Abstract
Adolescent individuals display altered behavioral sensitivity to ethanol, which may contribute to the increased ethanol consumption seen in this age-group. However, genetics also exert considerable influence on both ethanol intake and sensitivity. Thus far there is little research assessing the combined influence of developmental and genetic alcohol sensitivities. Sensitivity to the aversive effects of ethanol using a conditioned taste aversion (CTA) procedure was measured during both adolescence (P30) and adulthood (P75) in 8 inbred mouse strains (C57BL/6J, DBA/2J, 129S1/SvImJ, A/J, BALB/cByJ, BTBR T+tf/J, C3H/HeJ, and FVB/NJ). Adolescent and adult mice were water deprived, and subsequently provided with access to 0.9% (v/v) NaCl solution for 1h. Immediately following access mice were administered ethanol (0, 1.5, 2.25, 3g/kg, ip). This procedure was repeated in 72h intervals for a total of 5 CTA trials. Sensitivity to the aversive effects of ethanol was highly dependent upon both strain and age. Within an inbred strain, adolescent animals were consistently less sensitive to the aversive effects of ethanol than their adult counterparts. However, the dose of ethanol required to produce an aversion response differed as a function of both age and strain.
Introduction
The neurobiological alterations that occur during adolescence may cause altered sensitivities to alcohol in this age-group, that may, in turn, account for the differences in intake observed between adults and adolescents. Ethical constraints have limited research examining adolescent alcohol sensitivity in humans. However, research using rodents has identified many behaviors in which adolescents appear to show differential sensitivity to ethanol. For example, adolescent rodents exhibit reduced sensitivity to the anxiolytic (Varlinskaya and Spear, 2002; Hefner and Holmes, 2007), sedative/hypnotic (Little et al., 1996; Silveri and Spear, 1998; Silveri and Spear, 1999, Hefner and Holmes, 2007; Linsenbardt et al., 2009), hypothermic (Silveri and Spear, 2000), and motor impairing (White et al., 2002; Hefner and Holmes, 2007) effects of ethanol. However, adolescent rodents have displayed increased sensitivity to the locomotor stimulatory (Hefner and Holmes, 2007; Stevenson et al., 2008; Melón and Boehm, 2011), amnestic (Markwiese et al., 1998), ataxic (Linsenbardt et al., 2009) and the social faciliatory (Varlinskaya and Spear, 2002) actions of ethanol.
In addition to the developmental-based differences in ethanol sensitivity, there are also differences in sensitivity that can be attributed to genetic sources. For example, B6 and D2 mice, two of the most commonly used inbred mouse strains in alcohol research, display vast differences in their behavioral response to ethanol. Compared to D2 mice, B6 mice not only consume larger amounts of ethanol and display a preference for the drug in a 2-bottle choice procedure (McClearn and Rodgers, 1959; Yoneyama et al., 2008), but also display reduced sensitivity to ethanol induced withdrawal, (Crabbe et al., 1994), locomotor stimulation and sensitization (Phillips et al., 1994; Melón and Boehm, 2011), loss of righting reflex (Linsenbardt et al., 2009) and conditioned taste aversion (CTA; Broadbent et al., 2002).
Some of the aforementioned ethanol-related behaviors may serve as cues for ethanol drinking. Genetic or developmental differences in sensitivity to these behaviors may then account for some of the differences in ethanol intake observed among different ages and genetic populations. For example, the genetic variation in sensitivity to ethanol’s aversive effects as measured by CTA is negatively correlated with ethanol intake (Green & Grahame, 2008). In this classical conditioning procedure, the pharmacological (and/or withdrawal) effects of ethanol (unconditioned stimulus; US) are paired with a novel flavor (conditioned stimulus; CS). The extent to which the animal finds ethanol aversive will be reflected by the amount of the CS that is consumed. High drinking rodents will continue to consume the CS, despite previous pairings with ethanol, and may therefore be said to display reduced sensitivity to the aversive effects of ethanol. Selectively bred alcohol preferring rats (such as the P and HAD lines) display attenuated ethanol induced CTA compared to rats bred to avoid ethanol (NP and LAD lines; Badia-Elder et al., 1999; Froehlich et al., 1988). This pattern of high ethanol intake paired with low ethanol induced CTA response is also observed in the alcohol-preferring inbred B6 strain versus the alcohol avoiding inbred D2 strain (Broadbent et al., 2002). It should be noted, however, that a high enough dose of ethanol paired with the CS would result in a conditioned taste aversion regardless of age or line/strain.
Because adolescent rodents have been reported to be less sensitive than adults to the aversive effects of ethanol as measured by conditioned taste aversion (CTA; Vetter-O’Hagan et al., 2009; Anderson et al., 2010; Holstein et al., 2011), and because behavioral genetic studies have indicated that there is a strong negative association between ethanol induced CTA and consumption of the drug (Green & Grahame, 2008), the current work assessed ethanol induced CTA in adolescent (P30) and adult (P75) mice of 8 inbred mouse strains. The objective was to use a traditional behavioral genetic paradigm to assess the developmental differences in ethanol sensitivity. Comparing a panel of inbred strains known to differ in limited access ethanol intake (refer to Rhodes et al, 2007) for ethanol sensitivity during both adolescence and adulthood allows the determination of whether adolescents are generally more sensitive to ethanol’s effects than adults, or if the developmental differences in ethanol sensitivity differ depending on genetic background. It is expected that adolescents will display reduced sensitivity to ethanol induced CTA as compared to their adult counterparts. However, it is predicted that the highest drinking strains will show the greatest developmental difference in ethanol sensitivity (i.e., B6 adolescent mice will be less sensitive to ethanol than adults, but there will be little difference in ethanol sensitivity between D2 adolescent and adult mice).
Materials and Methods
Animals
C57BL/6J (B6), DBA/2J (D2), FVB/NJ (FVB), C3H/HeJ (C3), BTBR T+tf/J (BTBR), A/J (AJ), 129S1/SvLmJ (129) and BALB/cByJ (BALB) inbred mice were obtained from the Jackson Laboratory and bred in the AAALAC accredited animal research facility at Indiana University – Purdue University, Indianapolis (IUPUI). Mice were weaned at postnatal day 21–23, and were group-housed according to sex in clear polycarbonate shoebox mouse cages with a paper tissue as environmental enrichment and food and water available ad libitum except as indicated. Male mice were transferred to the behavioral testing room and singly housed at least one week prior to the initiation of experiments. Only male mice were tested in the current work (N=566). Mice were 30 (P30, adolescent) or 75 (P75, adult) days old at the start of testing. Animals were maintained on a reverse 12 hour light-dark cycle (lights out at 11 AM) with the temperature and humidity of the vivarium kept at approximately 20°C and 50%, respectively. All testing occurred during the animals’ dark cycle. Red incandescent overhead lights were continuously left on so that experimenters could navigate the procedure room after lights out. All procedures were approved by both the Binghamton University Animal Care and Use Committee and the IUPUI Animal Care and Use Committee, and conformed to National Institutes of Health Guidelines.
Ethanol Induced Conditioned Taste Aversion (CTA)
Procedures were similar to those described by Broadbent et al., (2002), with some slight variations. At 1PM on experimental day 1 (P29 or P74), mice were weighed and their water bottles were removed from the home cage. Water restriction was necessary to promote subsequent consumption of the CS (NaCl; see below). On experimental day 2, the first CTA trial was conducted. Starting at 11AM, mice were weighed and then provided with 1h access to the CS, 0.9% NaCl (physiological saline) solution. Immediately following the conclusion of NaCl access, mice were administered a paired injection of either saline or ethanol dissolved in saline (US; 0, 1.5, 2.25, or 3.0 g/kg, ip). Each ethanol dose was administered to a separate group of mice. Three hours following the ethanol injection, water bottles were returned to the home cage. On experimental day 3 (24h following the paired injection) mice were weighed and then were administered an unpaired injection; animals that were previously administered a paired ethanol injection received an unpaired saline injection, whereas animals that were previously administered a paired saline injection received an unpaired ethanol injection (3.0 g/kg). This procedure was repeated for a total of 5 conditioning trials. Therefore, mice were water deprived on experimental days 1, 4, 7, 10, and 13, conditioning trials/paired injections occurred on experimental days 2, 5, 8, 11, and 14, and unpaired injections occurred on experimental days 3, 6, 8, and 12. Weights were monitored daily. Mice that did not consume more NaCl solution than could be accounted for by leak at Trial 1 were excluded from the study.
Statistical Analysis
An overall analysis was performed using a repeated measures analysis of variance (ANOVA) with genotype, age and ethanol dose as between groups factors, and day (or time) as the within groups factor. Post hoc tests were performed to isolate significant effects when appropriate. Heritability (h2) estimates were calculated from the ANOVA summary table by dividing the sum of squares between strains by the total sum of squares (Grisel et al., 1997). Phenotypic correlations were calculated by determining Pearson product-moment correlation coefficients using the percent change from CTA Trial 1–3, 2.25g/kg ethanol dose strain means. Results were considered significant at p<0.05.
Results
Ethanol Induced Conditioned Taste Aversion
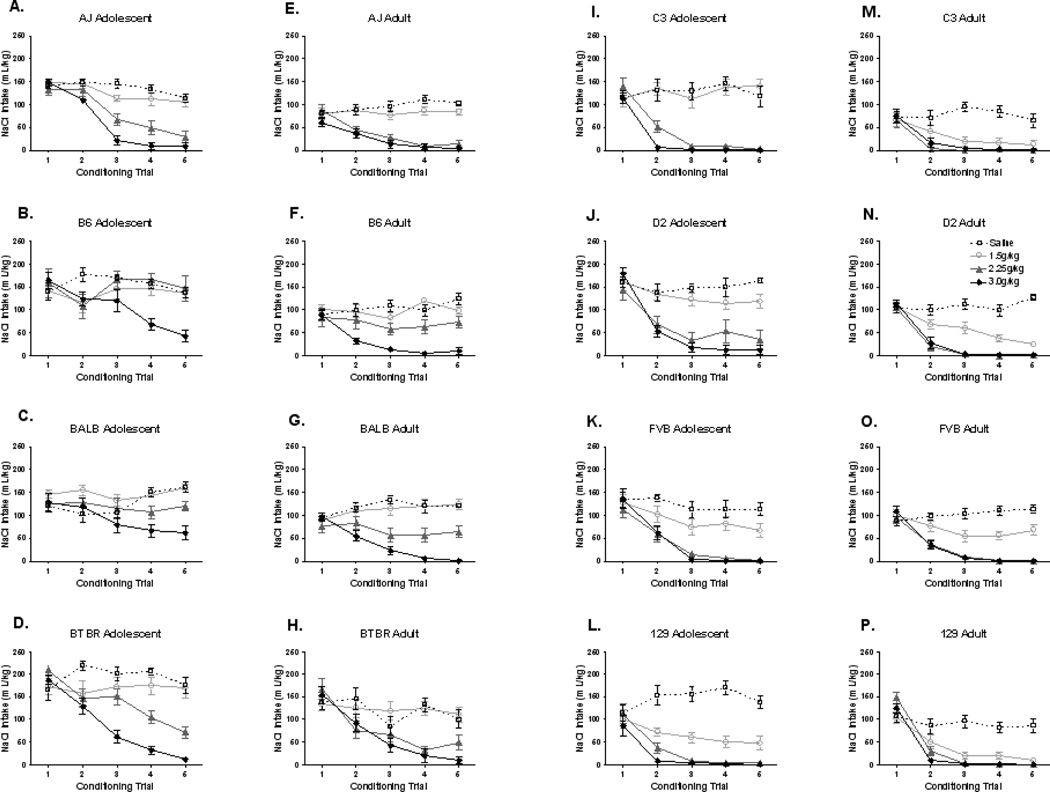
The NaCl intake data over each trial can be seen in Figures 1A–P. Group mean fluid intakes (mL/kg) on the first conditioning trial ranged from 70.3 – 181.6 mL/kg. The amount of the CS consumed on Trial 1 can influence the development of a CTA response. Therefore, only the mL NaCl intake at Trial 1 was analyzed to determine age and strain differences in NaCl solution consumption. NaCl consumption at Trial 1 was analyzed by a 3-way between measures ANOVA (strain × age × ethanol dose). A main effect of strain was detected, F(7,502)=17.6, p<0.0001. The pattern of trial 1 intakes by strain was as follows: BTBR>D2>B6=FVB=129=AJ=BALB≥C3. Newman Keuls post hoc tests indicated that BTBRs consumed the most fluid at Trial 1 (p’s<0.0001), followed by D2s (p’s<0.05). NaCl solution volumes did not significantly differ between B6s, FVBs, AJs, BALBs, and 129s. C3 mice consumed significantly less NaCl solution than B6s, FVBs, BTBRs, D2s, FVBs, and 129s (p’s<0.05). A main effect of age was also detected, F(1,502)=111.9, p<0.0001, indicating that adolescents consumed more NaCl solution per g body weight than did the adults. A strain × age interaction was also detected, F(7,502)=6.6, p<0.0001. Newman Keuls post hoc tests indicated that adolescents of the AJ, B6, BALB, BTBR, C3, and D2 strains consumed significantly more NaCl solution than their adult counterparts (p<0.01). Additionally, a trend was observed for the FVB adolescents to consume more than the FVB adults (p=0.06). No differences were observed between 129 adolescents and adults. No main effect of ethanol dose or interaction of ethanol dose with any other factor was detected. Heritability estimates for NaCl solution intake were derived from NaCl solution intake (mL/kg) strain means at Trial 1; the overall heritability estimate was 25% (30% for the adolescents and 40% for the adults).
Figure 1. Adolescent and adult mL/kg NaCl solution consumed across conditioned taste aversion trials.
(A) AJ adolescents, n’s: saline=8, 1.5g/kg=8, 2.25g/kg=6, 3.0g/kg=6. (B) B6 adolescents, n’s: saline=7, 1.5g/kg=7, 2.25g/kg=6, 3.0g/kg=7. (C) BALB adolescents, n’s: saline=6, 1.5g/kg=6, 2.25g/kg=6, 3.0g/kg=6. (D) BTBR adolescents, n’s: saline=9, 1.5g/kg=8, 2.25g/kg=7, 3.0g/kg=10. (E) AJ adults, n’s: saline=11, 1.5g/kg=10, 2.25g/kg=10, 3.0g/kg=11. (F) B6 adults, n’s: saline=10, 1.5g/kg=10, 2.25g/kg=7, 3.0g/kg=10. (G) BALB adults, n’s: saline=10, 1.5g/kg=11, 2.25g/kg=10, 3.0g/kg=10. (H) BTBR adults, n’s: saline=12, 1.5g/kg=12, 2.25g/kg=9, 3.0g/kg=10. (I) C3 adolescents, n’s: saline=6, 1.5g/kg=6, 2.25g/kg=7, 3.0g/kg=7. (J) D2 adolescents, n’s: saline=9, 1.5g/kg=10, 2.25g/kg=9, 3.0g/kg=9. (K) FVB adolescents, n’s: saline=10, 1.5g/kg=9, 2.25g/kg=9, 3.0g/kg=7. (L) 129 adolescents, n’s: saline=8, 1.5g/kg=9, 2.25g/kg=8, 3.0g/kg=8. (M) C3 adults, n’s: saline=9, 1.5g/kg=8, 2.25g/kg=8, 3.0g/kg=8. (N) D2 adults, n’s: saline=11, 1.5g/kg=12, 2.25g/kg=8, 3.0g/kg=12. (O) FVB adults, n’s: saline=9, 1.5g/kg=10, 2.25g/kg=10, 3.0g/kg=10. (P) 129 adults, n’s: saline=12, 1.5g/kg=14, 2.25g/kg=11, 3.0g/kg=11.
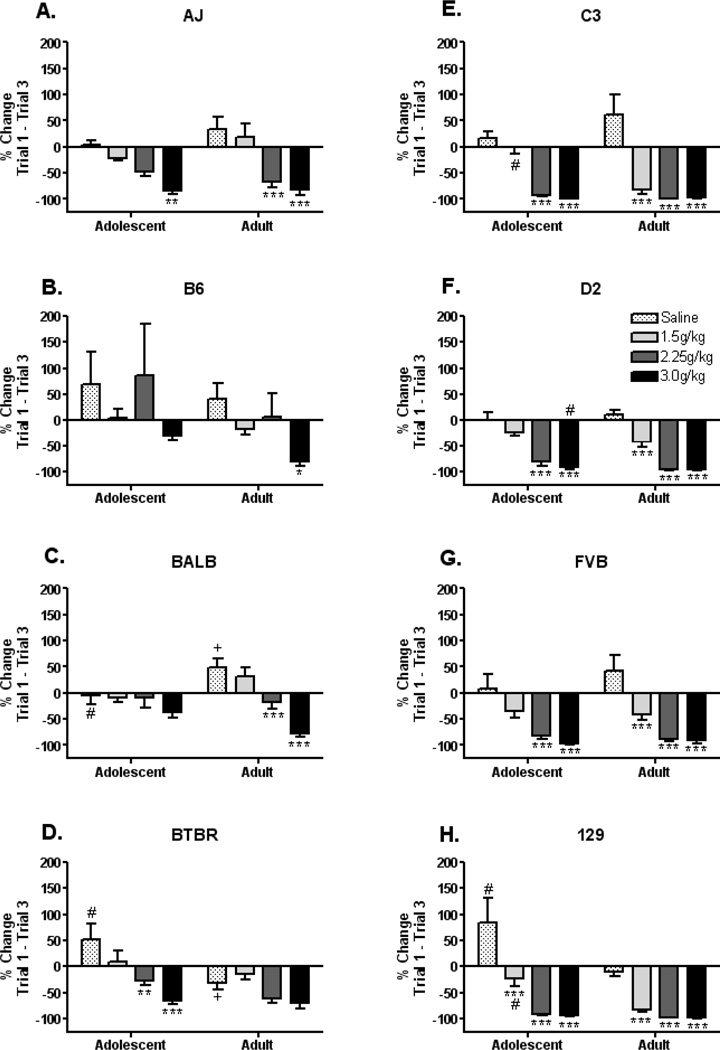
Age and strain differences were observed in NaCl intake at Trial 1, therefore data were normalized by creating a percent change score from Trial 1 to each subsequent Trial (Figure 2A–P). This strategy reduced the influence of strain and age differences in initial US (NaCl solution) intake on the taste aversion index. To prevent the influence of floor effects on the index of taste aversion, statistical analysis of age and strain differences focused on the percent change from Trial 1 to Trial 3, as inspection of the raw data in Figure 1 indicated that maximum CTA was typically achieved by Trial 3. The percent change from Trial 1 to Trial 3 was also used to calculate the heritability estimates. The overall heritability estimate of the CTA response was 40%. However, within adults the heritability estimate of ethanol induced CTA was 52%, while in adolescents the heritability estimate was 39%.
Figure 2. Ethanol induced conditioned taste aversion: percent change from trial 1 to trial 3.
(A) AJ adolescents display a CTA response at 3.0g/kg ethanol, while adults display a CTA response at 2.25 and 3.0g/kg ethanol. (B) B6 adolescents do not display a CTA response at any of the doses tested, however B6 adults display a CTA response at 3.0g/kg ethanol. (C) BALB adolescents do not display a CTA response at any of the doses tested, however BALB adults display a CTA response at 2.25 and 3.0g/kg ethanol. (D) BTBR adolescents display a CTA response at 2.25 and 3.0 g/kg ethanol, but BTBR adults do not display a significant CTA response at any of the doses tested, likely because they reduce NaCl intake following saline injections. (E) C3 adolescents display a CTA response at 2.25 and 3.0 g/kg ethanol, while C3 adults display a CTA response at all ethanol doses (1.5, 2.25 and 3.0 g/kg). (F) D2 adolescents display a CTA response at 2.25 and 3.0g/kg ethanol, while D2 adults display a CTA response at all ethanol doses (1.5, 2.25, 3.0 g/kg). (G) FVB adolescents display a CTA response at 2.25 and 3.0g/kg ethanol, while FVB adults display a CTA response at all ethanol doses (1.5, 2.25, 3.0 g/kg). (H) 129 adolescents and adults display a CTA response at all ethanol doses, however the adolescents do not display as large of a reduction at the 1.5g/kg ethanol dose as do the adults. (*=p<0.05, **=p<0.01, ***=p<0.001; the asterisks (*) indicates significant difference from saline control within age-group; # indicates significant age difference at the same dose; + indicates a significant change from zero in the saline treated group).
The between subject ANOVA for CTA by Trial 3 indicated a main effect of strain, F(7,502)=11.1, p<0.0001. The pattern of CTA response by strain was as follows: B6=BALB≤BTBR<AJ<FVB=C3=D2=129. Newman Keuls post hoc analyses indicated that although C3s, D2s, FVBs and 129s did not differ from each other, they showed the greatest reduction from Trial 1 (p’s<0.05) as compared to the other strains. B6s and BALBs did not differ from each other (showing no apparent change from Trial 1), but AJs showed a significantly greater reduction from Trial 1 as compared to these strains (p’s<0.01) and BTBRs displayed a trend (p’s=0.07). A main effect of age was also detected, F(1,502)=7.9, p<0.01, indicating that adults displayed a greater reduction in intake from Trial 1 as compared to adolescents. A main effect of ethanol dose was detected, F(3,502)=94.6, p<0.0001. Newman Keuls post hoc analyses indicated that each ethanol dose group was significantly different from every other (p’s<0.0001). The 3g/kg ethanol dose group showed the greatest reduction from Trial 1, followed by the 2.25 and 1.5g/kg groups, whereas the saline group showed a slight increase in intake compared to Trial 1.
A significant interaction of strain × age [F(7,502)=3.2, p<0.01] was detected. Newman Keuls post hoc tests revealed that B6 adolescents were significantly different (positive percent change from Trial 1) compared to all other age/strain groups (p’s<0.05), and that 129 adults showed a greater reduction from trial 1 as compared to 129 adolescents (p<0.05). No other important significant effects were detected. A significant interaction of strain × ethanol dose was also detected, F(21, 502)=2.6, p<0.001. Newman Keuls post hoc analyses indicated that compared to saline, B6s showed a significant CTA response at the 3.0g/kg dose (p<0.0001), and a trend at the 1.5g/kg dose (p=0.06), BALBs, BTBRs, FVBs and 129s showed a significant CTA response at the 3.0g/kg dose (p’s<0.001, 0.01, 0.05, and 0.001, respectively), AJs and D2s showed a significant CTA response at the 2.25 and 3.0g/kg ethanol doses (p’s<0.01, and 0.0001, respectively), and C3s showed a significant CTA response at all ethanol doses (p’s<0.001).
A significant strain × age × ethanol dose interaction was detected, F(21, 502)=1.7, p<0.05. Because only specific age at ethanol dose differences within a strain were of interest, planned comparisons were performed to isolate significant differences within the strain × age × ethanol dose interaction. Within each strain, adolescent and adult CTA responses were compared at each ethanol dose condition. Further, within each strain and age each ethanol dose response was compared to the saline response. Finally, saline groups within each age and strain were analyzed to determine if the percent change from Trial 1 significantly differed from zero.
The planned comparisons revealed that AJ adolescent mice treated with 3g/kg ethanol displayed a significant CTA response as compared to saline age-matched controls (p<0.01), and that AJ adults displayed a significant CTA response (as compared to age-matched saline controls) at the 2.25 and 3.0g/kg ethanol doses. No other significant effects were observed in this strain. B6 adolescents did not display a significant CTA response (as compared to saline adolescent controls) at any of the ethanol doses, although a trend towards a CTA response was observed at the 3g/kg ethanol dose (p=0.09). B6 adults displayed a significant CTA response only at the 3g/kg ethanol dose (p<0.05). BALB adolescents did not display a significant CTA response (compared to age matched controls) at any of the ethanol doses tested. However, BALB adults displayed a CTA response at the 2.25 and 3g/kg ethanol dose groups (p’s<0.001). Additionally, BALB adult and adolescent saline treated mice displayed significantly different changes in their NaCl intake (p<0.05), and trends towards differential changes in NaCl intake at the 1.5 and 3g/kg doses (p’s=0.07). BALB adult saline treated mice displayed a significant increase in NaCl intake, as compared with zero (i.e. no change; p<0.05). As compared to age matched saline controls, BTBR adolescent mice displayed a significant CTA response at the 2.25 and 3g/kg ethanol doses (p’s<0.01 and 0.0001, respectively), as well as a trend towards a CTA response in the 1.5g/kg dose (p=0.07). BTBR adults did not display a significant CTA response at any of the ethanol doses tested when compared to adult saline controls, however a trend towards a CTA response was observed in the 3g/kg dose (p=0.06). Additionally, adolescent and adult saline treated mice significantly differed in their change in NaCl intake (adolescents increased, while adults decreased their consumption over trials; p<0.0001), but specific ethanol doses did not differ between the two age groups, indicating a similar reduction in NaCl intake n adolescent and adult BTBR mice. When compared to age-matched saline controls, C3 adolescent mice displayed a significant CTA response to the 2.25 and 3g/kg ethanol doses (p’s<0.001), while C3 adults displayed a significant CTA response to all ethanol doses (p’s<0.0001). Additionally, 1.5g/kg ethanol treated adolescent and adult mice differed in their CTA response (p<0.01), with C3 adults displaying a greater reduction in NaCl intake than adolescents. A trend towards differential changes in adolescent and adult C3 saline treated mice was also observed (p=0.08), with C3 adults tending towards a greater increase in their NaCl consumption over trials compared to adolescents. D2 adolescent mice displayed significant CTA responses to 2.25 and 3g/kg ethanol dose (p’s<0.0001), and a trend towards a CTA response at the 1.5g/kg ethanol dose (p=0.06) as compared to adolescent saline controls. D2 adults displayed a significant CTA response at all ethanol doses tested (p’s<0.0001). Additionally, D2 adolescent and adult 3g/kg ethanol treated mice displayed differential changes in NaCl intake (p<0.0001) and a trend towards differential changes in NaCl intake at the 1.5g/kg dose (p=0.07), with adults displaying a greater reduction than adolescents at both doses. Compared to age-matched saline treated controls, FVB adolescents displayed a significant CTA response at the 2.25 and 3g/kg ethanol doses (p’s<0.001) and a trend at the 1.5g/kg dose (p=0.06), while FVB adults displayed a significant CTA response at all ethanol doses (p’s<0.0001). No other significant effects were observed in the FVBs. 129 adolescent and adult mice displayed significant CTA responses at all ethanol doses tested when compared to age-matched saline controls (p’s<0.0001). Additionally, both saline- and 1.5g/kg ethanol treated mice differentially changed their ethanol intake when compared to their respective matched ethanol doses across age (p’s<0.0001 and 0.01, respectively). Saline treated adolescent 129 mice showed a greater increase in NaCl consumption across trials compared to their adult counterparts, whereas 1.5g/kg ethanol treated adolescent 129 mice displayed a lesser reduction in NaCl consumption across trials compared to their adult counterparts.
Phenotypic Correlations
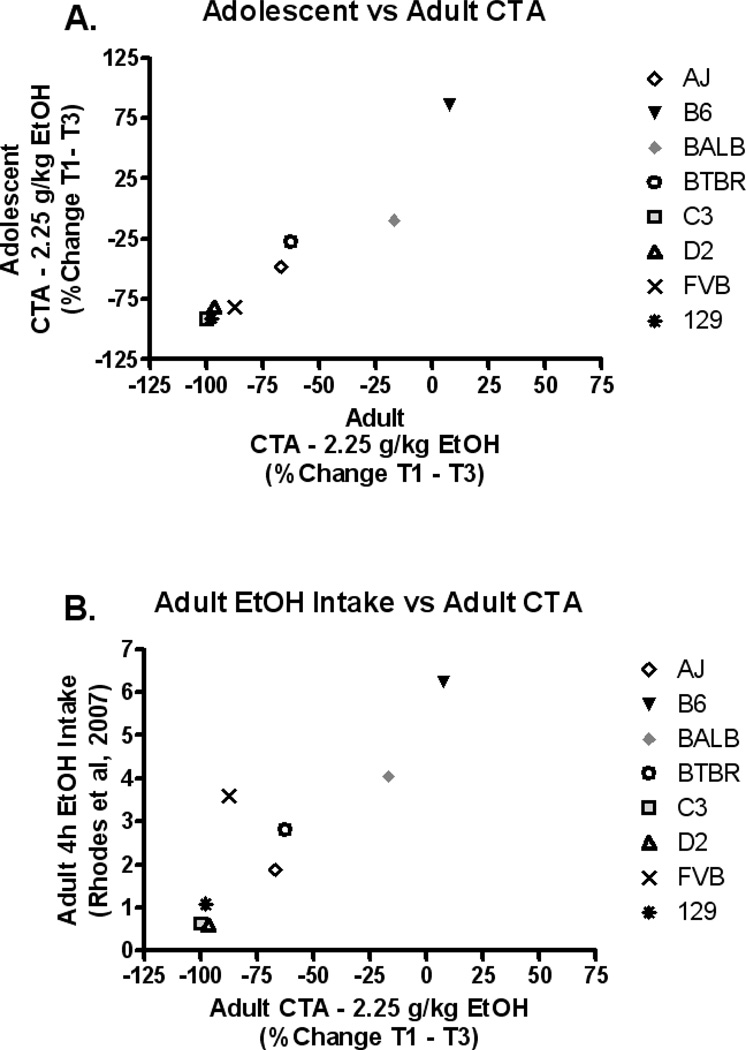
Strain means for the percent change from Trial 1 to Trial 3 at the 2.25g/kg ethanol dose were used to examine the relationship between adolescent and adult CTA response. The CTA response at the two ages was strongly positively associated (r=0.96, p<0.001; Figure 3A). To assess the relationship between adult CTA response and adult ethanol consumption, the strain means for the percent change from Trial 1 to Trial 3 at the 2.25g/kg ethanol dose in the current work were compared with 4h limited access ethanol intake strain means reported by Rhodes et al, (2007). Ethanol intake and CTA response were strongly negatively associated (r= −0.82, p<0.05; Figure 3B).
Figure 3. Phenotypic correlations.
(A) Adolescent and adult strain mean CTA responses are significantly correlated (percent change Trial 1–3, 2.25g/kg ethanol dose; r=0.96, p<0.001), suggesting that the same or very similar genes likely contribute to the behavior at both ages. (B) Adult strain mean CTA responses (percent change Trial 1–3, 2.25g/kg ethanol dose) is significantly negatively associated with adult limited access ethanol intakes reported by Rhodes et al, 2007 (4h male strain means; r= −0.82, p<0.05).
Discussion
This project sought to examine age- and genotype-dependent sensitivity to the aversive effects of ethanol. Consistent with results reported by Broadbent and colleagues (2002), the current work demonstrates that ethanol induced conditioned taste aversion (CTA) varied considerably by strain, suggesting that genetics exerts considerable influences over ethanol sensitivity in this behavior. Indeed, the overall heritability estimate in the current experiment suggests that 40% of the variation in ethanol induced CTA can be attributable to genotype, whereas Broadbent et al (2002) reported heritability estimates ranging 29–43%.
Ethanol-induced CTA was chosen to measure the aversive effects of ethanol because this behavioral measure has been consistently shown to be negatively correlated with ethanol intake and preference among genetic animal models (Green and Grahame, 2008; Broadbent et al., 2002; Rhodes et al., 2007; Froehilich et al., 1998; Quintanilla et al, 2001; Brunetti et al., 2002; Chester et al., 2003; Phillips et al., 2005; Horrowitz and Whitney, 1975; Risinger and Cunningham, 1992; 1995; 1998). Indeed, the current work replicated this negative association between ethanol intake (as reported by Rhodes et al., 2007) and sensitivity to ethanol-induced CTA in adult mice (r= −0.82, p<0.05).
Additionally, this work provides evidence that ethanol-induced CTA is mediated by very similar genetic means across age, as adolescent and adult sensitivity to ethanol induced CTA were strongly correlated (r=0.96, p<0.001). Furthermore, the current work demonstrates that within an inbred strain, adolescent mice display reduced sensitivity to ethanol’s aversive effects as compared to adults. This was clearly demonstrated in adolescent AJ, B6, BALB, C3, D2 and FVB mice, where higher doses were generally required to induce an ethanol-dependent CTA response and/or adolescents showed a reduced magnitude change in NaCl-solution consumption following ethanol dose as compared to adults. However, comparisons between strains show that the dose required to induce a CTA response among adolescent and adult animals is genotype dependent, and in some cases an adolescent may be more sensitive to ethanol’s aversive effects than an adult. For example, adolescent 129s displayed a CTA response at an ethanol dose as low as 1.5g/kg, however there were several adults of different inbred strains that required a higher ethanol dose before they displayed a CTA response (i.e., BALB, AJ, and B6).
Although adolescents of the AJ, B6, BALB, C3, D2 and FVB strains were clearly less sensitive to alcohol’s aversive effects than their adult counterparts, adolescent-typical reductions in ethanol-induced CTA were less obvious in the 129 and BTBR strains. Adolescents and adults of the 129 strain both displayed significant reductions in NaCl solution consumption at all ethanol dose-paired groups, which would seem to suggest equivalent ethanol-induced CTA responses across the age groups. However, it is important to note that at the 1.5g/kg ethanol-paired treated adolescent and adult mice significantly differed from one another. Adult 129s in this ethanol dose group displayed a greater magnitude change in the CTA response than did the dose-matched adolescent 129s. Therefore, adolescent and adult 129s do not display equivalent ethanol-induced CTA responses, but rather adolescent 129s are less sensitive to the development of an ethanol-induced CTA than are adults as is evidenced by an examination of the 1.5 g/kg ethanol-paired groups.
Whereas BTBR adolescents showed a reduction in NaCl-solution intake at the 2.25 and 3.0 g/kg ethanol doses compared to BTBR adolescent saline-treated mice, BTBR adults displayed no significant reduction in NaCl intake following ethanol administration compared to BTBR adult saline-treated mice. This would seem to imply that adolescent BTBR mice are more sensitive to the aversive effects of ethanol. However, it is important to note that adolescent and adult saline-treated mice showed differential changes in NaCl-solution consumption across trials. Adolescent BTBR mice increased their NaCl solution intake, while BTBR adult mice reduced their NaCl intake over trials. Moreover, the two saline-treated age groups significantly differed from each other. It is therefore possible that the positive change in NaCl consumption observed in the saline treated adolescent BTBR mice over-emphasizes the apparent ethanol-induced reduction in NaCl intake while the negative change in NaCl consumption observed in the saline-treated adult BTBR mice under-estimates the ethanol induced reduction in NaCl intake.
We chose to analyze and interpret our CTA results based on a percent change score from Trial 1 to Trial 3. This approach was intended to reduce any influence of differences in exposure to the unconditioned stimulus (US; NaCl solution). Both age and strain influenced the amount of NaCl solution consumed at Trial 1. Differences in exposure to the US can affect subsequent associations (Revillo et al, 2012). Because we were comparing multiple strains at two different ages, we thought it best to control for initial exposure to the US by transforming the data to a percent change from Trial 1. It is possible that this approach may have obscured some age or strain dependent ethanol effects, however we thought it best to control for any association differences between the strains/ages that were due to US exposure, since we cannot control for learning differences that are due to age or strain.
The observed differences in age and strain are thought to reflect inherent differences in sensitivity to the aversive effects of ethanol. However, it is possible that differences in ethanol-induced CTA responses are due to strain/age differences in learning the contingency or general differences in sensitivity to aversive stimuli. Compared to adults, adolescents tend to perform better on simple active avoidance tasks, but perform poorly in more complex learning tasks (Spear and Brake, 1983). Spear and Brake (1983) suggested that adolescent-typical hyperactivity may account for some of the age-dependent differences in learning tasks, as hyperactivity is an advantage in the simple active avoidance tasks, but a disadvantage in the more complex learning tasks. Schramm-Sapyta et al. (2006) report that adolescents are less sensitive to lithium chloride- (LiCl-) induced CTA, and suggest that this age effect could be due either to a failure to learn the association (as CTA is a task where hyperactivity would not be advantageous for forming CS/US associations), or the age effect could be due to a general insensitivity to aversive stimuli among adolescents.
Strain differences in LiCl-induced CTA have also been reported in the same direction as the strain differences in ethanol-induced CTA with D2 mice displaying a stronger CTA response to LiCl than B6 mice (Risinger and Cunningham, 2000). Additionally, rats selectively bred for proneness or resistance to cyclophosphamide-induced CTA has also been reported to display differential sensitivity to ethanol-induced CTA (Elkins et al., 1992). Elkins and colleagues (1992) observed that the selectively bred taste aversion prone (TAP) rats displayed a stronger ethanol-induced CTA response than taste aversion resistant (TAR) rats, despite the fact that ethanol CTA response was not a condition for selection. The TAP and TAR lines display similar abilities in other general learning paradigms, such as radial arm maze and bar pressing for food (Hobbs et al., 1993; Orr et al., 1997; 2004).
As mentioned previously, the ethanol-induced CTA measure was chosen to assess age and strain differences in ethanol sensitivity because it is predictive of ethanol consumption. Although strain/age differences in general aversion sensitivity and learning capabilities cannot be ruled out as alternative explanations for the results obtained in the present study, both aversion sensitivity and ability to form associations presumably could both influence ethanol consumption, either independently of, or in addition to, variations in ethanol sensitivity. For example, in addition to differing in drug-induced CTA, the TAR and TAP rat lines have been shown to differ in ethanol consumption, with TAP rats consuming less ethanol than TAR rats (Orr et al., 1997; 2004). This suggests that a general proneness (or resistance) to CTA can influence not only ethanol-induced CTA (Elkins et al., 1992), but also ethanol consumption.
Two limitations of the current study should be noted. First, in order to induce adequate NaCl solution consumption water deprivation procedures were utilized. The water deprivation presumably created a stressful situation for both the adolescent and adult animals. Importantly, stressors can sometimes differentially affect ethanol responses among adolescent and adult animals (Ristuccia et al., 2007; Song et al., 2007; Varlinskaya et al., 2010), although Anderson and colleagues (2010) reported that, at least for restraint and social isolation, stressor presentation did not significantly alter adolescent or adult responses to ethanol-induced CTA. Second, the 8 inbred strains in the current work were chosen based on their limited access ethanol intake reported by Rhodes et al, (2007). We were interested in maximizing behavioral diversity in regards to ethanol intake, and therefore chose 8 inbred strains that represent a spectrum of limited access ethanol intake. However, though these strains are behaviorally diverse, they are not necessarily genetically diverse. Future work should include additional inbred strains for age assessments of ethanol sensitivity that are more genetically diverse.
The current experiment demonstrated strain- and age-dependent alterations in sensitivity to ethanol’s aversive effects as measured by ethanol-induced CTA. Consistent with the hypothesis that adolescent animals are less sensitive to the aversive effects of ethanol, adolescents in general displayed reduced sensitivity to ethanol-induced CTA. Within strains, adolescents were less sensitive to ethanol’s aversive effects than adults; however, between strains this was not necessarily the case. The dose required to induce a CTA response among adolescent animals differed as a function of strain. Between strains, in some cases adolescents were more sensitive to ethanol’s effects than adults (i.e, adolescent 129s displayed a CTA response at the 1.5g/kg ethanol dose, but BALB and AJ adults required 2.25g/kg, and B6 adults required 3.0g/kg to display a CTA response). Future investigations into genetic- and age-dependent differences in ethanol sensitivity should increase the number of inbred strains used to allow for gene mapping. Additional behaviors, perhaps those associated with ethanol’s rewarding effects, should also be examined.
Acknowledgments
This work was supported in part by grants from NIAAA (AA015434, AA016789 to SLB and AA018910 to EMM), the Center for Development and Behavioral Neuroscience at Binghamton University, and the Indiana Alcohol Research Center at IUPUI.
Footnotes
The authors have no conflicts of interest to declare.
References
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male Sprague dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34(12):2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Woods JE, II, Murphy JM, Lumeng L, Li T-K. Ethanol- induced conditioned taste aversion in high alcohol drinking (HAD) and low alcohol drinking (LAD) rats. Alcohol Clin Exp Res. 1999;23:104A. doi: 10.1097/01.ALC.0000071929.17974.DA. (Abstract). [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Brunetti G, Carai MAM, Lobina C, Melis S, Serra S, Vacca G, et al. Differences in ethanol-induced conditioned taste aversion in Sardinian alcohol-preferring and Sardinian alcohol-nonpreferring rats. Alcohol. 2002;26(3):167–172. doi: 10.1016/s0741-8329(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Further evidence of an inverse-genetic relationship between innate differences in alcohol preference and alcohol withdrawal magnitude in multiple selectively bred rat lines. Alcohol Clin Exp Res. 2003;27(3):377–387. doi: 10.1097/01.ALC.0000056619.98553.50. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK, Buck KJ, Metten P. Use of recombinant inbred strains for studying genetic determinants of responses to alcohol. Alcohol Alcohol Supp. 1994;2:64–71. [PubMed] [Google Scholar]
- Elkins RL, Walters PA, Orr T. Continued development and unconditioned stimulus characterization of selectively bred lines of taste aversion prone and resistant rats. Alcohol Clin Exp Res. 1992;16:928–934. doi: 10.1111/j.1530-0277.1992.tb01895.x. [DOI] [PubMed] [Google Scholar]
- Elkins RL. Separation of taste aversion prone and taste aversion resistant rats through selective breeding: implications for individual differences in conditionability and aversion-therapy alcoholism treatment. Behav Neurosci. 1986;100:121–124. doi: 10.1037//0735-7044.100.1.121. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li T-K. Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav. 1988;31:215–222. doi: 10.1016/0091-3057(88)90336-x. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Badia-Elder NE, Zink RW, McCullough DE, Portoghese PS. Contribution of the opiod system to alcohol aversion and alcohol drinking behavior. J Pharmacol Exp Ther. 1998;287(1):284–292. [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: Is free choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Belknap JK, O’Toole LA, Helms ML, Wenger CD, Crabbe JC. Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. J Neurosci. 1997;17:745–754. doi: 10.1523/JNEUROSCI.17-02-00745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–322. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Hobbs SH, Walters PA, Shealy EF, Elkins RL. Radial-maze learning by lines of taste-aversion prone and taste aversion resistant rats. Bull Psychon Soc. 1993;31:171–174. [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35(10):1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrowitz GP, Whitney G. Alcohol-induced conditioned aversion: genotype specificity in mice (Mus musculus) J Comp Physiol Psychol. 1975;89(4):340–346. doi: 10.1037/h0076803. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SLII. Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- McClearn GE, Rodgers DA. Differences in alcohol preference among inbred strains of mice. Quart J Stud Alcohol. 1959;20:691–695. [Google Scholar]
- Melón LC, Boehm SL., II Role of Genotype in the Development of Locomotor Sensitization to Alcohol in Adult and Adolescent Mice: Comparison of the DBA/2J and C57BL/6J Inbred Mouse Strains. Alcohol Clin Exp Res. 2011;35:1351–1360. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr TE, Whitford-Stoddard JL, Elkins RL. Taste aversion prone (TAP) rats and taste aversion resistant (TAR) rats differ in ethanol self administration, but not in ethanol clearance or general consumption. Alcohol. 2004;33:1–7. doi: 10.1016/j.alcohol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Orr TE, Walters PA, Elkins RL. Differences in free-choice ethanol acceptance between taste aversion prone and taste aversion resistant rats. Alcohol Clin Exp Res. 1997;21:1491–1496. [PubMed] [Google Scholar]
- Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, et al. Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol induced conditioned taste aversion. Behav Neurosci. 2005;119(4):892–910. doi: 10.1037/0735-7044.119.4.892. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME. Differences in sensitivity to the aversive effects of ethanol in low-alcohol drinking (UChA) and high-alcohol drinking (UChB) rats. Alcohol. 2001;23(3):1777–1782. doi: 10.1016/s0741-8329(01)00128-8. [DOI] [PubMed] [Google Scholar]
- Revillo DA, Arias C, Spear NE. The unconditioned stimulus pre-exposure effect in preweanling rats in taste aversion learning: role of the training context and injection cues. Dev Psychobiol. 2012 doi: 10.1002/dev.21011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced hyperglycemia and conditioned taste aversion. Life Sci. 1992;50(16):PL113–PL118. doi: 10.1016/0024-3205(92)90463-y. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12(6):535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Rissinger FO, Cunningham CL. Ethanol-induced conditioned taste aversion in BXD recombinant inbred mice. Alcohol Clin Exp Res. 1998;22(6):1234–1244. [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. DBA/2J mice develop stronger lithium chloride-induced conditioned taste and place preference aversions than C57BL/6J mice. Pharmacol Biochem Behav. 2000;67:17–24. doi: 10.1016/s0091-3057(00)00310-5. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Hernandez M, Wilmouth CE, Spear LP. Differential expression of ethanol induced hypothermia in adolescent and adult rats induced by pretest familiarization to the handling/injection procedure. Alcohol Clin Exp Res. 2007;31(4):575–581. doi: 10.1111/j.1530-0277.2007.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent and adult rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism: Clinical and Experimental Research. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Song M, Wang X-Y, Zhao M, Wang X-Y, Zhai H-F, Lu L. Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcohol Clin Exp Res. 2007;31(12):2001–2005. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16(2):83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Besheer J, Hodge CW. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology. 2008;197:361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya E, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya E, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96(2):228–235. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter O’Hagen CS, Varlinskaya EL, Spear LP. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44(6):547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Truesdale M, Bae J, Ahmad S, Wilson W, Best P, Swartzwelder H. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Munillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42(3):149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]