entirely. The concern about this class of agents as risk factors for SPM first arose in studies of patients treated with lenalidomide as maintenance therapy post-ASCT. Interestingly, the SPMs included both hematological malignancies as well as solid tumors. Our group of patients had too few patients on lenalidomide therapy either pre- or post-ASCT to meaningfully examine this exposure, but thalidomide exposure was common. Hence thalidomide exposure was included in the case-control analysis and we did see a non-significant trend to increased risk with thalidomide use at any time, i.e. either pre- or post-ASCT. The large European trial series reviewed by Palumbo et al.saw an approximately 1% per year incidence of SPM in the melphalan and thalidomide group [18]. So while the association of SPMs with thalidomide exposure in our study did not reach statistical significance, the trend observed in our cohort has potential implications. It raises the question of whether SPM risk with immunomodulatory agents has more to do with the immunomodulatory class of drugs than with lenalidomide specifically.
Our large single institution series confirms that SPM is a concern post-autologous transplantion for myeloma. We were able to identify patients at higher risk for second malignancy based on age, and race. Previously identified risk factors for SPM include lenalidomide, low CD34+ cell dose, chemotherapy agent exposure (alkylators, epipodophyllotoxins), and radiation exposure. Lenalidomide has been known to impact the bone marrow microenvironment, as evidenced by poor stem cell mobilization with long-term lenalidomide exposure. In turn, low CD34+ stem cell yields have been associated with t-MDS/AML. In this study, however, we had very few cases of lenalidomide exposure and all cases of t-MDS/AML occurred before the year 2000, prior to the widespread use of lenalidomide. The trend in our case-control analysis toward an association of thalidomide exposure with SPM post-ASCT for myeloma may point to a class effect rather than a lenalidomide-specific effect. This potential class effect is consistent with data showing similar but slightly lower rates of SPM in thalidomide-exposed compared to lenalidomide-exposed patients [20]; however, the possible mechanism of action remains unclear, especially since thalidomide does not affect stem cell yields. As we enter the age of personalized medicine in myeloma in the context of improved survival with new drugs, we need to weigh the risks and benefits of exposure to these agents. Ultimately, one could envision the use of an algorithm incorporating host factors, disease-related factors and risk of SPM so that we can better tailor therapy.
Acknowledgments
This work was supported in party by funding from the Kennedy Klemens Fund [A.Y.K], NIH P30 CA33572 [Cancer Center], R01 CA078938 [S.B.], P01 CA 30206 [S.J.F.], and the Leukemia Lymphoma Society (2192) [S.B.].
Dr. Krishnan is on the speakers bureau for Celgene and Millenium, owns Celgene stock, and is a consultant for Celgene and Merck. Dr. Somlo is a consultant and speaker for Celgene and a speaker for Millenium. Dr. Sahebi received research funds from Millenium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol. 2010;28:830–834. doi: 10.1200/JCO.2009.25.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlogie B, Pineda-Roman M, van Rhee F, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112:3115–3121. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27:1788–1793. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 6.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 9.Mailankody S, Pfeiffer RM, Kristinsson SY, et al. Risk of acute myeloid leukemia and myelodysplastic syndromes after multiple myeloma and its precursor disease (MGUS) Blood. 2011;118:4086–4092. doi: 10.1182/blood-2011-05-355743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia S, Ramsay NK, Steinbuch M, et al. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87:3633–3639. [PubMed] [Google Scholar]
- 11.Micallef IN, Lillington DM, Apostolidis J, et al. Therapy-related myelodysplasia and secondary acute myelogenous leukemia after high-dose therapy with autologous hematopoietic progenitor-cell support for lymphoid malignancies. J Clin Oncol. 2000;18:947–955. doi: 10.1200/JCO.2000.18.5.947. [DOI] [PubMed] [Google Scholar]
- 12.Darrington DL, Vose JM, Anderson JR, et al. Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol. 1994;12:2527–2534. doi: 10.1200/JCO.1994.12.12.2527. [DOI] [PubMed] [Google Scholar]
- 13.Govindarajan R, Jagannath S, Flick JT, et al. Preceding standard therapy is the likely cause of MDS after autotransplants for multiple myeloma. Br J Haematol. 1996;95:349–353. doi: 10.1046/j.1365-2141.1996.d01-1891.x. [DOI] [PubMed] [Google Scholar]
- 14.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1140–1154. [Google Scholar]
- 15.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society. 1972;B34:187–220. [Google Scholar]
- 16.Attal M, Lauwers Vc, Marit G, et al. Maintenance Treatment with Lenalidomide After Transplantation for MYELOMA: Final Analysis of the IFM 2005-02. ASH Annual Meeting Abstracts. 2010;116:310. [Google Scholar]
- 17.McCarthy PL, Owzar K, Anderson KC, et al. Phase III Intergroup Study of Lenalidomide Versus Placebo Maintenance Therapy Following Single Autologous Hematopoietic Stem Cell Transplantation (AHSCT) for Multiple Myeloma: CALGB 100104. ASH Annual Meeting Abstracts. 2010;116:37. [Google Scholar]
- 18.Palumbo A, Larocca A, Zweegman S, et al. Second Primary Malignancies in Newly Diagnosed Multiple Myeloma Patients Treated with Lenalidomide: Analysis of Pooled Data in 2459 Patients. ASH Annual Meeting Abstracts. 2011;118:996. [Google Scholar]
- 19.Fenk R, Neubauer F, Bruns I, et al. Secondary primary malignancies in patients with multiple myeloma treated with high-dose chemotherapy and autologous blood stem cell transplantation. Br J Haematol. 2012;156:683–686. doi: 10.1111/j.1365-2141.2011.08905.x. [DOI] [PubMed] [Google Scholar]
- 20.Usmani SZ, Sexton R, Hoering A, et al. Second malignancies in total therapy 2 and 3 for newly diagnosed multiple myeloma: influence of thalidomide and lenalidomide during maintenance. Blood. 2012;120:1597–1600. doi: 10.1182/blood-2012-04-421883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergsagel DE, Bailey AJ, Langley GR, MacDonald RN, White DF, Miller AB. The chemotherapy on plasma-cell myeloma and the incidence of acute leukemia. N Engl J Med. 1979;301:743–748. doi: 10.1056/NEJM197910043011402. [DOI] [PubMed] [Google Scholar]
- 22.Kaldor JM, Day NE, Clarke EA, et al. Leukemia following Hodgkin's disease. N Engl J Med. 1990;322:7–13. doi: 10.1056/NEJM199001043220102. [DOI] [PubMed] [Google Scholar]