Abstract
A direct-diode pumped Ti:sapphire femtosecond oscillator is used to perform multiphoton imaging for the first time.
INTRODUCTION
The application base of multiphoton microscopy continues to grow encompassing a broad range of basic science applications and clinical diagnostics (Carriles et al. (2009), Sheetz & Squier (2009)). As a diagnostic tool, there is a need for less expensive laser sources and much work has been focused on producing easy to use laser platforms that are more cost effective. Many of these sources are fiber based, or are based on materials (e.g., Yb:KGW, Cr:LiCAF) (Sheetz, Hoover, Carriles, Kleinfeld, & Squier (2008), Sakadic et al. (2008), Kim, Song, Song, Kwon, Sung, & Kim (2012), Nie et al. (2012)) that can be pumped by semiconductor lasers that are centered at long wavelengths (780 nm and greater). While effective, these sources are often restricted in the wavelength range over which they can be tuned, and subsequently the ability to directly produce clean, extremely short (<20 fs) pulses if so desired. More so than any other gain media, Ti:sapphire based systems meet these broadest of nonlinear imaging criteria. However, they are expensive lasers and little has been done to help mitigate the cost of this laser source which is the workhorse of multiphoton microscopy.
NEW DESIGN
In this communication we address the issue of system cost by demonstrating for the first time, that directdiode pumped Ti:sapphire lasers (Durfee et al. (2012)) are indeed a viable alternative for nonlinear imaging applications. Currently, Ti:sapphire is pumped by expensive (>$25 k U.S.) sources at green wavelengths 514527 nm. However, as a result of the projection market, inexpensive (<$100 U.S.) semiconductor lasers centered at 445 nm with average powers in the range of Watts have recently become available. The emergence of these sources raises the question: can they be used to effectively pump Ti:sapphire without compromising the characteristics of this source, which make it the dominant laser for multiphoton imaging (wavelength range, average power, and pulse duration)?
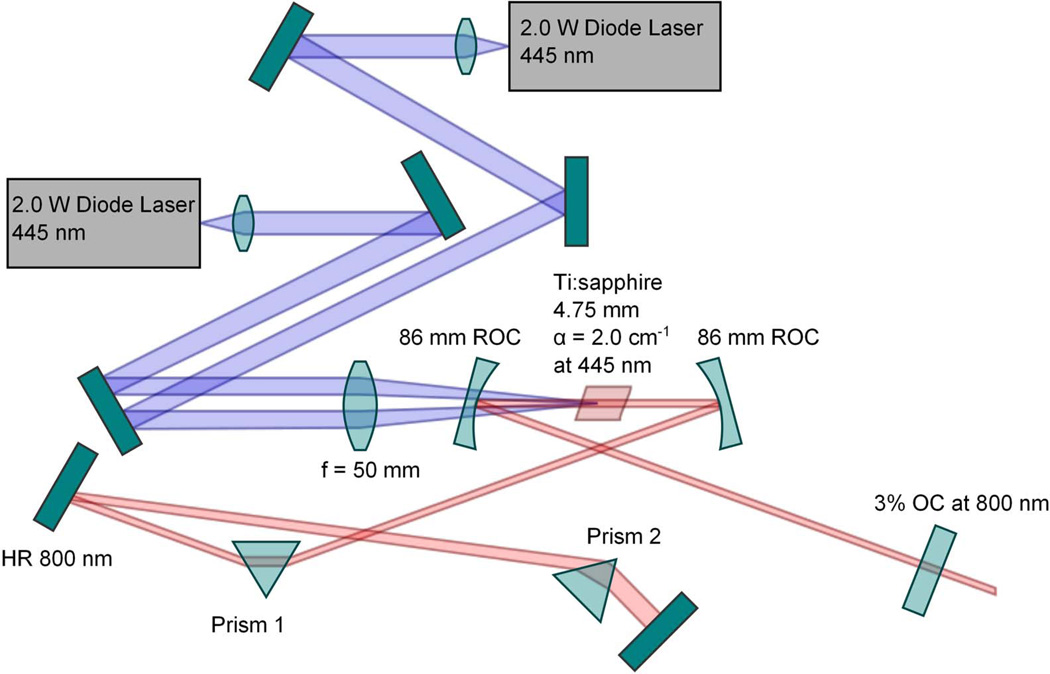
The challenge is twofold: the shorter pump wavelength translates to reduced absorption, and the asymmetry of the laser diode emitter will reshape the gain profile and possibly compromise the Kerrlens modelocking mechanism. A typical laser diode emitter features a facet with a 15:1 aspect ratio resulting in an emission profile that has a ~3.5:1 fast-to-slow axis divergence. Fortunately, as we have recently demonstrated, the advent of inexpensive aspheric lenses enables efficient delivery in a longitudinal pumping scheme which results in the production of a gain volume suitable to enable Kerrlens modelocking (Durfee et al. (2012)). Using two, 2 W 445 nm laser diodes, and 3% output coupling (See Fig. 1), we have produced up to 105 mW average power in a 100 MHz pulse train that features pulses with a 40 nm bandwidth, or 70 mW average power pulse trains of 140 nm bandwidth (this results in a transform limited pulse duration of 12 fs, limited by the mirror coatings in the laser resonator). This broad spectrum serves to illustrate two significant features of the directdiode pumped Ti:sapphire laser configuration. First, extremely short pulses can be produced. Using external dispersion compensation we have produced pulse durations as short as 15 fs as measured by second harmonic frequency resolved optical gating (Sheetz, Hoover, Carriles, Kleinfeld, & Squier (2008)). Second, it shows that the tunability of the laser is not compromised. This laser can be tuned throughout the wavelength range of the mirror set, from ~720 to 870 nm.
Figure 1.
Layout diagram of the directdiode pumped femtosecond Ti:sapphire laser.
DEMONSTRATION
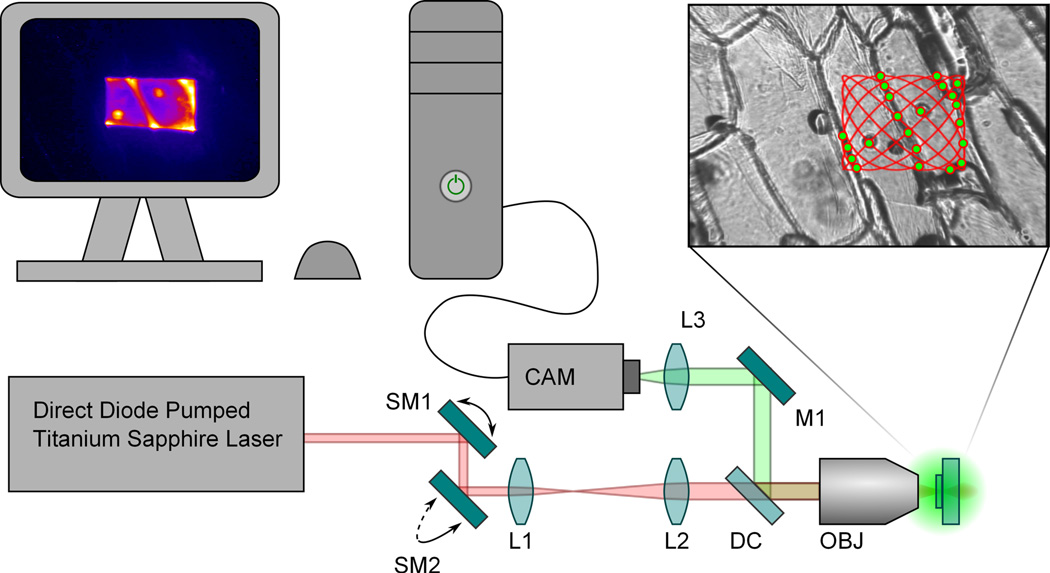
To demonstrate the utility of this source for imaging, we constructed a simple multiphoton microscope (see Fig. 2). The laser was operated in two modes: 50 fs (40 nm spectrum) and 15 fs (120 nm spectrum). In the case of the 15 fs pulses, a dispersive mirror set external to the oscillator was used to provide dispersion compensation. The pulse duration was measured at the output of laser cavity. The laser was directed through two galvanometric scan mirrors which are appropriately image relayed to the entrance pupil of the excitation objective. The power measured at the focus was 2 mW. The twophoton excitation fluorescence (TPEF) (Durfee et al. (2012)) is captured in the epiconfiguration and separated from the excitation beam with a dichroic (Chroma 725dcxr, 50% Transmission at 725 nm (Chroma, Bellows Falls, Vermont, USA)). The raster pattern created by the scan mirrors is a simple traveling Lissajou pattern optimized to provide uniform compensation for the various exposure times. The nondescanned TPEF signal is imaged to a Hamamatsu camera, model C5985 (Hamamatsu, Hamamatsu City, Shizuoka Pref., Japan).
Figure 2.
Diagram of the scanning microscope system. The upperright box contains a whitelight illuminated image of an onion skin with an overlay of a simple traveling Lissajou scan pattern. The green dots represent twophoton excitation and the final image is rendered in the upperleft computer screen (for detail see Fig. 5). SM1 and SM2: Scan mirrors for the x and y axes respectively. L1 and L2: Achromatic lenses which compromise the telecentric/beam expander relay system. DC: Chroma 725dcxr dichroic. M1: Fold mirror to the camera. L3: Focusing lens for the camera. CAM: Hamamatsu Chilled CCD Camera. OBJ: Excitation and Imaging Objective.
Axial Point Spread Function
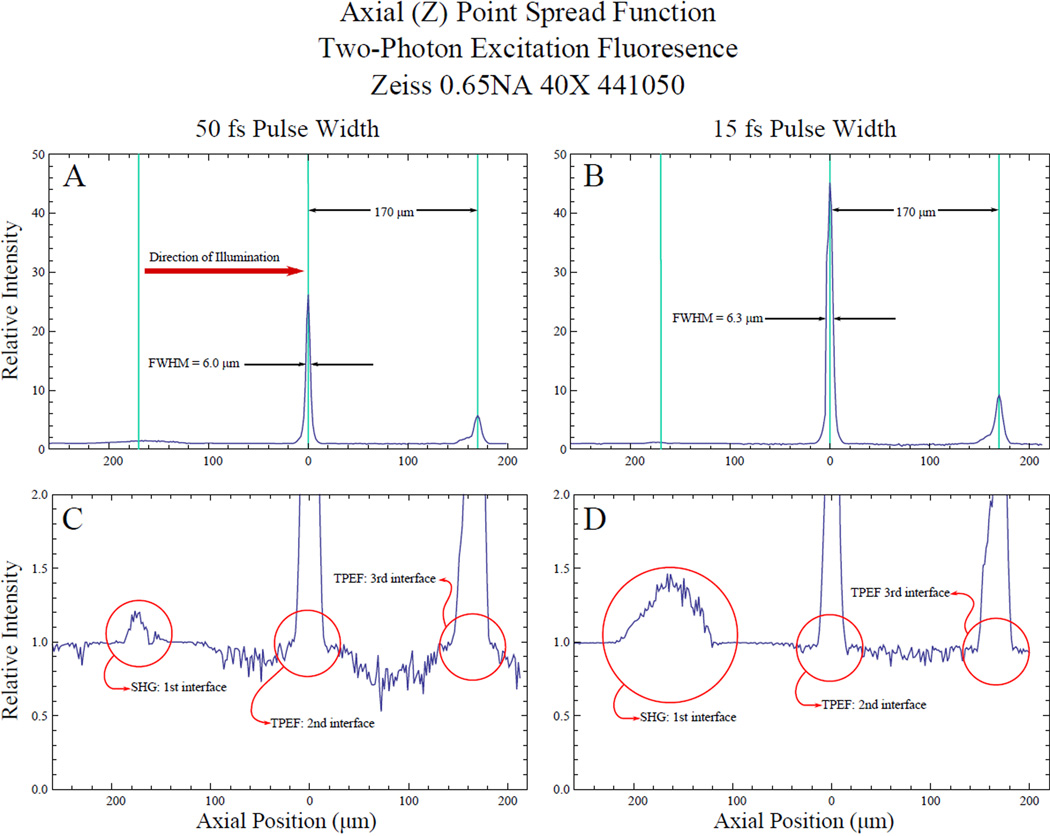
The axial point spread function of this system was measured as a function of pulse duration as shown in Fig. 3. A thin layer of fluorescent dye from a pink Avery® Hiliter® (Avery Dennison Corporation, Brea, CA, USA) was applied between the interfaces of a stack of two coverslips and a glass slide for these measurements. The highlighter is readily available and represents an easy, inexpensive way to create test samples. In this and the following samples described, the highlighter dye created TPEF at focus which was visible to the naked eye. The sectioning curve is made with a Zeiss 0.65 NA, 40× Achroplan taken with 50 fs pulses (Figure 3 graphs A and C). The axial sectioning is near the diffraction limit, given by, of 5.5 µm at the second interface (6.0 µm FWHM) as expected (Squier, Müller (2001)). When operated at the shorter pulse duration of 15 fs, the axial sectioning is compromised (Figure 3 graphs B and D) now measuring 6.3 µm FWHM. The increase in sectioning is indicative of the challenge of using extremely short pulses.
Figure 3.
Twophoton Excitation Fluorescence Response (axial point spread function) of fluorescent dye. Samples are composed of two coverslips and a glass slide with fluorescent dye in between each piece of glass. Graphs A and B show the TPEF response of the second and third interfaces. The coverslip thickness is used to calibrate the axial scale. The response at the second interface is used to characterize the axial resolution limit of the microscope system. Graphs C and D are magnifications of graphs A and B respectively and show the SHG response of the first interface of the sample. Graphs A and C are the axial PSF with a 50 fs pulse while graphs B and D are the axial PSF with a 15 fs pulse.
Two-photon excitation images
A series of TPEF images were recorded for a variety of specimens all stained with a red fluorescent highlighter. All images were taken with an average power of 2 mW measured at the focus. Exposure times are were 10 s or less per image.
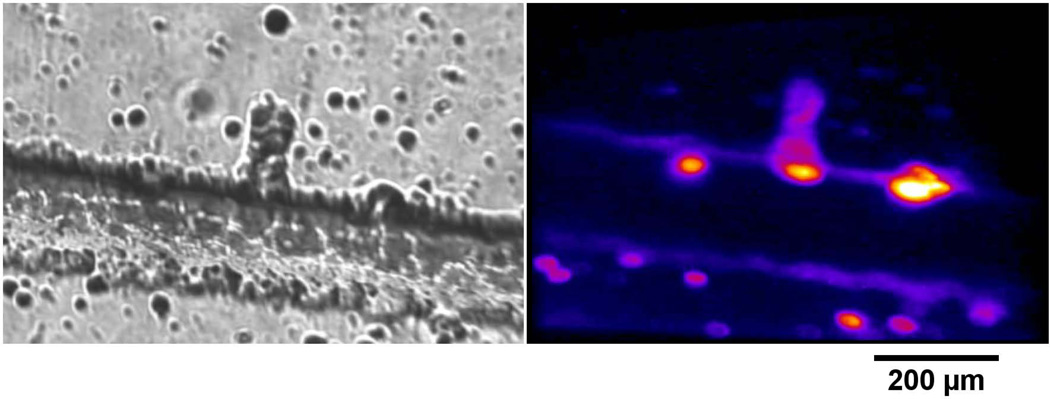
Figure 4 shows a femtosecond lasermachined channel in fused silica.
Figure 4.
Images of a femtosecond lasermachined channel in fused silica stained with fluorescent dye. Image A is the channel with white-light illumination. Image B is the TPEF response of the channel.
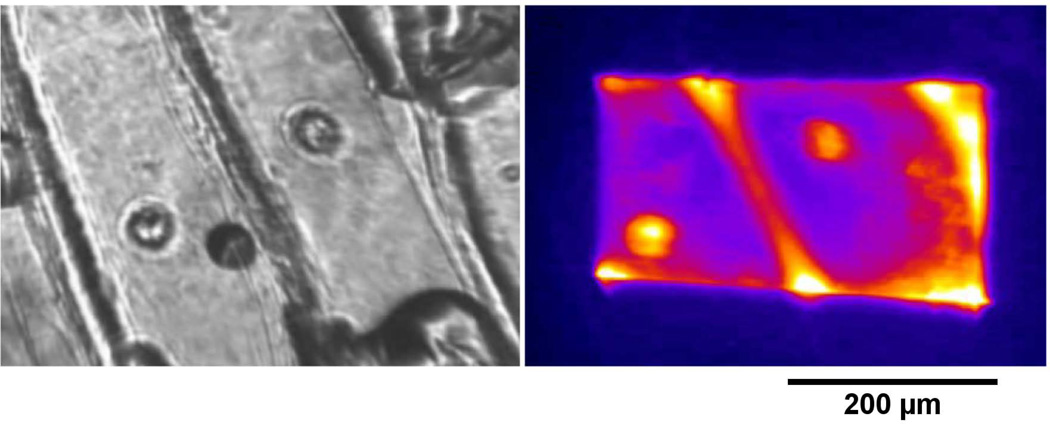
Figure 5 shows an onion skin. Figure 5 image A is the sample seen with whitelight illumination while figure 5 image B is the TPEF response of the same sample. The two nuclei in the center of image A may be seen in image B.
Figure 5.
Images of Allium cepa (onion) skin stained with fluorescent dye. Image A is A. cepa with whitelight illumination. Image B is the TPEF response of the stained skin.
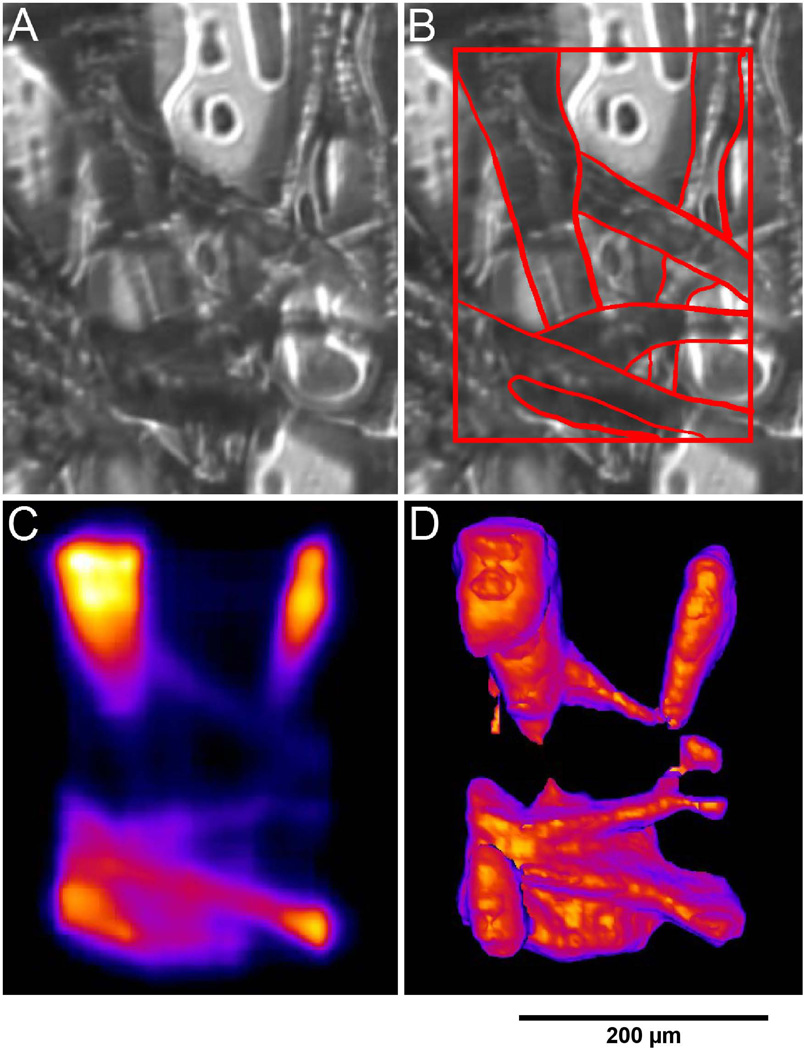
Figure 6 shows fluorescent dye stained cellulose. Images A, B and C show the sample with whitelight illumination and TPEF response respectively. This sample was incrementally scanned in depth, with 7 s exposure time per scan, so as to reconstruct a 3D representation shown in image D.
Figure 6.
Images of cellulose based fibers stained with fluorescent dye. Image A shows the fibers under whitelight illumination. Image B shows the same image as in A except the prominent features of the fibers have been outlined. Image C shows the TPEF response of the fibers. Image D is a 3D reconstruction of a series of TPEF images at different depths.
CONCLUSION
In this communication we have addressed the viability of a directdiode pumped Ti:sapphire system, with application to nonlinear microscopy. We demonstrate the utility of the laser for this application by imaging a variety of samples using twophoton excitation fluorescence. This new pumping paradigm for a Ti:sapphire gain medium provides a distinct advantage over existing Ti:sapphire systems, namely a greatly reduced "entry" cost for a multiphoton microscopy system. Significantly it does so without sacrificing a key feature of the Ti:sapphire laser: its ability to produce clean, extremely short pulses, which also translates to the tuning range of the source.
ACKNOWLEDGEMENTS
This work was funded by the National Institute of Biomedical Imaging and Bioengineering under the Bioengineering Research Partnership EB003832.
Bibliography
- Carriles R, Schafer DN, Sheetz KE, Field JJ, Cizek R, Barzda V, Sylvester AW, Squier JA. Invited review article: Imaging techniques for harmonic and multiphoton absorption fluorescence microscopy. Review of Scientific Instrument. 2009;80:081101. doi: 10.1063/1.3184828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee CG, Garlick J, Hill S, Squier JA, Kirchner M, Taft G, Shea K, Kapteyn H, Murnane M, Backus S. Direct diodepumped Kerrlens modelocked Ti:sapphire laser. Opt. Express. 2012;20:1367713683. doi: 10.1364/OE.20.013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DU, Song H, Song W, Kwon H-S, Sung M, Kim DY. Twophoton microscopy using an Yb3+-doped fiber laser with variable pulse widths. Opt. Express. 2012;20:1234112349. doi: 10.1364/OE.20.012341. [DOI] [PubMed] [Google Scholar]
- Nie B, Saytashev I, Chong A, Lie H, Arkhipov SN, Wise FW, Dantus M. Multimodal microscopy with sub30fs Yb fiber laser oscillator. Biomed. Opt. Express. 2012;3:17501756. doi: 10.1364/BOE.3.001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakadic S, Demirbas U, Mempel TR, Moore A, Ruvinskaya S, Boas DA, Sennaroglu A, Kaertner FX, Fujimoto JG. Multiphoton microsocpy with a low-cost and highly efficient Cr:LiCAF laser. Opt. Express. 2008;16:2084820863. doi: 10.1364/oe.16.020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz KE, Hoover EE, Carriles R, Kleinfeld D, Squier JA. Advancing multifocal nonlinear microscopy: development and application of a novel multibeam Yb:KGd(WO4)2 oscillator. Opt. Express. 2008;16:1757417584. doi: 10.1364/oe.16.017574. [DOI] [PubMed] [Google Scholar]
- Sheetz K, Squier J. Ultrafast Optics: Imaging and manipulating biological systems. Journal of Applied Physics. 2009;105:051101. [Google Scholar]
- Squier J, Müller M. High resolution nonlinear microscopy: A review of sources and methods for achieving optimal imaging. Review of Scientific Instruments. 2001;72:28552867. [Google Scholar]