Abstract
Global international trends in female breast cancer incidence have been described previously but no comparable analysis of male breast cancer incidence rates has been conducted. We obtained male and female case and population data using Cancer Incidence in Five Continents (CI5). We calculated age-adjusted sex-specific incidence rates and female-to-male incidence rate ratios (FMIRRs) and compared trends of such for the period 1988–2002. This analysis included 8,681 male breast cancer cases and 1.14 million female breast cancer cases. The highest male incidence rate was observed in Israel at 1.24 per 100,000 man-years, and the highest female incidence rate was observed in the USA at 90.7 per 100,000 woman-years. The lowest incidence rates for males (0.16) and females (18.0) were observed in Thailand. In general, male breast cancer incidence trends were variable; a minority of countries displayed evidence for an increase. In contrast, female incidence rates have been increasing in a majority of countries. The Pearson correlation coefficient (r) for male and female breast cancer incidence rates by country during 1988–2002 was 0.69. Male breast cancer rates were generally less than 1 per 100,000 man-years, in contrast to the much higher rates of female breast cancer, providing for an overall FMIRR of 122. The differences in both incidence rates and time trends between males and females may reflect sex differences in underlying risk factors, pathogenesis, and/or over-diagnosis. Conversely, the high correlation between male and female breast cancer incidences may indicate that both sexes share some common risk factors for breast cancer.
Keywords: Breast Neoplasms, Sex, Incidence, Trends, Epidemiology, Male, Female
Introduction
The vast majority of cancer types have a higher incidence rate in males relative to females 1, with one of the few and obvious exceptions to the rule being breast cancer. It is estimated, for example, that 232,620 people will be diagnosed with breast cancer in the USA in 2011, with less than 1% of these cases appearing in men 2. Breast cancer has traditionally been considered a female-specific disease, and lack of awareness of its occurrence in men may lead to diagnoses at later age and more advanced stage than in females 3.
A previous analysis using cancer incidence data from the United States Surveillance, Epidemiology and End Results (SEER) databases examined male breast cancer and compared this with female breast cancer 4. Similar breast cancer incidence trends among males and females were interpreted to suggest the existence of common breast cancer risk factors that influence both sexes. Although the evidence is equivocal as to whether men diagnosed with breast cancer have a worse prognosis, cancer-specific survival, and overall survival, compared with female breast cancer cases 3, 5–8, recent histopathologic 5, 7, 9, 10 and molecular 11–13 studies are persuasive to the thesis of heterogeneity in pathogenesis of disease by sex. In recent years there has been an increasing number of epidemiologic studies of male breast cancer, and we are slowly unraveling the risk factor profile of this rare disease 14–17. To aid hypotheses and direction of such research, we undertook the first global international comparison of female and male breast cancer incidence rates using data from Cancer Incidence in Five Continents (CI5) in order to evaluate sex-specific incidence trends as well as incidence rate ratios for the period 1988–2002.
Materials and Methods
Data were provided by the International Agency for Research on Cancer (IARC) and were derived from CI5 Volumes I–IX (1958–2002) 18. Female and male breast cancer case counts and their corresponding populations, in single calendar years, were obtained for each of 18 age-groups (0–4, 5–9,…, 80–84, 85+) for 88 registries of various ethnic groups in the Americas, Europe, Asia, and Oceania. We grouped these registries by country to enable calculation of sex-specific, age-standardized incidence rates for each of 25 countries for up to nine consecutive 5-year calendar periods (1958–1962, 1963–1967,…, 1998–2002). A listing of registry and time period coverage for each country is included in a Table 1. Incidence rates per 100,000 man or woman-years were age-adjusted to the World Standard Population 19, 20. The female-to-male incidence rate ratio (IRR) was calculated using the female age-adjusted incidence rate as the numerator and the male age-adjusted incidence rate as the denominator. Ninety-five percent confidence intervals for the female-to-male IRRs and sex-specific incidence rates were estimated for each 5-year calendar period as well as the total combined period for each country, according to the method of Tiwari et al 21.
Table 1.
Registries and time periods available for analysis.
| Country | Region | Time Period |
|---|---|---|
| Australia | Australia, New South Wales | 1983–2002 |
| Australia, South | 1978–2002 | |
| Australia, Tasmania | 1978–2002 | |
| Australia, Victoria | 1983–2002 | |
| Australia, Western | 1983–2002 | |
|
| ||
| Austria | Austria, Tyrol | 1988–2002 |
|
| ||
| Brazil | Brazil, Goiania | 1988–2002 |
|
| ||
| Canada | Canada, Alberta | 1973–2002 |
| Canada, British Columbia | 1978–2002 | |
| Canada, Manitoba | 1958–2002 | |
| Canada, New Brunswick | 1978–2002 | |
| Canada, Nova Scotia | 1978–2002 | |
| Canada, Prince Edward Island | 1983–2002 | |
| Canada, Newfoundland | 1978–2002 | |
| Canada, Ontario | 1978–2002 | |
| Canada, Saskatchewan | 1968–2002 | |
|
| ||
| Colombia | Colombia, Cali | 1983–2002 |
|
| ||
| Costa Rica | Costa Rica | 1983–2002 |
|
| ||
| Denmark | Denmark | 1978–2002 |
|
| ||
| Ecuador | Ecuador, Quito | 1988–2002 |
|
| ||
| Estonia | Estonia | 1968–2002 |
|
| ||
| France | France, Bas-Rhin | 1978–2002 |
| France, Calvados | 1978–2002 | |
| France, Doubs | 1978–2002 | |
| France, Haut-Rhin | 1988–2002 | |
| France, Herault | 1988–2002 | |
| France, Isere | 1979–2002 | |
| France, Somme | 1983–2002 | |
| France, Tarn | 1983–2002 | |
|
| ||
| Iceland | Iceland | 1958–2002 |
|
| ||
| India | India, Mumbai | 1978–2002 |
| India, Chennai | 1983–2002 | |
|
| ||
| Israel | Israel: Jews | 1963–2002 |
|
| ||
| Italy | Italy, Florence | 1985–2002 |
| Italy, Parma | 1978–2002 | |
| Italy, Ragusa Province | 1983–2002 | |
| Italy, Romagna | 1988–2002 | |
| Italy, Torino | 1985–2002 | |
| Italy, Lombardy, Varese province | 1978–2000* | |
| Italy, Modena | 1988–2002 | |
|
| ||
| Japan | Japan, Miyagi Prefecture | 1978–2002 |
| Japan, Osaka Prefecture | 1963–2002 | |
|
| ||
| Netherlands | The Netherlands, Eindhoven | 1973–2002 |
|
| ||
| Philippines | Philippines, Manila | 1983–2002 |
|
| ||
| Poland | Poland, Cracow City | 1978–2002 |
| Poland, Warsaw city | 1988–2002 | |
|
| ||
| Singapore | Singapore: Chinese | 1968–2002 |
| Singapore: Malay | 1968–2002 | |
|
| ||
| Slovakia | Slovakia | 1973–2002 |
|
| ||
| Spain | Spain, Granada | 1985–2002 |
| Spain, Murcia | 1983–2001* | |
| Spain, Navarra | 1973–2002 | |
| Spain, Tarragona | 1983–2001* | |
| Spain, Zaragoza | 1978–2000* | |
|
| ||
| Switzerland | Switzerland, Geneva | 1973–2002 |
| Switzerland, St Gall-Appenzell | 1983–2002 | |
|
| ||
| Thailand | Thailand, Chiang Mai | 1983–2002 |
|
| ||
| UK | UK, England, Merseyside and Cheshire | 1978–2002 |
| UK, England, North Western | 1979–2002 | |
| UK, England, Oxford | 1985–2002 | |
| UK, England, Birmingham & West Midlands Region | 1979–2002 | |
| UK, England, Yorkshire | 1983–2002 | |
| UK, Scotland | 1978–2002 | |
|
| ||
| USA | USA, SEER (9 registries) | 1978–2002 |
Time period ends before 2002. Italicized country names indicate data are regional or restricted by ethnicity.
In each of the male and female data extractions, populations for age-group 85+ or both age-groups 80–84 and 85+ were collapsed into the preceding age-group in several registries from developing countries. Consequently, incidence rates were age adjusted in five-year categories (0–4 years, 5–9, 10–14, etc) with the final category being ages 75+ years; the world standard population weight for the age group 75+ years was calculated by summing the weights for the age groups 75–79, 80–84, and 85+. The starting year in which data were available varied by registry, thereby reducing periods of coverage for certain countries from the maximum possible of 45-years (see Table 1 for details). Cancer incidence data for the period 1988–2002 were available from at least one registry for each country included in this analysis, thus these years constituted the primary analytic period. The number of registries and period covered by each respective registry varied by country. Countries for which data were restricted to regions or ethnicities are presented in italic font in tables and figures.
International trends in male and female breast cancer incidences were assessed, tabulated, and plotted for the period 1988–2002. Estimated annual percentage changes (EAPCs) were estimated by fitting a least squares regression to the natural logarithm of rates based on three-year groupings for the period 1988–2002, with ninety-five percent confidence intervals calculated using the t-distribution 22. Sex-specific incidence rates were also evaluated stratified by age-group (<50, ≥50 years) and by the three most recent 5-year calendar periods (1988–92, 1993–97, 1998–2002). Countries with the highest male breast cancer incidence rates during the period 1958–2002 were graphed separately with female breast cancer incidence rates and male-to-female IRRs for further investigation. Additional analyses were conducted which assessed the correlations between male and female breast cancer incidence rates, and between sex-specific breast cancer rates and health expenditure per capita for the year 2000 23. Incidence rates were also stratified by health expenditure per capita. We also compared sex-specific breast cancer incidence rates and MFIRRs for periods pre- (1978–1982) and post- (1998–2002) introduction of national screening programs for women. For this analysis we selected all countries that had introduced a national screening program prior to 1996 24–28 as to allow time for implementation to assess the effects of such on incidence rates during 1998–2002. Of the countries available for analysis, only Columbia and Poland did not have a national screening program and were considered to be relatively unaffected by opportunistic screening prior to 2003, and only Poland had cancer incidence data for the period 1978–1982. Thus Poland was also assessed in this subgroup analysis as a “negative control”. Stata (StataCorp LP, 2011, release 11.2) was used for data processing and analysis, and SigmaPlot (Systat Software, Inc., 2008, version 11.0) was used to produce graphs.
Results
Table 2 shows overall sex-specific, age-adjusted incidence rates and female-to-male IRRs for the period 1988–2002 for each country, as well as their respective 95% confidence intervals. The highest female breast cancer incidence rates occurred in the USA (90.7 per 100,000 woman-years), and the lowest rate was seen in Thailand (18.0). Breast cancer incidence in males was highest in Israel (1.24 per 100,000 man-years) and lowest in Thailand (0.16). The female-to-male IRR was highest in Singapore (205) and lowest in the Philippines (55). Variability in the IRR values did not appear to be explained by continent. As can be seen from the EAPCs, female breast cancer incidence rates have increased for all countries included in this analysis. This was statistically significant for 18 of the 25 countries assessed (Table 2), while consistent trends across five-year time periods were observed for 21 of the 25 countries (Supplementary Table 1). Of particular note are the EAPCs for Thailand (4.17), Singapore (3.47), Costa Rica (3.25), and Brazil (3.21). Conversely, in our assessment of male breast cancer incidence rates there appeared to be much less consistency in trends across the five-year periods displayed—nine (Philippines, Austria, Denmark, Estonia, Poland, Spain, UK, Canada, Australia) of the 25 countries presented consistent patterns (Supplementary Table 1). EAPCs indicated that the greatest increases in incidence were in Austria (12.04), Brazil (10.45), and Costa Rica (5.47), while the greatest decreases were in Australia (−3.38), Poland (−2.28), and Singapore (−1.96). However, it should be noted that EAPC confidence intervals for male breast cancer incidence trends were wide, a factor attributable to the small numbers of cases that accrue each year of this rare disease.
Table 2.
Sex-specific, Age-adjusted Breast Cancer Incidence Rates and Female-to-male Incidence Rate Ratios, All Ages, 1988–2002
| Population (years) | Female Cases (n ) | Females
|
Male Cases (n ) | Males
|
Female-to-Male Incidence Rate Ratio (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Incidence Rate (95% CI) | EAPC (95%CI) | Incidence Rate (95% CI) | EAPC (95%CI) | ||||
| Asia | |||||||
| India | 20,814 | 26.8 (26.4, 27.2) | 0.63 (−0.47, 1.75) | 321 | 0.42 (0.37, 0.47) | 0.84 (−1.93, 3.69) | 64.3 (57.5, 72.3) |
| Israel | 35,725 | 87.2 (86.3, 88.2) | 2.32 (1.13, 3.53) | 488 | 1.24 (1.13, 1.36) | 0.35 (−6.59, 7.82) | 70.4 (64.3, 77.5) |
| Japan | 37,846 | 30.4 (30.1, 30.7) | 2.35 (0.81, 3.92) | 221 | 0.18 (0.16, 0.21) | 1.06 (−2.24, 4.47) | 168.7 (148.2, 193.8) |
| Philippines | 13,171 | 54.2 (53.2, 55.1) | 0.66 (−0.92, 2.26) | 172 | 0.98 (0.83, 1.16) | 2.10 (−2.81, 7.25) | 55.0 (47.0, 65.2) |
| Singapore | 10,815 | 47.2 (46.3, 48.2) | 3.47 (3.18, 3.77) | 43 | 0.23 (0.17, 0.31) | −1.96 (−9.93, 6.73) | 204.5 (153.8, 285.3) |
| Thailand | 2,078 | 18.0 (17.3, 18.8) | 4.17 (2.72, 5.63) | 17 | 0.16 (0.09, 0.26) | - | 111.3 (71.7, 195.6) |
| Europe | |||||||
| Austria | 5,168 | 68.9 (66.9, 70.9) | 1.05 (−0.15, 2.26) | 31 | 0.45 (0.30, 0.66) | 12.04 (−0.16, 25.74) | 151.8 (107.7, 229.6) |
| Denmark | 51,074 | 80.0 (79.2, 80.7) | 1.13 (0.56, 1.70) | 349 | 0.54 (0.48, 0.60) | 0.59 (−1.70, 2.94) | 148.1 (133.0, 165.8) |
| Estonia | 7,540 | 41.7 (40.7, 42.7) | 2.64 (1.32, 3.98) | 64 | 0.49 (0.37, 0.62) | 1.02 (−0.06, 2.11) | 85.7 (67.7, 111.9) |
| France | 51,487 | 83.6 (82.8, 84.3) | 1.59 (1.18, 2.01) | 455 | 0.77 (0.70, 0.85) | −0.32 (v3.76, 3.24) | 108.4 (98.8, 119.5) |
| Iceland | 1,908 | 78.1 (74.5, 81.8) | 0.91 (−3.10, 5.09) | 23 | 0.93 (0.58, 1.42) | −1.74 (−20.66, 21.69) | 84.2 (56.8, 137.1) |
| Italy | 54,097 | 77.6 (76.9, 78.3) | 2.61 (1.82, 3.41) | 560 | 0.83 (0.76, 0.91) | 0.67 (−5.43, 7.16) | 93.0 (85.4, 101.7) |
| Netherlands | 8,748 | 83.1 (81.3, 84.9) | 2.29 (0.92, 3.69) | 50 | 0.51 (0.38, 0.68) | 0.45 (−5.15, 6.39) | 163.4 (125.3, 222.0) |
| Poland | 15,681 | 50.9 (50.1, 51.7) | 2.06 (0.22, 3.94) | 122 | 0.50 (0.42, 0.60) | −2.28 (−5.67, 1.23) | 101.3 (85.2, 122.5) |
| Slovakia | 23,474 | 42.5 (42.0, 43.1) | 2.19 (1.25, 3.15) | 220 | 0.48 (0.42, 0.55) | 1.89 (−2.58, 6.57) | 87.9 (77.1, 101.0) |
| Spain | 21,216 | 53.1 (52.3, 53.8) | 2.48 (1.52, 3.46) | 200 | 0.50 (0.43, 0.58) | 3.85 (−0.95, 8.89) | 106.4 (92.2, 124.0) |
| Switzerland | 8,307 | 75.7 (74.0, 77.5) | 2.15 (1.51, 2.79) | 55 | 0.55 (0.41, 0.73) | 1.29 (−7.91, 11.41) | 136.9 (105.4, 185.0) |
| UK | 212,665 | 76.4 (76.1, 76.8) | 1.27 (0.09, 2.47) | 1,373 | 0.50 (0.47, 0.53) | 0.63 (−1.43, 2.72) | 152.5 (144.4, 161.3) |
| America | |||||||
| Brazil | 2,689 | 45.9 (44.2, 47.8) | 3.21 (1.90, 4.53) | 20 | 0.48 (0.29, 0.74) | 10.45 (2.09, 19.50) | 96.0 (63.6, 160.9) |
| Canada | 184,771 | 79.4 (79.0, 79.7) | 0.32 (−0.43, 1.07) | 1,307 | 0.59 (0.56, 0.62) | 1.57 (−1.51, 4.75) | 134.5 (127.3, 142.3) |
| Colombia | 4,538 | 40.5 (39.3, 41.8) | 2.54 (0.15, 4.99) | 20 | 0.23 (0.14, 0.35) | 0.09 (−23.62, 31.16) | 176.8 (117.7, 295.0) |
| Costa Rica | 6,296 | 32.0 (31.2, 32.8) | 3.25 (2.12, 4.39) | 54 | 0.30 (0.22, 0.39) | 5.47 (−7.62, 20.41) | 107.9 (83.4, 145.2) |
| Ecuador | 2,212 | 29.0 (27.8, 30.3) | 0.87 (−0.96, 2.73) | 19 | 0.29 (0.17, 0.46) | 0.95 (−16.27, 21.72) | 99.7 (65.3, 170.1) |
| USA | 241,875 | 90.7 (90.3, 91.0) | 0.57 (−0.07, 1.22) | 1,693 | 0.72 (0.69, 0.76) | 0.30 (−2.10, 2.75) | 125.8 (119.8, 132.3) |
| Oceania | |||||||
| Australia | 114,877 | 78.1 (77.6, 78.6) | 2.11 (0.46, 3.79) | 804 | 0.55 (0.51, 0.59) | −3.38 (−5.94, −0.74) | 143.1 (133.5, 153.9) |
Cancer Incidence in Five Continents: July 2011 Submission (1988–2002). Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity.
Abbreviations: CI, confidence interval; EAPC, estimated annual percentage change. “-“ not able to be estimated due to one or more 3-year period having zero counts of cases. EAPCs in bold indicate statistical significance at alpha=0.05.
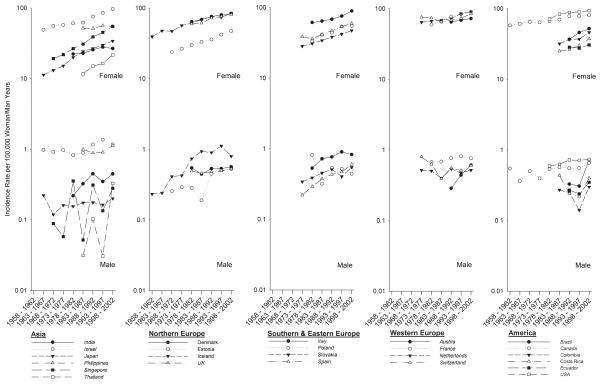
Figure 1 depicts trends in female and male breast cancer incidences for each continent (except Oceania) by country for the full period of analysis (1958–2002) for all ages. For a majority of countries, male breast cancer incidence trends are either difficult to discern, due to volatility attributable to small numbers (Singapore, Thailand), or show some evidence of an increase (India, Iceland, Italy, Spain, Brazil). However, even those countries which indicate an increase, caution is warranted as these rates are still based on small numbers. Female breast cancer rates appear to be steadily increasing over time regardless of continent. Countries that have high or low female breast cancer incidence rates tend to have correspondingly high or low male breast cancer incidence rates. In Northern Europe, the lowest female breast cancer incidence rates and lowest male breast cancer incidence rates over time were consistently reported by Estonia. In Southern/Eastern Europe, Italy had the highest incidence of male breast cancer and female breast cancer. Male breast cancer incidence is markedly higher in North American countries compared with countries in South and Central America (except for Brazil in the most recent time period), and this observation is also true for female breast cancer. For countries that had the highest, and thus most stable, male breast cancer incidence rates, we plotted sex-specific incidence rates and female-to-male IRRs for individuals of all ages (Supplementary Figure 1). For each of these countries, the female-to-male IRR over time represented a mirror image of male breast cancer incidence, indicating that this statistic is largely a function of the percentage change in male breast cancer incidence between each time period being large, relative to the percentage change in female breast cancer incidence rates.
Figure 1.
Trends in Sex-specific, Age-adjusted Incidence Rates for selected countries Stratified by Continent, All Ages, 1958–2002.
Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity.
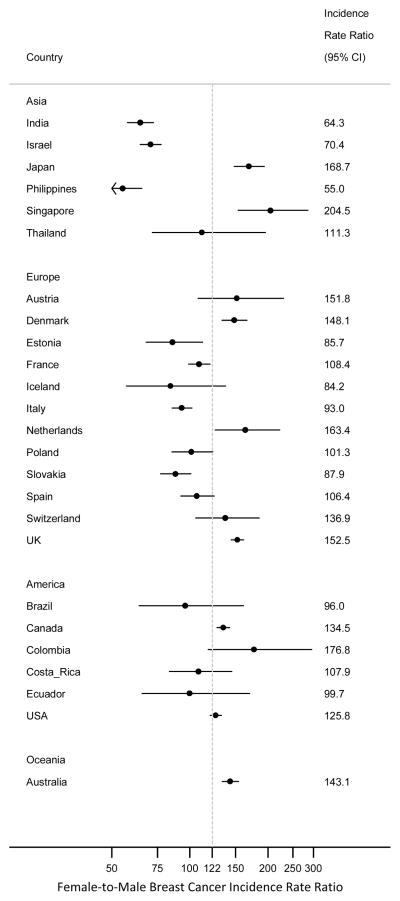
A forest plot consisting of international female-to-male IRRs and corresponding 95% confidence intervals for the period 1988–2002 for individuals of all ages is shown in Figure 2. This graph illustrates how each country’s IRR differs from each other as well as the same metric calculated for all countries combined (i.e., a world estimate). As can be seen, there is wide range of IRRs in each continent, which reiterates the observation that IRR differences are not explained by continent. For many countries, the IRR estimates are significantly different from the world female-to-male IRR estimate for individuals of all ages, 1988–2002, of 122 (95%CI: 119, 124), which is the result of large differences in both male and female breast cancer rates within and between country. World estimates of female and male breast cancer incidence rates, that contributed to this IRR, were 68.5 (95%CI:68.3, 68.6) per 100,000 woman-years and 0.56 (95%CI:0.55, 0.57) per 100,000 man-years, respectively.
Figure 2.
Forest plot of Female-to-male Breast Cancer Incidence Rate Ratios, All Ages, 1988–2002.
The grey dashed line represents the female-to-male IRR of 122 for all countries combined.
Abbreviation: CI, confidence interval.
We also stratified sex-specific, age-adjusted incidence rates by age-group (<50, & ≥50 years) for the period 1988–2002 (Supplementary Table 2). We found male breast cancer incidence was highest in Iceland in the <50 years age-group and highest in Israel in the ≥50 years age-group. These are distinct from the countries which have historically had the highest rates of female breast cancer—France in the <50 years age-group and the USA in the ≥50 years age-group. The lowest female and male incidence rates were generally observed in Thailand, regardless of age. Even though the rankings of countries for female and male breast cancer did not align perfectly, the Pearson correlation coefficient r for male and female breast cancer incidence rates during 1988–2002 for all ages was 0.69 (95%CI: 0.41, 0.85; p<0.0001; Figure 3). Differences in sex-specific IRRs (incidence rates for ages ≥50 years / incidence rates for ages <50 years) demonstrated that the impact of age on male breast cancer rates was greater, when comparing older to younger age-groups, than that observed for female breast cancer. For example, in the US the male IRR was 31.6 compared with the female IRR of 11.0 (Supplementary Table 2).
Figure 3.
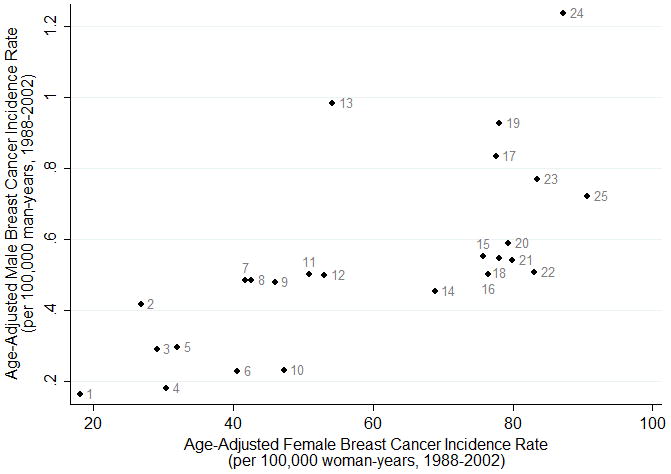
Correlation between Age-Adjusted Male Breast Cancer Incidence Rate and Female Breast Cancer Incidence Rate, All Ages, 1988–2002.
1Thailand, 2India, 3Ecuador, 4Japan, 5Costa Rica, 6Colombia, 7Estonia, 8Slovakia, 9Brazil, 10Singapore, 11Poland, 12Spain, 13Philippines, 14Austria, 15Switzerland, 16United Kingdom, 17Italy, 18Australia, 19Iceland, 20Canada, 21Denmark, 22Netherlands, 23France, 24Israel, 25United States.
Supplementary Figure 2 portrays the relationships between sex-specific breast cancer incidence rates for individuals aged ≥50 years, 1998–2002, and health expenditure per capita for the year 2000. There was a positive association between age-adjusted female breast cancer incidence rates and health expenditure per capita (r=0.68, 95% CI: 0.41, 0.85; p<0.0001), with Japan a notable outlier. There was no significant relationship observed between age-adjusted male breast cancer incidence rates and health expenditures (r=0.19, 95%CI: −0.23, 0.54; p=0.37). These correlations were similar when using individuals of all ages (data not shown). Lastly, assessment of incidence rates by health expenditure per capita indicated higher rates of female and male breast cancer, and higher FMIRRs, for the second and third tertiles compared with the first tertile (Supplementary Table 3).
An analysis of incidence rates from countries that introduced national screening programs for women shows that female breast cancer incidence rates increased by approximately 35% from 60 to 81 per 100,000 women-years between the periods 1978–1982 and 1998–2002 (Supplementary Table 4). Conversely, equivalent rates for the same time interval in Poland—a country that had not introduced a national screening program or widely utilized opportunistic screening during the period analyzed—increased 65% from 33 to 55 per 100,000 woman-years.
Discussion
In this analysis of CI5 data, we have shown that male breast cancer rates are generally less than 1 per 100,000 man-years, in contrast to the much higher rates of female breast cancer, a fact underscored by the high female-to-male IRR of 122 for all countries combined. Although some of the EAPC’s for male breast cancer incidence during the period 1988–2002 were large, confidence in these estimates is wide due to the rarity of this disease. Conversely, female breast cancer incidence has been consistently increasing in a majority of countries assessed. Finally, the high correlation between male and female breast cancer incidences (r=0.69, 95%CI: 0.41, 0.85; p<0.0001) may indicate that some risk factors have common effects in both men and women.
Although this study has shown male breast cancer incidence rates to be universally low, relative to female breast cancer, there still existed dramatic and substantial variability between countries. The highest overall age-adjusted rates occurred in Israel (1.08 per 100,000 man-years), followed closely by the Philippines (0.99), Italy (0.80), and then France (0.75). The lowest rates of male breast cancer were recorded in Thailand (0.14), then Japan (0.17), Singapore (0.19) and Colombia (0.24). Part of the reason for such variability in rates may be population genetic susceptibility. For example, three founder mutations in BRCA1 (185delAG and 5382insC) and BRCA2 (6174delT) genes have been observed at higher frequency (>2% in total) in the Ashkenazi Jewish population than in the general USA population 29, 30. A population bottleneck effect has been suggested to explain the increased frequencies of these haplotypes in the Ashkenazi Jewish population, as genetic drift and founder effects can quickly occur in small interbreeding populations, resulting in reduced genetic variation 31. Previous studies have associated mutations in BRCA1 and BRCA2 with male breast cancer, which may explain the high male breast cancer incidence in Israel 32 and warrant further genetic studies in other populations with relatively high incidence rates. Although such studies should not be restricted to loci implicated in female breast cancer, the good correlation between male and female breast cancer incidence rates (r=0.69) may indicate that genetic susceptibility to this disease is similar in men and women. In order to utilize powerful agnostic approaches to determine whether male-specific genetic susceptibility breast cancer loci exist, it will be necessary to obtain a large numbers of cases—a difficult hurdle to overcome given the rarity of this malignancy in men.
The high correlation between female and male breast cancer incidence rates could indicate that environmental exposures which increase breast cancer risk are similar between men and women. Indeed, akin to female breast cancer, male breast cancer has been associated with conditions which imply a hormonal pathogenesis of disease including Klinefelter syndrome, obesity, physical inactivity, and diabetes 14, 15, 17, 33, 34. In addition, it has been suggested that male breast cancer may be etiologically comparable to late-onset female breast cancer, given similar prognostic characteristics, age-frequency distribution, and age-specific incidence patterns 35, with sex steroid hormone-independent risk factors driving carcinogenesis 4. These hypotheses deserve further attention. Conversely, within-country temporal trends for men and women have not always been equivalent and this may suggest sex-specific risk factors due to differences in exposure or biological processing 36. The most stark examples of sex-disparities in breast cancer incidence trends presented herein include Japan (202% increase in female breast cancer rates, 10% decrease in male breast cancer rates), Israel (97% increase in female breast cancer rates, 19% increase in male rates), Austria (12% increase in female breast cancer rates, 113% increase in male rates) and India (20% increase in female breast cancer rates, 104% increase in male rates). Although a degree of caution is warranted in interpretation of such comparisons, given the relative instability of male rates, these observations are suggestive of different risk factor profiles for men and women. Indeed, major risk factors for female breast cancer stems from the varying reproductive and menstrual behavior of women which are obviously unique to this sex 3. In addition, although there is evidence both for 3, 5, 6 and against 7, 8 the thesis that male breast cancer patients have worse prognosis, cancer-specific survival, and overall survival, compared with female breast cancer cases, histopathologic evidence for differences in the frequency of breast cancer subtypes between men and women is strong and compelling 5, 7, 9, 10. Moreover, recent molecular analyses of male and female breast cancer have provided evidence for differences in the transcriptome 11, 12 and cancer genome 13, which may also support the idea of heterogeneity in pathogenesis of disease by sex.
Some of the perceived differences in breast cancer incidence trends between men and women could be an artificial effect created by the availability and/or use of screening modalities (e.g., self-exam, mammography) 37–39. There is evidence that high-income countries, such as the USA, may enhance diagnosis or even that some tumors may be the result of “over-diagnosis” 40; that is, diagnosis of cancers that would never have caused symptoms or death. In support of these propositions, we found a strong correlation (r=0.68, 95% CI: 0.41, 0.85; p<0.0001) between female breast cancer incidence rates (for the period 1998–2002 and ages ≥50 years) and health-care expenditure per capita for the year 2000; Japan’s outlying status may be attributable to protective effects conferred by soy foods, which are rich in isoflavones 41. For males, the correlation with health-care expenditure was much weaker (r=0.19), as may be expected given the absence of breast cancer screening programs for men. The finding that Polish female breast cancer incidence rates increased more than that of countries which introduced national screening programs, during the time period analyzed, could be interpreted as evidence against the idea of over-diagnosis, although such international comparisons could be complicated by differential use of self- and/or clinical breast examinations, differential underlying trends of incident disease, and differential ascertainment of disease. Within-country analyses are likely to better inform on the extent of over-diagnosis of breast cancer 40.
Previous analyses of US SEER data have indicated that rates of male breast cancer have undergone a minor increase since the late 1970s 4, 35, 42, 43. Similar analyses have been conducted for five European registries and Singapore 3, and for the UK 44 but age standardized incidence rates from these studies suggest that only Singapore has had a slight increase in male breast cancer incidence, with the rest of the countries appearing to have stable rates. In our analyses presented herein, which covers a much larger number of countries and periods of time than any previous study, we observed positive EAPCs for a majority of countries, with those from Austria (EAPC=12.04), Brazil (10.45), and Costa Rica (5.47) of particular note. However, the rarity of this malignancy in men does not provide for high confidence in these estimates and a degree of caution is warranted in their interpretation.
There is evidence to suggest that between-country differences in female breast cancer incidence rates may be attributable to variability in exposure to breast cancer risk factors. For example, temporal trends demonstrate that female breast cancer incidence rates undergo gradual changes which would suggest changes in environmental exposures rather than a change in diagnostic procedures or introduction of a new screening test. In addition, studies have found that breast cancer risk is associated with country of residence rather than country of origin—Shin et al. 45 reported that breast cancer incidence among Asian-American women residing in the US was 1.5–4 times higher than what was found in the women’s own countries of origin. Moreover, the timing of ancestral migration has also been shown to affect this risk 46. These effects of Western lifestyles and exposures may include reproductive factors, such as delayed childbearing, early age at menarche, or late age at menopause; a higher fat diet 47; and/or use of hormonal replacement therapy for menopause 48. Geographic differences in breast cancer incidence rates presented herein could be leveraged in the design of future migrant studies to aid determination of female, and possibly male, risk factors for this malignancy.
Strengths of this study include the use of Cancer Incidence in Five Continents data which has high data quality standards that have been applied across all volumes 18. The use of female-to-male IRRs may also be viewed as a strength because this metric is less affected by changes over time in tumor definitions, coding assignments or diagnostic accuracy, relative to absolute differences in incidence rates 49; however, female-to-male IRRs of breast cancer should be interpreted with caution as they are still vulnerable to effects of sex-related differences in early detection practices, health care utilization, illness behavior, and physician behavior 50, and the effect of such may be exacerbated given the rarity of this tumor in men. Female-to-male IRRs are also limited by their dimensionless scale, thus we also present sex-specific incidence rates to enable comparisons of absolute differences. Lastly, varying calendar periods of cancer registry coverage reduced parts of our analysis to the shorter period of 1988–2002, in order to enable a fair comparison of statistics presented.
In this analysis of 8,681 male breast cancer cases and 1.14 million female breast cancer cases, we have shown that male breast cancer rates are generally less than 1 per 100,000 man-years, in contrast to the much higher rates of female breast cancer, a fact underscored by the high female-to-male IRR of 122 for all countries combined. In addition, this study has shown that female breast cancer incidence has been increasing over time for a majority of countries assessed, while male breast cancer incidence trends are more variable, with only a minority of countries presenting evidence for an increase. Variability in the female-to-male IRR may reflect enhanced diagnosis, and potentially “over-diagnosis”, in females and/or sex differences in exposures and pathogenesis. Conversely, the high correlation between male and female breast cancer incidences may indicate that both sexes share common risk factors for this disease.
Supplementary Material
Trends in Sex-specific, Age-adjusted Incidence Rates and Female-to-male Incidence Rate Ratios for selected countries, All Ages, 1958–2002.
- Israel
- Philippines
- Italy
- France
- Iceland
- United States
Abbreviation: FM IRR, female-to-male incidence rate ratio. Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity.
Correlation between Sex-specific, Age-Adjusted Breast Cancer Incidence Rates and Health Expenditure per Capita for the Year 2000, Ages ≥50 Years, 1998–2002.
- Age-adjusted Female Breast Cancer Incidence Rate
- Age-adjusted Male Breast Cancer Incidence Rate
1Thailand, 2India, 3Ecuador, 4Japan, 5Costa Rica, 6Colombia, 7Estonia, 8Slovakia, 9Brazil, 10Singapore, 11Poland, 12Spain, 13Philippines, 14Austria, 15Switzerland, 16United Kingdom, 17Italy, 18Australia, 19Iceland, 20Canada, 21Denmark, 22Netherlands, 23France, 24Israel, 25United States.
Sex-specific, Age-adjusted Breast Cancer Incidence Rates and Female-to-male Incidence Rate Ratios, All Ages, Stratified by Period (1988–1992, 1993–1997, 1998–2002).
Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity. Abbreviations: CI, confidence interval; EAPC, estimated annual percentage change. “-“ not able to be estimated due to one or more 3-year period having zero counts of cases. EAPCs in bold indicate statistical significance at alpha=0.05.
Sex-specific, Age-adjusted Breast Cancer Incidence Rates and Sex-specific Incidence Rate Ratios Stratified by Age-Group (<50 vs. ≥50 years), 1988–2002.
Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity. Abbreviations: IRR, incidence rate ratio; CI, confidence interval.
Sex-specific, Age-adjusted Breast Cancer Incidence Rates and Female-to-male Incidence Rate Ratios by Health Expenditure per Capita (US$, Year 2000), Ages ≥50 Years, 1998–2002.
Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. 1Includes the countries (in order of health expenditure per capita from lowest to highest) India, Philippines, Ecuador, Thailand, Colombia, Estonia, Poland, and Slovakia. 2Includes the countries Brazil, Costa Rica, Singapore, Spain, Israel, Iceland, Italy, Australia, and UK. 3Includes the countries Netherlands, Canada, France, Austria, Denmark, Japan, Switzerland, and USA. Abbreviations: CI, confidence interval.
Sex-specific, Age-adjusted Breast Cancer Incidence Rates and Female-to-male Incidence Rate Ratios, All Ages, Stratified by Period (1978–1982, 1998–2002).
Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity. 1These estimates combine cases and populations of Iceland, Netherlands and UK. Abbreviations: CI, confidence interval.
Novelty and Impact.
This paper is the first international analysis of male and female breast cancer incidence rates. It uses data available only through IARC as many of the male breast cancer rates are not published in previous CI5 volumes and the case counts and populations are not publicly available. There is much interest in the causes of male breast cancer and whether they are similar to female breast cancer or constitute a totally different disease. This international comparison of male and female breast cancer incidence rates provides the first global epidemiologic data in relation to such questions. We show that global patterns of male and female breast cancer are similar which may indicate shared risk factors and homogeneity of disease between the sexes. Conversely, there are some sex disparities and outliers of female-to-male incidence rate ratios which indicate that sex-specific risk factors/carcinogenic processes may also play a role in the pathogenesis of this disease.
Acknowledgments
FUNDING
Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
There are no financial disclosures from any of the authors.
References
- 1.Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, Devesa SS, McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–82. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Miao H, Verkooijen HM, Chia KS, Bouchardy C, Pukkala E, Laronningen S, Mellemkjaer L, Czene K, Hartman M. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29:4381–6. doi: 10.1200/JCO.2011.36.8902. [DOI] [PubMed] [Google Scholar]
- 4.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28:232–9. doi: 10.1200/JCO.2009.23.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greif JM, Pezzi CM, Klimberg VS, Bailey L, Zuraek M. Gender Differences in Breast Cancer: Analysis of 13,000 Breast Cancers in Men from the National Cancer Data Base. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2479-z. [DOI] [PubMed] [Google Scholar]
- 6.Gnerlich JL, Deshpande AD, Jeffe DB, Seelam S, Kimbuende E, Margenthaler JA. Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol. 2011;18:1837–44. doi: 10.1245/s10434-010-1468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaaban AM, Ball GR, Brannan RA, Cserni G, Benedetto AD, Dent J, Fulford L, Honarpisheh H, Jordan L, Jones JL, Kanthan R, Maraqa L, et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat. 2012;133:949–58. doi: 10.1007/s10549-011-1856-9. [DOI] [PubMed] [Google Scholar]
- 8.Thalib L, Hall P. Survival of male breast cancer patients: population-based cohort study. Cancer Sci. 2009;100:292–5. doi: 10.1111/j.1349-7006.2008.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman MT, Tung KH, Wilkens LR. Comparative epidemiology of breast cancer among men and women in the US, 1996 to 2000. Cancer Causes Control. 2006;17:127–36. doi: 10.1007/s10552-005-5384-y. [DOI] [PubMed] [Google Scholar]
- 10.Burga AM, Fadare O, Lininger RA, Tavassoli FA. Invasive carcinomas of the male breast: a morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Arch. 2006;449:507–12. doi: 10.1007/s00428-006-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson I, Nilsson C, Berglund P, Lauss M, Ringner M, Olsson H, Luts L, Sim E, Thorstensson S, Fjallskog ML, Hedenfalk I. Gene expression profiling of primary male breast cancers reveals two unique subgroups and identifies N-acetyltransferase-1 (NAT1) as a novel prognostic biomarker. Breast Cancer Res. 2012;14:R31. doi: 10.1186/bcr3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callari M, Cappelletti V, De Cecco L, Musella V, Miodini P, Veneroni S, Gariboldi M, Pierotti MA, Daidone MG. Gene expression analysis reveals a different transcriptomic landscape in female and male breast cancer. Breast Cancer Res Treat. 2011;127:601–10. doi: 10.1007/s10549-010-1015-8. [DOI] [PubMed] [Google Scholar]
- 13.Johansson I, Nilsson C, Berglund P, Strand C, Jonsson G, Staaf J, Ringner M, Nevanlinna H, Barkardottir RB, Borg A, Olsson H, Luts L, et al. High-resolution genomic profiling of male breast cancer reveals differences hidden behind the similarities with female breast cancer. Breast Cancer Res Treat. 2011;129:747–60. doi: 10.1007/s10549-010-1262-8. [DOI] [PubMed] [Google Scholar]
- 14.Brinton LA, Carreon JD, Gierach GL, McGlynn KA, Gridley G. Etiologic factors for male breast cancer in the U.S. Veterans Affairs medical care system database. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinton LA, Richesson DA, Gierach GL, Lacey JV, Jr, Park Y, Hollenbeck AR, Schatzkin A. Prospective evaluation of risk factors for male breast cancer. J Natl Cancer Inst. 2008;100:1477–81. doi: 10.1093/jnci/djn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynge E, Afonso N, Kaerlev L, Olsen J, Sabroe S, Ahrens W, Eriksson M, Guenel P, Merletti F, Stengrevics A, Suarez-Varela M, Costa-Pererra A, et al. European multi-centre case-control study on risk factors for rare cancers of unknown aetiology. Eur J Cancer. 2005;41:601–12. doi: 10.1016/j.ejca.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Johnson KC, Pan S, Mao Y. Risk factors for male breast cancer in Canada, 1994–1998. Eur J Cancer Prev. 2002;11:253–63. doi: 10.1097/00008469-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, Forman D. Fifty years of cancer incidence: CI5 I–IX. Int J Cancer. 2010;127:2918–27. doi: 10.1002/ijc.25517. [DOI] [PubMed] [Google Scholar]
- 19.Segi M. Cancer Mortality for Selected Sites in 24 Countries (1950–57)ed. Sendai: Tohoku University School of Public Health; 1960. [Google Scholar]
- 20.Doll R, Payne P, Waterhouse J. Cancer incidence in five continents: a technical reported. Berlin: Springer-Verlag (for UICC); 1966. [Google Scholar]
- 21.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–69. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. Introduction to SEER*Stat. Bethesda, MD: 2008. [Google Scholar]
- 23.The World Bank. Health expenditure per capita (current US$) The World Bank; [Google Scholar]
- 24.International Cancer Screening Network. Vol. 2012 National Cancer Institute; [Google Scholar]
- 25.European Cancer Observatory. 2012 [Google Scholar]
- 26.IARC. IARC Scientific Publications No 160. Lyon: IARC; 2007. Cancer Incidence in Five Continents. Vol. IX ed. [Google Scholar]
- 27.IARC. Cancer Incidence in Five Continents. IARC Sci Publ. 2002;VIII:1–781. [PubMed] [Google Scholar]
- 28.IARC. Cancer Incidence in Five Continents. Lyon: International Agency for Research on Cancer; 1997. Vol. VIIed. [Google Scholar]
- 29.Roa BB, Boyd AA, Volcik K, Richards CS. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet. 1996;14:185–7. doi: 10.1038/ng1096-185. [DOI] [PubMed] [Google Scholar]
- 30.Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–8. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 31.Im KM, Kirchhoff T, Wang X, Green T, Chow CY, Vijai J, Korn J, Gaudet MM, Fredericksen Z, Shane Pankratz V, Guiducci C, Crenshaw A, et al. Haplotype structure in Ashkenazi Jewish BRCA1 and BRCA2 mutation carriers. Hum Genet. 2011;130:685–99. doi: 10.1007/s00439-011-1003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai YC, Domchek S, Parmigiani G, Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811–4. doi: 10.1093/jnci/djm203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ewertz M, Holmberg L, Tretli S, Pedersen BV, Kristensen A. Risk factors for male breast cancer--a case-control study from Scandinavia. Acta Oncol. 2001;40:467–71. doi: 10.1080/028418601750288181. [DOI] [PubMed] [Google Scholar]
- 34.Hsing AW, McLaughlin JK, Cocco P, Co Chien HT, Fraumeni JF., Jr Risk factors for male breast cancer (United States) Cancer Causes Control. 1998;9:269–75. doi: 10.1023/a:1008869003012. [DOI] [PubMed] [Google Scholar]
- 35.Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res. Treat. 2004;83:77–86. doi: 10.1023/B:BREA.0000010701.08825.2d. [DOI] [PubMed] [Google Scholar]
- 36.Shaaban AM, Ball GR, Brannan RA, Cserni G, Benedetto AD, Dent J, Fulford L, Honarpisheh H, Jordan L, Jones JL, Kanthan R, Maraqa L, et al. A comparative biomarker study of 514 matched cases of male and female breast cancer reveals gender-specific biological differences. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1856-9. [DOI] [PubMed] [Google Scholar]
- 37.Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152–61. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 38.Jonsson H, Johansson R, Lenner P. Increased incidence of invasive breast cancer after the introduction of service screening with mammography in Sweden. Int J Cancer. 2005;117:842–7. doi: 10.1002/ijc.21228. [DOI] [PubMed] [Google Scholar]
- 39.Verkooijen HM, Bouchardy C, Vinh-Hung V, Rapiti E, Hartman M. The incidence of breast cancer and changes in the use of hormone replacement therapy: a review of the evidence. Maturitas. 2009;64:80–5. doi: 10.1016/j.maturitas.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Welch HG, Black WC. Overdiagnosis in Cancer. J Natl Cancer Inst. 2010;102:605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 41.Iwasaki M, Tsugane S. Risk factors for breast cancer: epidemiological evidence from Japanese studies. Cancer Sci. 2011;102:1607–14. doi: 10.1111/j.1349-7006.2011.01996.x. [DOI] [PubMed] [Google Scholar]
- 42.Hill TD, Khamis HJ, Tyczynski JE, Berkel HJ. Comparison of male and female breast cancer incidence trends, tumor characteristics, and survival. Ann Epidemiol. 2005;15:773–80. doi: 10.1016/j.annepidem.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Stang A, Thomssen C. Decline in breast cancer incidence in the United States: what about male breast cancer? Breast Cancer Res. Treat. 2008;112:595–6. doi: 10.1007/s10549-007-9882-3. [DOI] [PubMed] [Google Scholar]
- 44.Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat. 2009;115:429–30. doi: 10.1007/s10549-008-0053-y. [DOI] [PubMed] [Google Scholar]
- 45.Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, Nishino Y, Sobue T, Chen CJ, You SL, Mirasol-Lumague MR, Law SC, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010;21:1777–85. doi: 10.1007/s10552-010-9604-8. [DOI] [PubMed] [Google Scholar]
- 46.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–27. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 47.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321:624–8. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–4. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 49.Nicholson WJ, Davis DL. Analyses of changes in the ratios of male-to-female cancer mortality. A hypothesis-generating exercise. Ann N Y Acad Sci. 1990;609:290–7. doi: 10.1111/j.1749-6632.1990.tb32076.x. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 50.Wingard DL. The sex differential in morbidity, mortality, and lifestyle. Annu Rev Public Health. 1984;5:433–58. doi: 10.1146/annurev.pu.05.050184.002245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trends in Sex-specific, Age-adjusted Incidence Rates and Female-to-male Incidence Rate Ratios for selected countries, All Ages, 1958–2002.
- Israel
- Philippines
- Italy
- France
- Iceland
- United States
Abbreviation: FM IRR, female-to-male incidence rate ratio. Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity.
Correlation between Sex-specific, Age-Adjusted Breast Cancer Incidence Rates and Health Expenditure per Capita for the Year 2000, Ages ≥50 Years, 1998–2002.
- Age-adjusted Female Breast Cancer Incidence Rate
- Age-adjusted Male Breast Cancer Incidence Rate
1Thailand, 2India, 3Ecuador, 4Japan, 5Costa Rica, 6Colombia, 7Estonia, 8Slovakia, 9Brazil, 10Singapore, 11Poland, 12Spain, 13Philippines, 14Austria, 15Switzerland, 16United Kingdom, 17Italy, 18Australia, 19Iceland, 20Canada, 21Denmark, 22Netherlands, 23France, 24Israel, 25United States.
Sex-specific, Age-adjusted Breast Cancer Incidence Rates and Female-to-male Incidence Rate Ratios, All Ages, Stratified by Period (1988–1992, 1993–1997, 1998–2002).
Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity. Abbreviations: CI, confidence interval; EAPC, estimated annual percentage change. “-“ not able to be estimated due to one or more 3-year period having zero counts of cases. EAPCs in bold indicate statistical significance at alpha=0.05.
Sex-specific, Age-adjusted Breast Cancer Incidence Rates and Sex-specific Incidence Rate Ratios Stratified by Age-Group (<50 vs. ≥50 years), 1988–2002.
Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity. Abbreviations: IRR, incidence rate ratio; CI, confidence interval.
Sex-specific, Age-adjusted Breast Cancer Incidence Rates and Female-to-male Incidence Rate Ratios by Health Expenditure per Capita (US$, Year 2000), Ages ≥50 Years, 1998–2002.
Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. 1Includes the countries (in order of health expenditure per capita from lowest to highest) India, Philippines, Ecuador, Thailand, Colombia, Estonia, Poland, and Slovakia. 2Includes the countries Brazil, Costa Rica, Singapore, Spain, Israel, Iceland, Italy, Australia, and UK. 3Includes the countries Netherlands, Canada, France, Austria, Denmark, Japan, Switzerland, and USA. Abbreviations: CI, confidence interval.
Sex-specific, Age-adjusted Breast Cancer Incidence Rates and Female-to-male Incidence Rate Ratios, All Ages, Stratified by Period (1978–1982, 1998–2002).
Rates are per 100,000 woman/man years and age-adjusted to the World Standard Population. Italicized country names indicate data are regional or restricted by ethnicity. 1These estimates combine cases and populations of Iceland, Netherlands and UK. Abbreviations: CI, confidence interval.