Abstract
Background
During early development avian embryos are easily accessible in ovo for transplantations and experimental perturbations. However, these qualities of the avian embryonic model rapidly wane shortly after embryonic day (E)4 when the embryo is obscured by extraembryonic membranes, making it difficult to study developmental events that occur at later stages in vivo.
Results and Conclusions
In this study, we describe a multistep method that involves initially windowing eggs at E3, followed by dissecting away extraembryonic membranes at E5 to facilitate embryo accessibility in ovo until later stages of development. The majority of the embryos subjected to this technique remain exposed between E5 and E8, then become gradually displaced by the growing allantois from posterior to anterior regions. Exposed embryos are viable and compatible with embryological and modern developmental biology techniques including tissue grafting and ablation, gene manipulation by electroporation, and protein expression. This technique opens up new avenues for studying complex cellular interactions during organogenesis and can be further extrapolated to regeneration and stem cell studies.
Keywords: Late-stage chick embryo, in ovo, extraembryonic membranes, dissection, cell injections
INTRODUCTION
The chick embryo is a valuable model organism used to study vertebrate development. Besides their availability and low cost of fertilized eggs, early stage chick embryos are readily accessible in ovo for visualization and experimental manipulations. During the initial 3 days of incubation, a chick embryo, which begins as a blastodisk, undergoes gastrulation, neurulation and several morphogenetic movements to form distinct cranial and trunk structures. Within this period of development, the embryos can be easily accessed and manipulated at different developmental time points by ‘windowing’ the egg-shell, then re-incubating until the desired late embryonic stages. Using this approach, several techniques such as DiI injections (del Barrio and Nieto, 2002) and tissue transplantation (Le Douarin, 1973; Goldstein, 2006; Lwigale and Schneider, 2008) or tissue ablation (Summerbell, 1974; Bénazéraf et al., 2010) have been extensively utilized to study cell fate and pattern formation. In addition, gain- and loss-of-function studies involving electroporation of DNA, RNAi, morpholinos, and virus constructs are now widely used in molecular studies involving chick embryos (see reviews by Nakamura, et al., 2004; Sauka-Spengler and Barembaum, 2008).
Despite their versatility as model organisms for studying developmental biology, chick embryos are rapidly surrounded by extraembryonic membranes, making it difficult for experimental manipulations in ovo past embryonic day (E)5. The chick embryo is initially covered by a transparent vitelline membrane that separates it from the albumen. Beginning at about HH stage 12 (Hamburger and Hamilton, 1951), the amnion and chorion membranes overlap the forebrain and tail bud by growing towards each other and fuse to cover the entire embryo by stage 18. At about this time another membrane, the allantois, forms as a balloon-like structure in the hind-gut region of the embryo. The allantois fuses with the chorion to form the chorioallantoic membrane, which stores nitrogenous waste and is involved in respiration and calcium transport. The chorioallantoic membrane grows rapidly and covers most of the embryo by stage 20 (E5). During early development, embryos can be easily accessed through the vitelline membrane, or chorion, and by dissecting the amniotic membrane. However, accessing an E5 or older embryo through the overlaying chorioallantoic membrane is lethal. Therefore most developmental biology studies using chick embryos in ovo are limited to relatively early stages of development, prior to critical periods of organogenesis that may require different signals and cellular interactions.
Several genes associated with cellular interactions and differentiation during organogenesis of the eye, ear, brain, skin, and tissues such as bones and cartilages, are either transiently expressed or initiate expression during later stages of embryogenesis. To increase accessibility to later stages (older than E4), methods such as ex ovo culture of chick embryos have been developed (New, 1955; Auerbach et al., 1974; Dugan et al., 1991; Datar and Bhonde, 2005; El-Ghali et al., 2010). While these methods overcome the complications that arise from adhering of the embryo to the eggshell and increase access to the embryo, the chorioallantoic membranes expand and obscure the embryo from manipulation at later stages. In addition, chick embryos cultured ex ovo show severe retardation in growth probably due to Ca2+ deficiency, which is naturally provided by the eggshell (Luo and Redies, 2005).
Until now there have been no attempts to address the obstacles that impede manipulation of the late-stage chick embryos in ovo. Current studies of organogenesis can only be conducted in vitro on tissue that is isolated from the influence of the endogenous embryonic environment, which may affect gene expression and crucial cellular interactions. In this study, we report a novel method that further strengthens the chick embryo as a model for developmental biology. This stepwise method combines preparation of fertilized chick eggs at different developmental time points with careful dissection and relocation of extraembryonic membranes to increase accessibility to embryos at late stages of development. Embryos exposed using this technique are viable and easily accessible in ovo for manipulation of tissues during organogenesis. We show that different regions of the exposed E7 embryos are easily accessible for manipulations such as injection with RCAS-GFP expressing cells. After two days of re-incubation, the injected cells can be tracked by monitoring GFP expression in various tissues. Late-stage embryos exposed by this technique can be manipulated using standard developmental approaches to study gene function, cellular interactions, tissue regeneration, and stem cell potential during organogenesis.
RESULTS AND DISCUSSION
Overview of Preparations for Late-stage Embryonic Manipulations
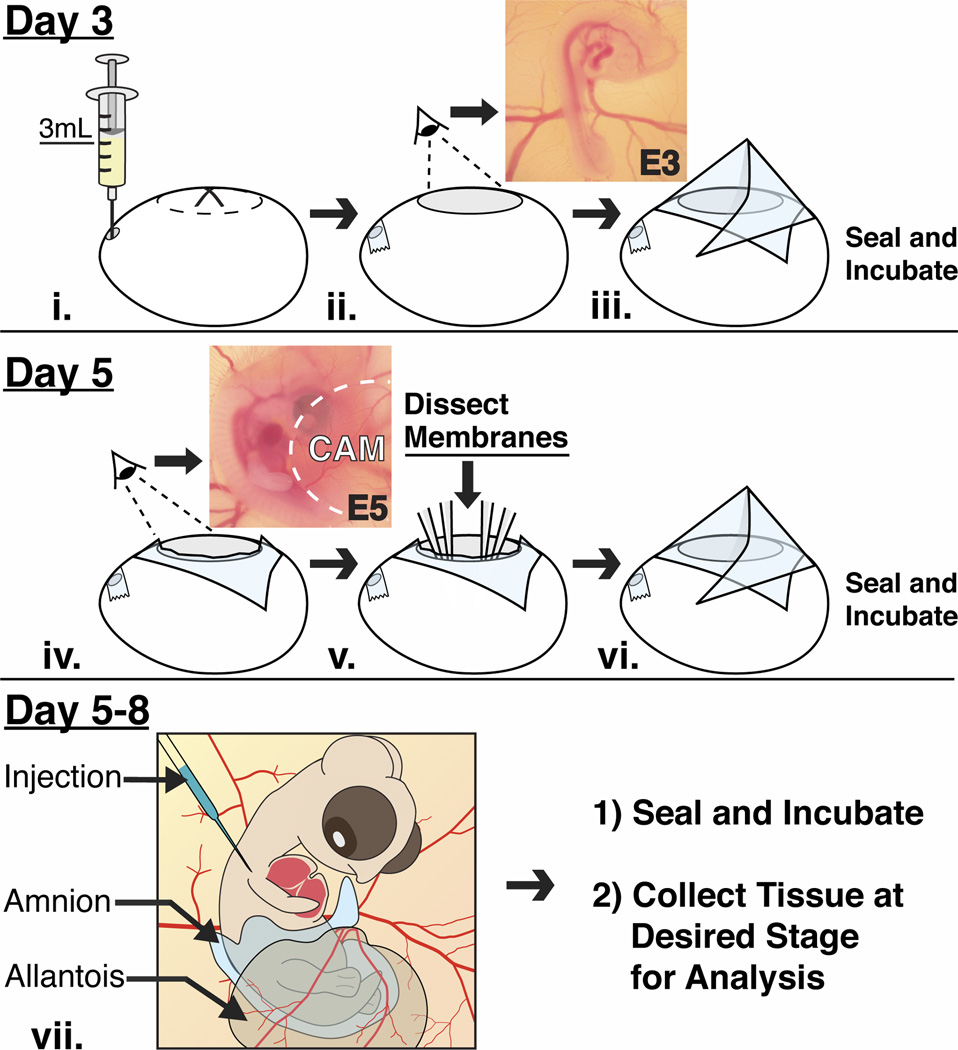
Eggs were incubated in a horizontal position for approximately 72 hours to obtain E3 embryos. A small hole was made with blunt forceps on the narrow end of the egg through which 3–4 ml of albumen were removed with a syringe (Fig. 1i). Eggs were windowed along the long axis by carefully breaking away pieces of the eggshell with curved forceps. At this stage embryos were visible and easily accessible through the amniotic membrane (Fig. 1ii). Two-three drops of Ringer’s solution containing Pencillin/Streptomycin antibiotics (50 U/ml and 50 µg/ml respectively) were added to the E3 embryos and the eggs were sealed and re-incubated on their side (Fig. 1iii). Windowing at E3 is critical to avoid problems associated with attachment of the embryo and extraembryonic membranes to the eggshell by E4 (Luo and Redies, 2005).
Fig. 1.
A schematic diagram showing treatment of the eggs and embryos at different stages of development. (i–iii) After 3 days of incubation, albumen is withdrawn from the narrow end of the egg, which is then windowed to reveal an intact E3 chick embryo and surrounding vasculature. The eggs are sealed with transparent tape and re-incubated. (iv–vi) On day 5, the tape is cut to reveal the E5 embryo that is partially covered by the CAM. The amnion is dissected and the CAM is displaced away to expose the embryo. The exposed embryo can be manipulated at this stage or re-incubated if later stages are desired. (vii) The exposed embryos do not show any defects and are accessible in ovo between E5–E8 and they can be manipulated and re-incubated until a desired time point.
Two days after windowing, the tape was cut away with scissors to access E5 embryos. At this stage embryos were covered by the amnion, and partially covered by the chorioallantoic membranes (Fig. 1iv). These membranes were carefully dissected away from the embryo as described in detail below (Fig. 1v). Two-three drops of Ringer’s solution were added to the exposed E5 embryo. At this point embryos are accessible for further experimental manipulation, or the eggs can be sealed and re-incubated if later stages of development are desired (Fig. 1vi and 1vii).
Microdissections of the Extraembryonic Membranes to Expose the E5 Embryo
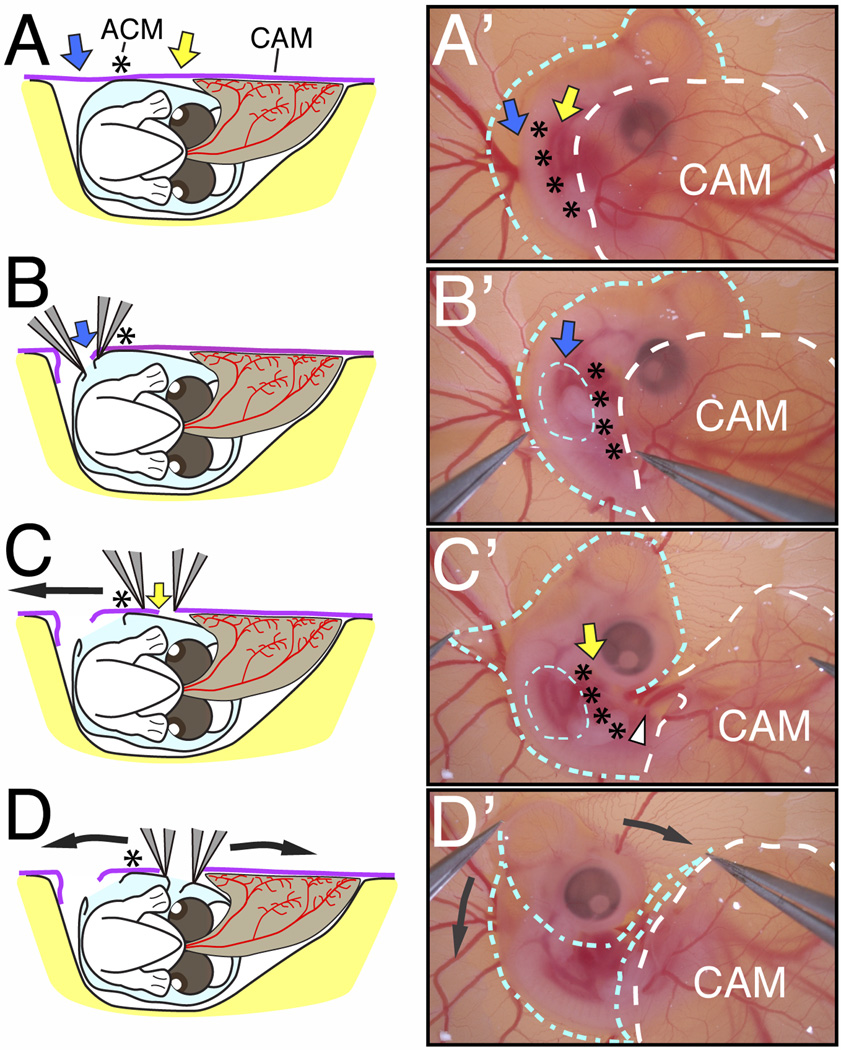
By E5, all major extraembryonic membranes including the amnion, chorion, and allantois are well developed and envelop the chick embryo. The allantois extends outward from the gut region of the embryo (Fig. 2A; brown membrane) and fuses with the chorion (Fig. 2A; purple membrane), to form the chorioallantoic membrane (CAM, Fig. 2A’). The CAM rapidly extends over the developing embryo (dotted white line, Fig. 2A’), obscuring the ventral and posterior regions of the embryo. At this stage, the apex of the amniotic membrane is fused to the chorionic membrane creating the amniochorionic membrane (ACM, Fig. 2A, asterisk). Separation of the ACM from CAM is crucial for extended exposure of the embryo during subsequent developmental stages. Although these membranes can be dissected at an earlier stage, i.e. E4, we found that performing these dissections before the establishment of the CAM reduces accessibility to embryos at later stages due to the outgrowth of the allantois.
Fig. 2.
Exposure of the E5 chick embryo in ovo. (A–D) Schematics of the embryo viewed from the posterior showing the surrounding amnion (light blue), the chorion (purple) and the vascularized allantois (brown) during membrane dissections. (A’–D’) Bright-field images of the embryo showing the location of the amnion (blue outline) and chorioallantoic membrane (white outline) during membrane dissections. The amnion and chorion are dissected as indicated by the blue and yellow arrows in the regions lateral to the ACM (asterisks). Following the separation of the embryo and surrounding amnion from the CAM (C’), the amnion is peeled away from the cranial region (D’, curved black arrows). The posterior region of the embryo slides out of the amnion and the entire embryo becomes exposed.
To ensure exposure and viability of the embryos, two critical steps have to be followed while dissecting the extraembryonic membranes. First, use fine forceps to pierce the chorion and tear a small hole in the amnion above the region of the forelimb (blue arrow and dotted blue circle, Fig. 2B and B’). This introduces slack in the chorion and amnion, which enables the dissection of a narrow, non-fused region of the chorion between the CAM and the ACM (yellow arrow, Fig. 2A and C). Second, start slightly ventral to the ACM (yellow arrow, Fig. 2C) and carefully separate the ACM from the CAM in a rostrocaudal direction (Fig. 2C and C’). These membranes are successfully separated when the CAM is displaced away from the embryo, which exposes the allantoic artery and vein (white arrowhead, Fig. 2C’). Following this separation, grasp the amniotic membrane in the mid-cranial region of the embryo and gently pull dorsoventrally (Fig. 2D and 2D’; black arrows). This dissection removes the amniotic membrane and fully exposes the embryo. The dissected amnion and intact CAM are gently displaced to the right side of the embryo, which permits their subsequent growth away from the embryo. The exposed embryo can be manipulated at E5 or re-incubated until later stages.
Accessibility and Survival of Embryos after Membrane Dissection
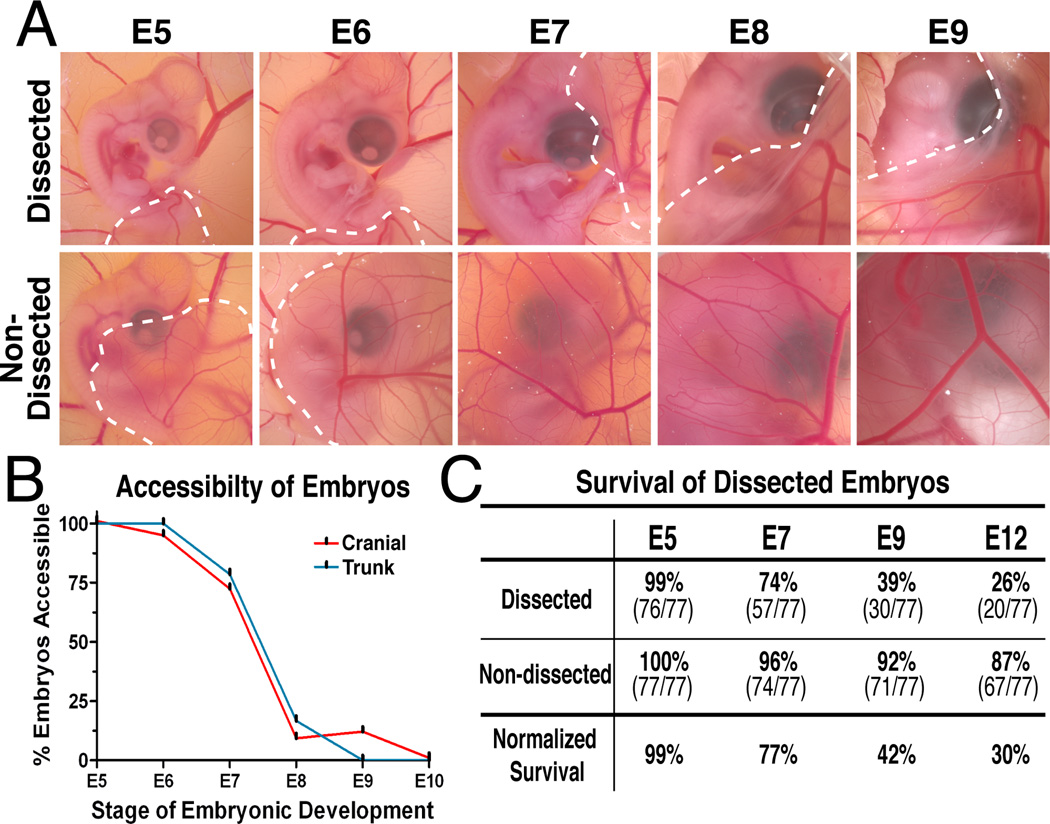
After membrane dissections, embryos were re-incubated and monitored daily for accessibility and mortality. The majority of the embryos (80%) remained fully exposed between E5 and E7 (Fig. 3A and B). The cranial region (brain, ear, eye, maxillo-mandibular process) and trunk region (limbs, ectoderm/skin, spinal cord) were easily accessible for manipulation. Between E8 and E9, the expanding chorioallantoic membrane begins to cover most of the embryos (Fig. 3A), however tissues in the cranial region remain accessible in 15% of the embryos through E9 (Fig. 3B). In the non-dissected control embryos, the chorioallantoic membrane becomes highly vascularized and grows over the embryo, which increasingly reduces accessibility to the embryo starting at E5 (Fig. 3A). In our experience, all embryos accessed directly through the chorioallantoic membrane die within 24 hours. This is possibly due to massive bleeding from injured blood vessels and concomitant leakage of the nitrogenous waste stored in the allantois into the embryonic cavity.
Fig. 3.
Exposure and survival of embryos at different stages of development. (A) Following membrane dissection, the majority of the embryos are exposed between E5–E7, before they are gradually obscured by the CAM (white dotted line) by E9. (B) The cranial and trunk regions of embryos are accessible for in ovo manipulation between E5–E7. By E9, access to the embryo is limited in most cases to the cranial region. (C) The majority of the exposed embryos are viable between E5–E7 and approximately a third of the embryos are viable during the critical period of organogenesis. The normalized numbers of surviving exposed embryos are reported as a percentage of surviving non-dissected controls at each stage.
Removal of the amnion and displacement of the chorioallantoic membrane does not appear to affect normal development of the embryos. The quality of eggs greatly affects the survival of both exposed and unexposed embryos. The survival rate of the exposed embryos was calculated as a percentage of surviving non-dissected embryos (Fig. 3C). Almost all windowed embryos are viable at E5 (99%, data not shown). The survival rate for exposed embryos remained high at E6 (95%) and at E7 (77%). Approximately half of the exposed embryos (42%) remained viable at E9, and about one third were alive at E12 (30%). In comparison to reported survival rates of about 50% after in ovo manipulation (Chapman et al., 2005; Brown et al., 2012), the current method provides ample numbers of exposed embryos that can be manipulated between E5–E8 and examined after organogenesis at about E12. This added window of opportunity for in ovo experimentation is during the dynamic period of organogenesis when complex cellular interactions occur between different tissues to form specialized organs. For example, the neural crest cells and ectoderm form the cornea (Lwigale et al., 2005; Creuzet et al., 2005); retinal tissues undergo sequential waves of dynamic gene expression essential for cell differentiation (Trimarchi et al., 2008); neural crest mesenchyme and otic placode form the inner ear (Brown et al., 2005; Wood et al., 2010; Zou et al., 2012); cranial and skeletal bones undergo chondrogenesis (Le Liévre, 1978; Merino et al., 1999), and the skin forms as a result of signaling between the ectoderm and underlying mesenchyme (Wessells, 1961; Mottet and Jensen, 1968; Rouzankina et al., 2004). Furthermore, approximately 6% of the exposed embryos were viable at E17 (3 days prior to hatching), the latest stage examined in this study. These numbers are relatively fewer than at E12, but indicate that this technique is applicable to other manipulations such as regeneration and stem cell studies, which may necessitate analysis at later stages.
Cell Injections into Exposed Embryos
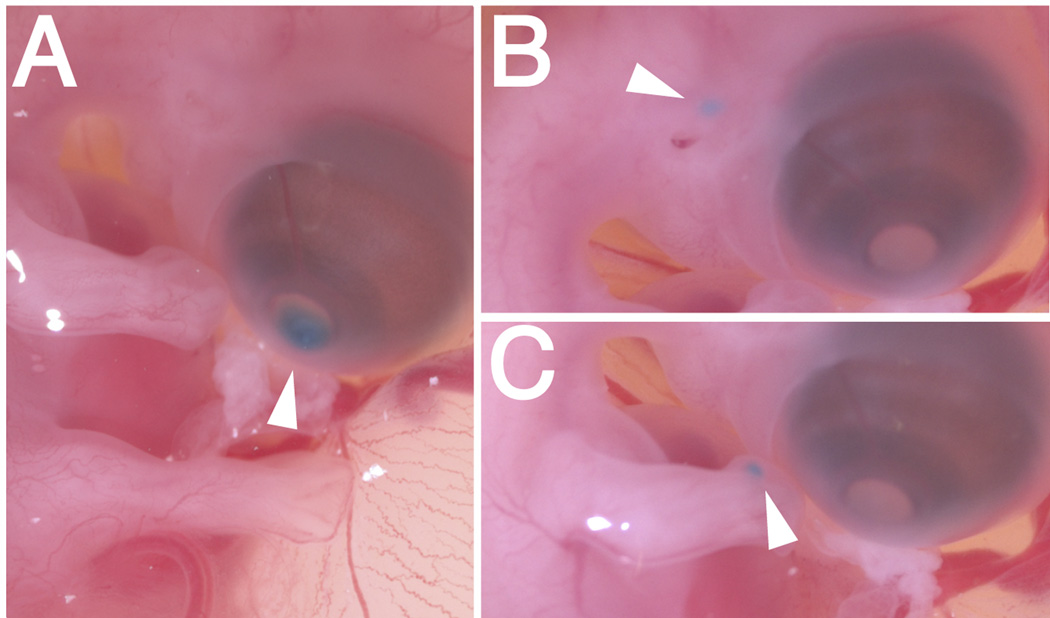
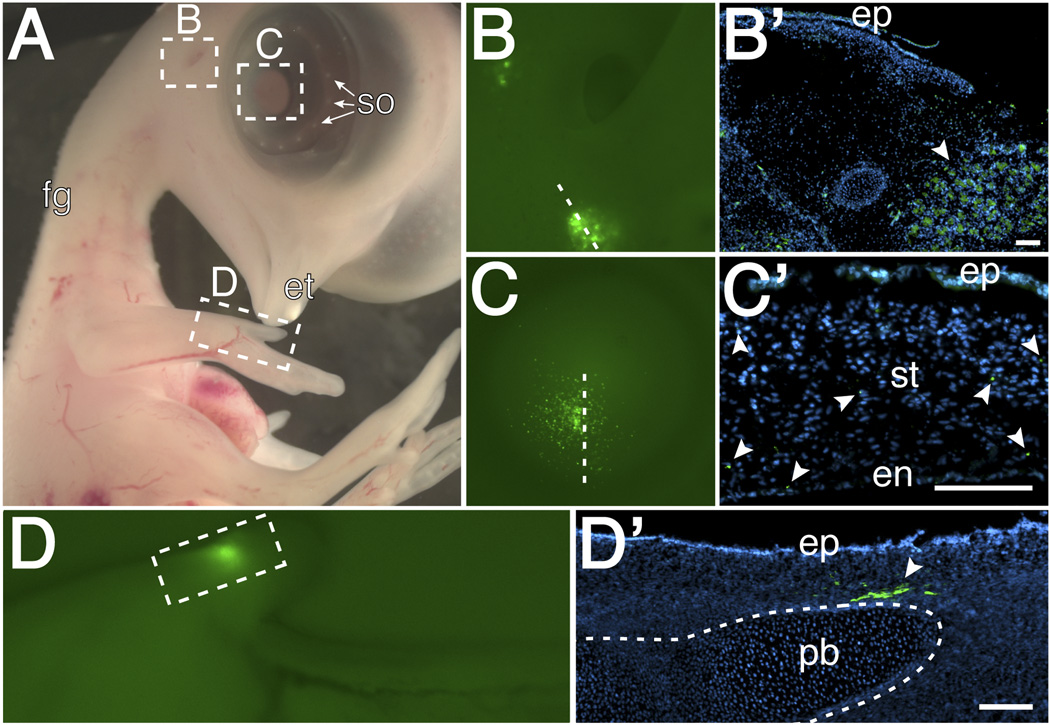
The exposed embryos are easy to manipulate using the tools that are available to developmental biologists. The effects of such manipulations can be assessed after re-incubation until a desired time point. Here we show one possible application of this method by injecting chick DF-1 cells expressing RCAS-GFP into different locations of exposed embryos that were re-incubated until E7. Embryos were injected in the cornea (Fig. 4A), mesenchyme adjacent to the ear (Fig. 4B), or into the forelimb mesenchyme (Fig. 4C), then re-incubated for additional two days.
Fig. 4.
Cell injections into E7 exposed embryos. Cells expressing RCAS-GFP were mixed with food dye and injected as indicated by the arrowheads into (A) the cornea, (B) mesenchyme adjacent to the ear, and (C) mesenchyme of the forelimb.
By E9, embryos exhibit several distinct features characteristic of this stage such as feather germs, scleral ossicles of the eye, and the egg tooth (Fig. 5A; Hamburger and Hamilton, 1951). Under fluorescent light, GFP-positive cells are visible in the ear region (Fig. 5B), in the cornea (Fig. 5C), and forelimb (Fig. 5D). GFP-positive cells could also be localized in cross-sections through these tissues (Fig. 5B’–D’). These results validate the method of cell injection into various tissues and the survival of the exposed embryo after this manipulation. A possible caveat of this approach is that some cells become post-mitotic whereas RCAS viral gene expression requires cell proliferation. In such cases, this technique can be used in combination with other well-established experimental techniques used for transient gene expression or knockdown such as electroporation with DNA, antisense morpholino oligonucleotide, and RNA interference constructs. Exposed embryos can also be used for implantation with protein-soaked beads and tissue ablation and grafts. Any of these techniques can be applied to the exposed tissues or organs on the right hand side of the embryo, leaving the unperturbed left side to serve as control.
Fig. 5.
Embryo collected 2 days after cell injections showing: (A) Normal development in absence of amnion. Under fluorescence, GFP expression was observed in (B and B’) the mesenchyme adjacent to the ear, (C and C’) the stroma region of the cornea, and (D and D’) the limb mesenchyme adjacent to a presumptive phalangeal bone. Abbreviations: fg-feather germs, et-egg tooth, so-scleral ossicles, ep-epithelium, ststroma, en-endothelium, pb-phalangeal bone. scale bar=100µm
Therefore, by extending the in ovo access to chick embryos during late stages of development, we provide a new avenue for various research interests in developmental biology. This method can also be further extrapolated and used in conjunction with current genetic manipulation and protein expression techniques, or combined with in vitro studies, thus adding to the versatility of the chick embryo as a model organism for studying developmental biology.
EXPERIMENTAL PROCEDURES
Embryos
Fertilized White Leghorn chicken eggs (TAMU, College Station, TX) were incubated at 38°C until needed. Eggs were windowed at E3 (HH stage 19–20, Hamburger and Hamilton, 1951) as described (Gammill and Krull, 2011; Griswold and Lwigale, 2012), sealed with transparent packing tape and then re-incubated at 38°C. After two days post-incubation, eggs were windowed again by cutting the tape and the extraembryonic membranes were dissected to expose the embryos at E5. Exposed embryos can be manipulated at E5 or re-incubated for later stages. For example, here we re-incubated the E5 exposed embryos and injected them with RCAS-GFP expressing cells at E7.
Cell injections
DF-1 chicken fibroblasts (ATTC, Manassas, VA) were infected with RCAS-GFP virus as previously described (Himly et. al, 1998). The resulting RCAS-GFP expressing cells were combined with food dye, loaded into pulled glass needle, and pressure-injected using Picospritzer III® (Parker Hannifin Corporation, Mayfield Heights, OH) until food dye was visible. Cells were injected into the eye, mesenchyme adjacent to the ear, and the limb mesenchyme at E7. After cell injection, into exposed embryos, eggs were sealed with transparent tape and re-incubated until E9.
Immunohistochemistry
Tissues from the injected sites were dissected out, collected in Ringer’s solution and fixed overnight in 4% paraformaldehyde at 4°C. After several washes in phosphate buffered solution (PBS), tissues were embedded in gelatin and cryosectioned at 8–10µm. Sections were immunostained with 1: 2000 dilution, anti-GFP antibody (Covance, Richmond, CA) to enhance the fluorescent signal, then counterstained with 4',6-diamidino-2-phenylindole (DAPI) using standard protocols.
Embryo Survival
Extraembryonic membrane dissection was preformed at E5 (n= 77). Eggs were sealed with tape, re-incubated at 38°C and percent survival was monitored each day after dissection until E18. Control eggs (windowed but not dissected to expose embryos, n= 77) were treated identically as exposed embryos including windowing at E3 and remaining outside the incubator for the same duration as dissected eggs.
Embryo Exposure
Following removal of extraembryonic membranes at E5, embryos were re-incubated and monitored daily to evaluate the regions of the body that are accessible for manipulation during further development. The cranial and trunk regions were scored for each embryo and recorded as percentage of the viable exposed embryos examined daily between E5–E17.
Key findings.
A multistep technique to dissect extraembryonic membranes away from the developing chick embryo.
This technique permits access to late-stage chick embryos (between E5 and E8) in ovo, which is otherwise difficult, as the extraembryonic membranes obscure chick embryos shortly after E4.
Exposed late-stage chick embryos are viable and can be manipulated using standard developmental biology techniques to study organogenesis.
ACKNOWLEDGMENTS
We thank Chelsey McKenna, Ravi Munjaal, and Sinu Agrawal for critical reading of the manuscript; Benjamin Deneen for the kind gift of the RCAS-GFP construct; Ravi Munjaal for technical assistance with RCAS-GFP virus. Research was supported in part by NIH EY018050 and EY022158 (PYL).
REFERENCES
- Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974;41:391–394. doi: 10.1016/0012-1606(74)90316-9. [DOI] [PubMed] [Google Scholar]
- del Barrio MG, Nieto MA. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development. 2002;129:1583–1593. doi: 10.1242/dev.129.7.1583. [DOI] [PubMed] [Google Scholar]
- Brown CY, Eom DS, Amarnath S, Agarwala S. A simple technique for early in vivo electroporation of E1 chick embryos. Dev Dyn. 2012;241:545–552. doi: 10.1002/dvdy.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ST, Wang J, Groves AK. Dlx gene expression during chick inner ear development. J Comp Neurol. 2005;483:48–65. doi: 10.1002/cne.20418. [DOI] [PubMed] [Google Scholar]
- Bénazéraf B, Francois P, Baker RE, Denans N, Little ND, Pourquié O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–252. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SC, Lawson A, Macarthur WC, Wiese RJ, Loechel RH, Burgos-Trinidad M, Wakefield JK, Ramabhadran R, Mauch TJ, Schoenwolf GC. Ubiquitous GFP expression in transgenic chickens using a lentiviral vector. Development. 2005;132:935–940. doi: 10.1242/dev.01652. [DOI] [PubMed] [Google Scholar]
- Creuzet S, Vincent C, Couly G. Neural crest derivatives in ocular and periocular structures. Int J Dev Biol. 2005;49:161–171. doi: 10.1387/ijdb.041937sc. [DOI] [PubMed] [Google Scholar]
- Datar S, Bhonde RR. Shell-less chick embryo culture as an alternative in vitro model to investigate glucose-induced malformations in mammalian embryo. Rev Diabet Stud. 2005;2:221–227. doi: 10.1900/RDS.2005.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan JD, Jr, Lawton MT, Glaser B, Brem H. A new technique for explanation and in vitro cultivation of chicken embryos. Anat Rec. 1991;229:125–128. doi: 10.1002/ar.1092290114. [DOI] [PubMed] [Google Scholar]
- El-Ghali N, Rabadi M, Ezin AM, De Bellard ME. New methods for chicken embryo manipulations. Microsc Res Tech. 2010;73:58–66. doi: 10.1002/jemt.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill LS, Krull CE. Embryological and genetic manipulation of chick development. Methods Mol Biol. 2011;770:119–137. doi: 10.1007/978-1-61779-210-6_5. [DOI] [PubMed] [Google Scholar]
- Goldstein RS. Transplantation of Human Embryonic Stem Cells to the Chick Embryo. Methods Mol Biol. 2006;331:137–151. doi: 10.1385/1-59745-046-4:137. [DOI] [PubMed] [Google Scholar]
- Griswold SL, Lwigale PY. Analysis of neural crest migration and differentiation by cross-species transplantation. J Vis Exp. 2012;7:3622. doi: 10.3791/3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Himly M, Foster DN, Bottoli I, Iacovoni JS, Vogt PK. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. A biological cell labeling technique and its use in experimental embryology. Dev Biol. 1973;30:217–222. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- Le Liévre CS. Participation of neural crest-derived cells in the genesis of the skull in birds. J Embryol Exp Morph. 1978;47:17–37. [PubMed] [Google Scholar]
- Luo J, Redies C. Ex ovo electroporation for gene transfer into older chicken embryos. Dev Dyn. 2005;233:1470–1477. doi: 10.1002/dvdy.20454. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Cressy PA, Bronner-Fraser M. Corneal keratocytes retain neural crest progenitor cell properties. Dev Biol. 2005;288:284–293. doi: 10.1016/j.ydbio.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Schneider RA. Other chimeras: quail-duck and mouse-chick. Methods Cell Biol. 2008;87:59–74. doi: 10.1016/S0091-679X(08)00203-3. [DOI] [PubMed] [Google Scholar]
- Merino R, Macias D, Gañan Y, Economides AN, Wang X, Wu Q, Stahl N, Sampath KT, Varona P, Hurle JM. Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev Biol. 1999;206:33–45. doi: 10.1006/dbio.1998.9129. [DOI] [PubMed] [Google Scholar]
- Mottet NK, Jensen HM. The differentiation of chick embryonic skin: An electron microscopic study with a description of peculiar epidermal cytoplasmic ultrastructure. Exp Cell Res. 1968;52:261–283. doi: 10.1016/0014-4827(68)90564-8. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Katahira T, Watanabe Y, Funahshi J. Gain- and loss-of-function in chick embryos by electroporation. Mech Dev. 2004;121:1137–1143. doi: 10.1016/j.mod.2004.05.013. [DOI] [PubMed] [Google Scholar]
- New DAT. A new technique for the cultivation of the chick embryo in vitro. J Embryol Exp Morph. 1955;3:320–332. [Google Scholar]
- Rouzankina I, Abate-Shen C, Niswander L. Dlx genes integrate positive and negative signals during feather bud development. Dev Biol. 2004;265:219–233. doi: 10.1016/j.ydbio.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Barembaum M. Gain- and loss-of-function approaches in the chick embryo. Methods Cell Biol. 2008;87:237–256. doi: 10.1016/S0091-679X(08)00212-4. [DOI] [PubMed] [Google Scholar]
- Summerbell D. A quantitative analysis of the effect of excision of the AER from the chick limb-bud. J Embryo Exp Morph. 1974;32:651–660. [PubMed] [Google Scholar]
- Trimarchi JM, Harpavat S, Billings NA, Cepko CL. Thyroid hormone components are expressed in three sequential waves during development of the chick retina. BMC Dev Biol. 2008;8:1–20. doi: 10.1186/1471-213X-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells NK. An analysis of chick epidermal differentiation in situ and in vitro in chemically defined media. Dev Biol. 1961;3:355–389. doi: 10.1016/0012-1606(61)90052-5. [DOI] [PubMed] [Google Scholar]
- Wood JL, Hughes AJ, Mercer KJ, Chapman SC. Analysis of chick (Gallus gallus) middle ear columella formation. BMC Dev Biol. 2010;10:1–12. doi: 10.1186/1471-213X-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Mak SS, Liu HZ, Han DY, Zhuang HX, Yang SM, Ladher RK. Induction of the chick columella and its integration with the inner ear. Dev Dyn. 2012;241:1104–1110. doi: 10.1002/dvdy.23788. [DOI] [PubMed] [Google Scholar]