Abstract
Cocaine is an inhibitor of the dopamine, norepinephrine, and serotonin reuptake transporters. Because its administration would therefore elevate signaling of all these three neurotransmitters, many studies have been aimed at attributing individual effects of cocaine to specific transmitter systems. Using mice with a cocaine insensitive dopamine transporter (DAT-CI mice), we previously showed that cocaine-induced dopamine elevations were necessary for its rewarding and stimulating effects. In this study, we observe that DAT-CI mice exhibit cocaine-conditioned place aversion, and that its expression depends on their genetic background. Specifically, DAT-CI mice backcrossed to the C57Bl/6J strain background did not display a preference or an aversion to cocaine, whereas DAT-CI mice that were on a mixed 129S1/SvImJ × C57Bl/6J (129B6) background had a robust conditioned place aversion to cocaine. These results indicate that while inhibition of the dopamine transporter (DAT) is necessary for cocaine reward, other cocaine targets and neurotransmitter systems may mediate the aversive properties of cocaine. Furthermore, the aversive effect of cocaine can be observed in the absence of a DAT-mediated rewarding effect, and it is affected by genomic differences between these two mouse strains.
Keywords: Aversion, Addiction, Cocaine, Dopamine, Reward
Introduction
The primary molecular actions of cocaine are the inhibition of the dopamine, norepinephrine, and serotonin transporters; with additional low-affinity targets that seem less relevant to cocaine’s behavioral effects at moderate doses (Han & Gu 2006; Ritz et al. 1987). The behavioral effects of cocaine include motor stimulation and reinforcement in rodents. In humans cocaine produces an intense euphoria and is considered very addictive.
Cocaine is also known to have negative psychomotor effects after the peak of euphoria, which are thought to indirectly reinforce repeated drug taking (Ahmed & Koob 2005; Leventhal et al. 2011; Newton et al. 2003). However, if the negative effects are more concurrent with reward, they would be expected to compete with reward and discourage drug taking. There is an observable approach-avoidance response conflict in rats for cocaine, due apparently to its anxiogenic properties (Guzman & Ettenberg 2007; Koob 1999). This behavior is evidence of such a competition.
Normally, if drugs of abuse are administered to mice in a conditioned place-preference (CPP) paradigm, they result in a positive preference for the drug-conditioned context – indicating that it was a rewarding experience. However, if the animal is placed into the conditioning apparatus with some delay after cocaine is administered, a negative preference or conditioned place aversion (CPA) for the drug-conditioned context is expressed. This is true for many drugs, even if they are generally addictive (Fudala & Iwamoto 1990). On the other hand, evidence for an acute aversive effect in the place-preference paradigm is lacking.
Additionally, the balance between rewarding and aversive effects may be influenced by genetics. This seems to be the case for methamphetamine in a model where genetically divergent subpopulations of mice display differences in total consumption. The high-consuming group has been shown to be less sensitive to the aversive effects of methamphetamine (Wheeler et al. 2009), and to have gene expression differences for DARPP-32, GLUR1, and GABB1 (Palmer et al. 2005).
In this study, we sought to investigate whether an acute aversive effect could be observed for cocaine and how strain backgrounds may modulate this effect. Previously, we created knock-in mice with a cocaine-insensitive dopamine transporter (DAT-CI mice). It was shown that inhibition of the dopamine transporter is necessary for cocaine reward – as evidenced by the lack of CPP and self-administration of the drug (Thomsen et al. 2009; Tilley et al. 2009). Initially, while the DAT-CI line was still on a mixed 129B6 background, the mice displayed an aversive response to cocaine (Chen et al. 2006). This aversive response seemed to be lost in various experiments performed after backcrossing to the C57 strain for more than 10 generations – when the mice displayed neither a preference nor aversion to cocaine. It was unclear whether this was truly due to strain genetics, or due to epigenetic or experimental differences that may have also changed over time. We therefore recreated a mixed 129B6 background DAT-CI mouse line, from contemporaneous C57-congenic breeders in order to directly test the influence of strain backgrounds in side-by-side experiments.
Methods
Animals Subjects
In these studies, a knock-in mouse line containing three point mutations (L104V/F105C/A109V) in the dopamine transporter gene was used. These mice (DAT-CI mice) were generated as described previously (Chen et al. 2006). Briefly, a site-specific targeting construct was generated by PCR assembly. The construct was introduced into mouse embryonic stem (ES) cells derived from the 129/SvJ strain (129 mice), and positive ES cell clones were used to generate the chimeric founder mice (Yale University Core Facility). These chimeras were then crossed to wild-type C57Bl/6J mice (C57 mice). Heterozygous F1 offspring would be on a 1:1 mixture of C57 and 129 background at this point, due to successful germline transmission of the 129/SvJ derived ES-cell DNA.
Group 1 mice (DAT-CI n = 14, and 8 controls; Wild-Type n = 8, and 10 controls) were directly derived from sibling pairings of the F1 heterozygotes described above. These mice were then bred to a C57-congenic CRE recombinase-expressing mouse line (Jackson Laboratories, B6.FVB-Tg(EIIa-cre)C5379Lmgd/J) in order to remove the Neomycin (Neo) selection marker that is flanked by two loxP sites. Heterozygous breeders from the Neo-removed (F2) progeny were used to generate wild-type and homozygous mutant mice for the experiments depicted in figure 1. These mice would contain an approximately 1:1 ratio of 129 and C57 backgrounds.
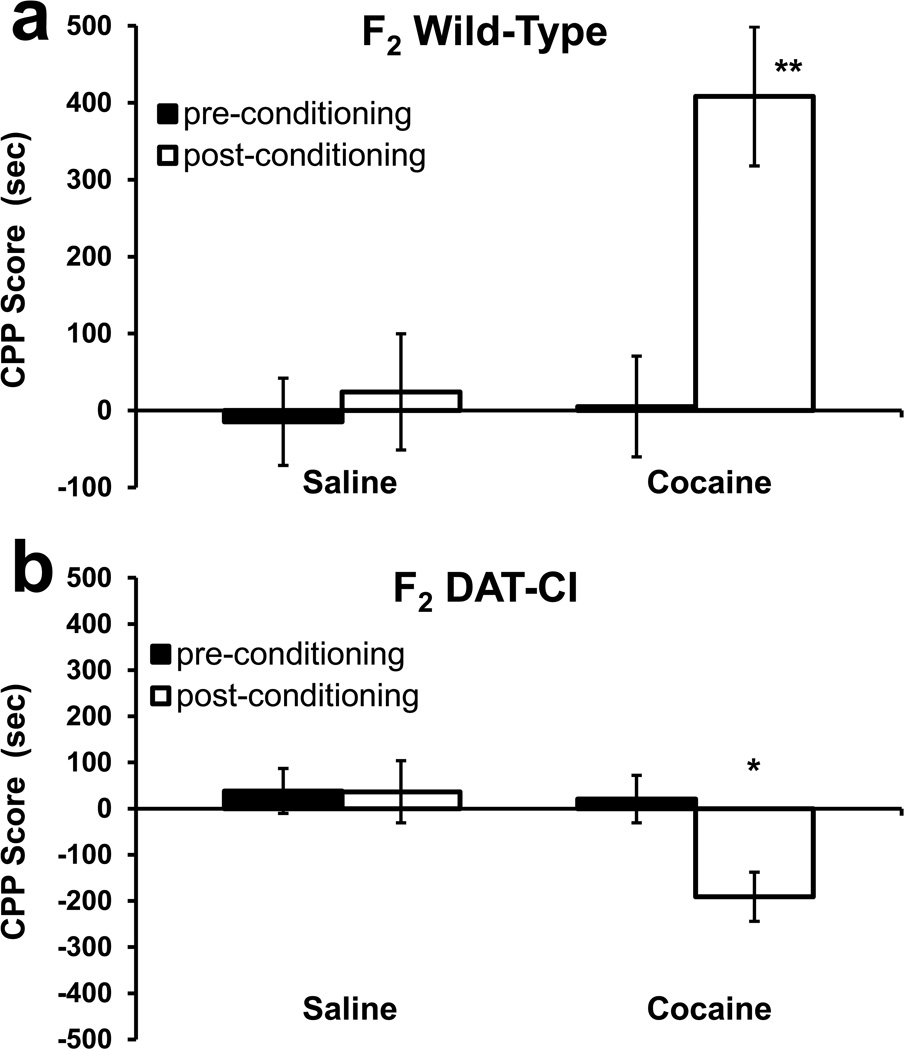
Figure 1. Conditioned Place-Preference of Group 1 Mice.
Mean ± SEM of CPP scores. Group 1 mice were of an early backcrossing generation (F2) and have a mixed genetic background consisting of a roughly 1:1 ratio of the 129 and C57 strains. CPP scores are defined as the time spent in the drug-designated environment minus the time spent in the saline-designated environment. Control mice receive saline in both environments. (a) Wild-type mice (cocaine: n = 8, saline: n = 10) show a robust CPP induced by 20 mg/kg cocaine. (b) DAT-CI mice (cocaine: n = 14, saline: n = 8) showed significant CPA induced by 20 mg/kg cocaine. All statistics are two-way ANOVA comparisons of the time bias (CPP score) observed post-conditioning (open bars) relative to saline-conditioned control mice within the figure (*, p < 0.05; **, p < 0.01).
Group 2 mice (DAT-CI n = 8, and 8 controls; Wild-Type n = 8, and 8 controls) were generated by backcrossing the F2 generation described above to wild-type C57Bl/6J mice for an additional twelve generations, producing F14 mice. Heterozygous male and female F14 mice were paired to generate wild-type and homozygous mutant mice for the experiments depicted in figure 2. These mice share greater than 99.9% of their genetic background with the C57Bl/6J strain.
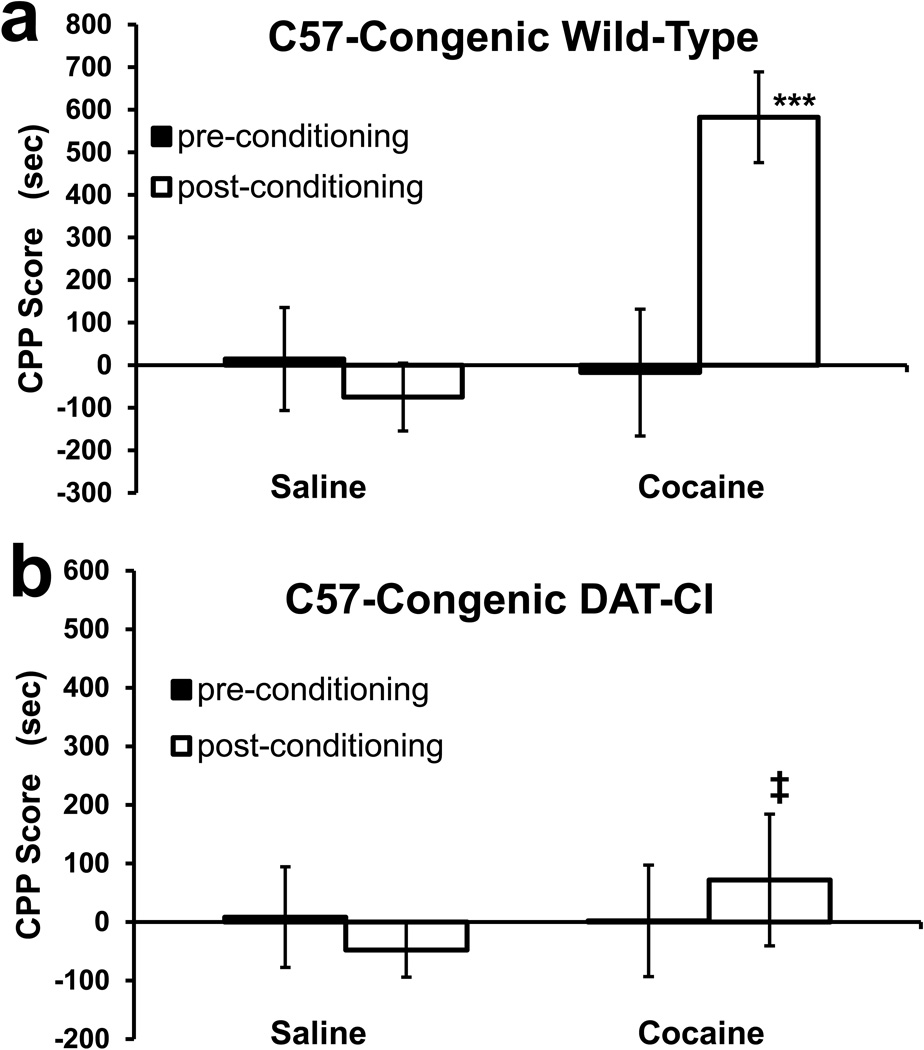
Figure 2. Conditioned Place-Preference of Group 2 Mice.
Mean ± SEM of CPP scores. Group 2 mice have been backcrossed to the C57 strain for 14 generations (F14) and have a >99.9% C57-congenic background. (a) Wild-type control mice (cocaine: n = 7, saline: n = 8) show a robust CPP induced by 20 mg/kg cocaine. (b) DAT-CI littermates (cocaine: n = 8, saline: n = 8) failed to show CPP or CPA to 20 mg/kg cocaine. All statistics denoted by asterisks are two-way ANOVA comparisons of the time bias (CPP score) observed post-conditioning (open bars) relative to saline-conditioned control mice (***, p < 0.001). An a priori hypothesis test was conducted for the between group (2 and 3) comparison of the DAT-CI mice responses (‡, p < 0.05).
Group 3 mice (DAT-CI n = 8, and 8 controls; Wild-Type n = 16, and 8 controls) were generated by outcrossing the C57-congenic DAT-CI mice described above with wild-type 129S1/SvImJ for one generation. The heterozygous offspring contain a 1:1 mixture of C57 and 129 backgrounds, and were sibling paired in order to generate DAT-CI mutants. Their wild-type and homozygous mutant progeny were used in experiments depicted in figure 3. The full designation of the strain is (129S1/SvImJ × C57BL/6J)F2 and it is abbreviated as 129B6.
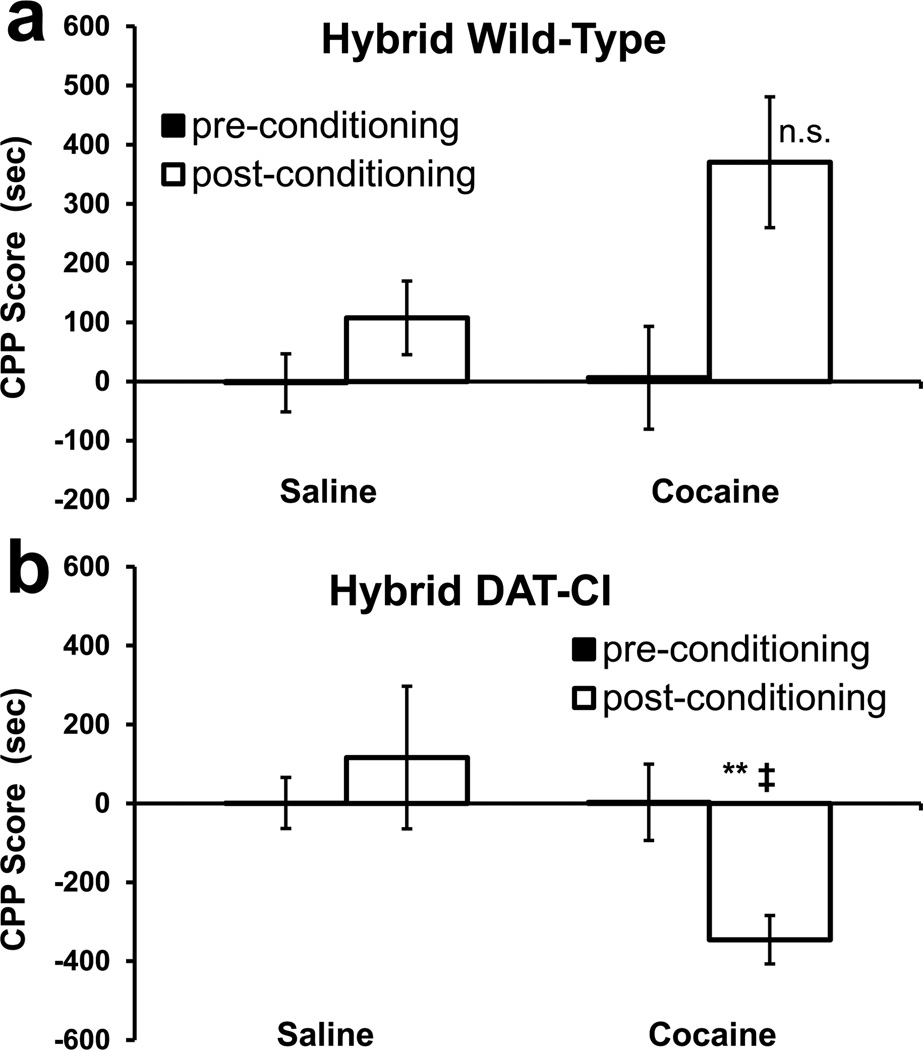
Figure 3. Conditioned Place-Preference of Group 3 Mice.
Mean ± SEM of CPP scores. Group 3 mice have a roughly 1:1 ratio of 129 and C57 strain backgrounds, via outcrossing the C57-congenic group 2 mice to 129S1/SvImJ mice. (a) Group 3 wild-type control mice (cocaine: n = 16, saline: n = 8) show an apparently large trend toward CPP induced by 20 mg/kg cocaine (p = 0.070), but this result is not statistically significant due to larger variations than in the inbred mice. (b) 129B6 DAT-CI littermates (cocaine: n = 8, saline: n = 8) show a robust CPA induced by 20 mg/kg cocaine in contrast to group 2 DAT-CI mice (a priori contrast, denoted by ‡; p < 0.05). All other statistics are comparisons of the time bias (CPP score) observed post-conditioning (open bars) relative to saline-conditioned control mice (*, p < 0.05; **, p < 0.01).
All mice were kept in standard housing conditions, including ad libitum access to food/water and 12 hours each of dark/light. Only male mice were used, and all mice were between 6–12 weeks of age at the time of behavioral testing. All animal procedures were approved by The Ohio State University Internal Laboratory Animal Care and Use Committee (ILACUC).
Drugs Administered
Cocaine hydrochloride was provided by the National Institute on Drug Abuse drug supply program. Cocaine was dissolved in 0.9% sterile saline and injected intraperitoneally (i.p.) at a dose of 20 mg/kg and a volume of 0.1 mL/10g body weight.
Conditioned Place-Preference Test
Conditioned place-preference/aversion testing was performed in 12.5 cm × 42.5 cm acrylic boxes subdivided into three interconnected compartments: two side compartments (12.5 cm × 17.5 cm) and a center compartment (12.5 cm × 7.5 cm). The three compartments were made visually and tactilely distinct from one another by cues, creating three different “environments”. An unbiased paradigm (balanced with respect to pre-conditioning preferences) consisting of a pre-conditioning preference test, a conditioning phase, and a post-conditioning preference test was performed as described previously (Tilley et al. 2009). For the pre/post conditioning preference tests, mice had access to all three compartments. Their preference was defined as the difference in time spent in one side compartment versus the other. Mice were assigned to receive cocaine in one environment or the other, based on their natural preference determined during the pre-conditioning test. The assignments were selected such that the average natural preference of each experimental group was minimized, by counterbalancing equal-sized subgroups.
On the first day of conditioning, half the mice received a 20 mg/kg cocaine injection (i.p) and half received the vehicle (0.9 % saline). The mice were then immediately confined for 30 minutes to the environment assigned to them for that day/treatment. Each mouse received the alternate treatment (in alternating environments) on subsequent days, for a total of 8 days.
After conditioning (paradigm day 10) mice were tested for their post-conditioning preference using the same unconfined apparatus configuration as the pre-test. Pre/post-test sessions were performed without an injection. During these sessions, mouse behavior was recorded by overhead cameras and analyzed by the AnyMaze software (Stoelting Co.). The time spent in the drug-designated compartment subtracted from the time in the vehicle-designated compartment is a mouse’s preference, or CPP score. Any change in the preference between the pre and post-conditioning tests is thought to be due to the rewarding action of the drug, which can have either a positive or negative reward valence (punishment). Additional groups, for which vehicle was administered in both environments, were also tested as controls. Any change in the bias between the pre and post-conditioning tests in these groups are indicative of noise in the paradigm.
Statistical Analysis
Statistics were performed using SPSS statistical software (ver. 19, IBM). Two-way ANOVA was performed, with drug treatment and genotype as fixed factors and with CPP score as the dependent measure. The CPP score was defined as time spent in the drug-designated compartment minus the time spent in the vehicle-designated compartment during the 30-minute preference tests. One-way ANOVA was then performed within genotypes having significant main effects and the Bonferroni-Dunn procedure was used to determine which groups had a significant conditioning effect of the drug. An a priori (simple) contrast was performed in order to compare between the group 2 and 3 DAT-CI responses. A significance level of p < 0.05 was used for all tests.
Results
We observed that DAT-CI mice displayed an aversive response to cocaine inconsistently: with an early experiment finding robust CPA to cocaine and a more recent experiment failing to replicate this effect. In this study, we sought to investigate whether genetic drift was responsible for this inconsistency. We compared the cocaine-induced conditioned place-preference/aversion response of three groups of DAT-CI mice. Group 1 mice are from early generations of breeding – before complete backcrossing – and therefore contain a mixture of C57 and 129 strain backgrounds. Group 2 mice are from later generations – after backcrossing – and therefore on a C57-congenic background. We created group 3 mice in order to test the hypothesis that behavioral differences between groups 1 and 2 are due to their genetic differences, and are not an effect of changing experiment or other environmental factors. Therefore, group 1 mice were analyzed separately in a one way ANOVA, and groups 2 and 3 mice were analyzed in a two way ANOVA in order to detect a strain interaction.
Group 1
Group 1 mice were homozygous DAT-CI mice and their wild type littermates. These mice were studied before the extensive backcrossing procedure and thus had a mixed background of 129 and C57 strains as described in detail in the “Methods” section. The mice were examined with the CPP/CPA procedure and the results are shown in figure 1. Two-way analysis of variance (ANOVA) of CPP/CPA post-test scores (open bars in figures) was performed within this data set (Fig 1) – with genotype and drug treatment as between-subject factors. There was no main effect of drug (F1, 36 = 1.211; p = 0.278), but there was a main effect of genotype (F1, 36 = 16.964; p < 0.001) and a genotype × drug interaction (F1, 36 = 18.389; p < 0.001). Bonferroni-Dunn post hoc test show that there was a significant CPP (p < 0.01) in the group 1 wild-type controls, and a significant CPA (p < 0.05) in the DAT-CI littermates for 20 mg/kg cocaine.
Groups 2 and 3
Group 2 mice are C57-congenic and derived from group 1, by backcrossing with wild-type C57 mice for 12 generations. Group 3 mice were generated by crossing group 2 mice with wild-type 129S1/SvImJ mice, and thus they are analogous to group 1 in that both groups are on a 129B6 mixed strain background. These groups were examined with the CPP/CPA procedure and the results are shown in figures 2 and 3. Two-way ANOVA of CPP/CPA post test scores was performed within these data sets – with genetics and drug treatment as between-subject factors; with genetics meaning the true genotype, involving both the mutation and strain. There was no main effect of drug (F1, 63 = 3.231; p = 0.078), but there was a main effect of genetics (F3, 63 = 4.977; p < 0.005) and a genetics × drug interaction (F3, 63 = 7.758; p < 0.001).
Bonferroni-Dunn post hoc tests show that there was no significant effect of 20 mg/kg cocaine (compared to saline) in group 2 DAT-CI mice (Fig 2b; p = 0.470), but there was a significant CPP in their wild-type controls (Fig 2a; p < 0.001). Importantly, Bonferroni-Dunn post hoc tests show that there was a significant CPA in group 3 DAT-CI mice (Fig 3b; p < 0.01). However, the large apparent CPP of their wild-type controls did not reach statistical significance (Fig 3a; p = 0.070). This is apparently due to a larger variance in this group, which is perhaps a result of the genetic heterogeneity of 129B6 mice compared to C57-congenic mice.
A priori hypothesis testing shows that group 2 and group 3 DAT-CI mice had significantly different responses to 20 mg/kg cocaine (F1, 63 = 6.417; p < 0.05) with group 3 having significant CPA and group 2 having no response to cocaine. Since group 3 wild-type mice had a reduced CPP score compared to group 2 wild-types, a similar a priori hypothesis test was conducted on the wild-type mice. This test does not indicate that there was a significant difference between the wild-type control mice of the two groups, in response to 20 mg/kg cocaine (F1, 63 = 2.00; p = 0.162).
Discussion
In the present study, we observe that mice with a cocaine insensitive dopamine transporter (DAT-CI mice) express aversion to cocaine – or indifference to it – depending on their genetic background. Other studies, involving delayed conditioning, had shown that an aversive effect can be observed for addictive drugs (Fudala & Iwamoto 1990). In contrast, we report here that DAT-CI mice on a mixed 129B6 background (groups 1 and 3) have a robust conditioned place aversion to immediate conditioning with cocaine. Since our conditioning was not delayed, we conclude that cocaine can have an aversive action that is to some degree acute and concomitant with the rewarding effect, as opposed to a delayed withdrawal effect.
This issue of timing is an important detail in the hedonic allostasis theory of addiction. The theory states that drug-induced negative states may counter intuitively promote drug-taking, if and only if a drug’s aversive effects are delayed relative to its rewarding effects (Ahmed & Koob 2005). In this case, the drug would be taken again for its initially rewarding effects – in order to delay the negative state once again. This mechanism is then used to explain the escalation of intake over time. Indeed, the sensitivity to methamphetamine’s aversive effect has been correlated with total intake in a mouse model of high versus low consumption (Shabani et al. 2011).
The current experimental results are helpful in updating or extending the theory on the aspect of timing of the opponent (CPP/CPA) processes. Apparently, the rewarding and aversive processes may not be much separated in time, as is commonly thought when equating aversion with the withdrawal syndrome. In wild-type littermates, where the DAT-mediated rewarding effect is present, a robust CPP is induced by immediate conditioning with cocaine. In contrast, a robust CPA is induced by immediate cocaine conditioning in the 129B6 DAT-CI mice. This is an important detail, because it suggests that cocaine’s aversive effects are to some degree concurrent with its rewarding effect in wild-type mice.
In this regard, experiments involving strains of readily available wild-type mice, especially hybrid mice, may be sufficient to study factors involved in reward and aversion on the genetic level. The DAT-CI mutation affords the unique ability to observe the effects of these genetic factors on the behavioral level (O’Neill & Gu 2012). For example, eliminating DAT inhibition promotes an aversive effect of cocaine in group 1 and group 3 DAT-CI mice. This is not the case in group 2 DAT-CI mice, where cocaine induces neither a CPP nor a CPA. A previous study which used multiple doses of cocaine in C57-congenic (group 2-like) DAT-CI mice also did not observe CPA (Tilley et al. 2009). Therefore, it appears that the unique loss of both CPP and CPA in group 2 DAT-CI mice is a fully qualitative difference due to genetics.
We can therefore hypothesize that the aversive actions of cocaine are normally mediated by its non-DAT targets, but that these actions are not readily manifest in the C57 strain. Interestingly, we found in another study that the DAT/NET inhibitor methylphenidate does not induce either reward or aversion (Tilley & Gu 2008). Studies using the cocaine conditioned taste aversion test in transporter knock-out mice support the idea that inhibition of NET and/or SERT – as opposed to DAT – are primarily involved in aversion (Jones et al. 2009). Furthermore, the reinforcing potency of amphetamine (but not of cocaine) has been inversely correlated with SERT binding (Ritz & Kuhar 1989), indicating that serotonin neurotransmission may play a complicated role in the negative effects of psychostimulants. Taken together, these data suggest that NET and/or SERT inhibition are involved in cocaine’s aversive effect.
Studies with genetically heterogeneous mice, such as mice in group 1, could also identify biomarkers of addiction risk. Several genes such as cFos, Slc6a4 (SERT), and Htr3a (a serotonin receptor) have been correlated with differences in methamphetamine sensitivity across strains (Wheeler et al. 2009). Differences in genes such as these may influence cocaine aversion or any effects mediated through inhibition of SERT and NET. Therefore, strain differences in modifier genes may explain the observed differences in CPA behavior, even though both strains contain the same mutation in DAT. It is important to realize that to some degree, even inbred mice presumed to be genetically homogenous are not so, due to retrotranspositions; and there are certainly different transpositions in the C57 and 129 strains (Akagi et al. 2008).
Not all cocaine-induced negative states proceed rapidly after inhibition of a cocaine target. Most models accept that addiction involves maladaptive, cumulative, drug-induced neurological changes termed “metaplasticity,” within the reward circuit. The mechanisms involved may include long, slow-acting plasticity such as synaptic and whole-cell remodeling, or neurodegeneration (Achat-Mendes et al. 2005; Lee et al. 2011; Russo et al. 2010). However, an acute aversive effect and a delayed or protracted antireward process are not mutually exclusive, and may play different roles in the drug response. The current data suggest an acute action of cocaine as the cause for its negative effects – when they are defined as negative reinforcement, or aversion.
In summary, DAT-CI mice can express an aversion to cocaine, and this effect is modulated by the genetic background. The aversive effect is to some degree concurrent with the rewarding effect that is expressed in wild-type controls. These findings are important because the aversive effect observed may be the reason for certain individuals having low propensity to cocaine addiction. The DAT-CI mouse strains can be used further, in molecular studies, to identify the differences in the genetic background that are critical in modifying their behavioral responses. Furthermore, behavioral studies that build upon this characterization, with a manipulation of the norepinephrine system for example, may uncover the mechanism of drug-induced aversion. Studies such as these could identify therapeutic strategies that exploit a more potent, acute aversive response in order to halt an affected individual’s drug taking.
Acknowledgements
The authors would like to acknowledge Dawn Han and Michael Chee for their valuable assistance. This work was supported by grants from the NIH (DA014610 and DA020124; PI: HG).
Footnotes
There are no financial conflicts of interest.
References
- Achat-Mendes C, Ali SF, Itzhak Y. Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacology. 2005;30:1128–1137. doi: 10.1038/sj.npp.1300675. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology (Berl) 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Akagi K, Li J, Stephens RM, Volfovsky N, Symer DE. Extensive variation between inbred mouse strains due to endogenous L1 retrotransposition. Genome Res. 2008;18:869–880. doi: 10.1101/gr.075770.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh SA, McDowell CS, Stavnezer AJ, Denenberg VH. A behavioral and neuroanatomical assessment of an inbred substrain of 129 mice with behavioral comparisons to C57BL/6J mice. Brain Res. 1999;836:38–48. doi: 10.1016/s0006-8993(99)01586-3. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Conditioned aversion after delay place conditioning with amphetamine. Pharmacol Biochem Behav. 1990;35:89–92. doi: 10.1016/0091-3057(90)90209-z. [DOI] [PubMed] [Google Scholar]
- Guzman D, Ettenberg A. Runway self-administration of intracerebroventricular cocaine: evidence of mixed positive and negative drug actions. Behav Pharmacol. 2007;18:53–60. doi: 10.1097/FBP.0b013e3280144ac9. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Hall FS, Uhl GR, Rice K, Riley AL. Differential involvement of the norepinephrine, serotonin and dopamine reuptake transporter proteins in cocaine-induced taste aversion. Pharmacol Biochem Behav. 2009;93:75–81. doi: 10.1016/j.pbb.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Lee J, Parish CL, Tomas D, Horne MK. Chronic cocaine administration reduces striatal dopamine terminal density and striatal dopamine release which leads to drug-seeking behaviour. Neuroscience. 2011;174:143–150. doi: 10.1016/j.neuroscience.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Gelernter J, Oslin D, Anton RF, Farrer LA, Kranzler HR. Agitated depression in substance dependence. Drug Alcohol Depend. 2011;116:163–169. doi: 10.1016/j.drugalcdep.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Tervo KE, Ling W. Irritability following abstinence from cocaine predicts euphoric effects of cocaine administration. Addict Behav. 2003;28:817–821. doi: 10.1016/s0306-4603(01)00273-8. [DOI] [PubMed] [Google Scholar]
- O’Neill B, Gu HH. Amphetamine-induced locomotion in a hyperdopaminergic ADHD mouse model depends on genetic background. Pharmacol Biochem Behav. 2012 doi: 10.1016/j.pbb.2012.09.020. http://dx.doi.org/10.1016/j.pbb.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm Genome. 2005;16:291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248:1010–1017. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011;10:625–636. doi: 10.1111/j.1601-183X.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009;331:204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, Gu HH. The effects of methylphenidate on knockin mice with a methylphenidate-resistant dopamine transporter. J Pharmacol Exp Ther. 2008;327:554–560. doi: 10.1124/jpet.108.141713. [DOI] [PubMed] [Google Scholar]
- Tilley MR, O'Neill B, Han DD, Gu HH. Cocaine does not produce reward in absence of dopamine transporter inhibition. Neuroreport. 2009;20:9–12. doi: 10.1097/WNR.0b013e32831b9ce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8:758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]