Abstract
Articular loading is an important factor in the joint degenerative process for individuals with anterior cruciate ligament (ACL) rupture. Evaluation of loading for a population that exhibits neuromuscular compensation for injury requires an approach which can incorporate individual muscle activation strategies in its estimation of muscle forces. The purpose of this study was to evaluate knee joint contact forces for patients with ACL deficiency using an EMG-driven modeling approach to estimate muscle forces. Thirty (30) athletes with acute, unilateral ACL rupture underwent gait analysis after resolving range of motion, effusion, pain and obvious gait impairments. Electromyography was recorded bilaterally from 14 lower extremity muscles and input to a musculoskeletal model for estimation of muscle forces and joint contact forces. Gait mechanics were consistent with previous reports for individuals with ACL-deficiency. Our major finding was that joint loading was altered in the injured limb after acute ACL injury; patients walked with decreased contact force on their injured knee compared to their uninjured knee. Both medial and lateral compartment forces were reduced without a significant change in the distribution of tibiofemoral load between compartments. This is the first study to estimate medial and lateral compartment contact forces in patients with acute ACL rupture using an approach which is sensitive to individual muscle activation patterns. Further work is needed to determine whether this early decreased loading of the injured limb is involved in the development of osteoarthritis in these patients.
INTRODUCTION
The risk for developing early knee osteoarthritis increases with anterior cruciate ligament (ACL) injury, and within 10–20 years, as many as 50% of those with injured knees will demonstrate radiographic osteoarthritis, significant pain and functional limitation.1,2 Altered joint loading is an important factor in the development of osteoarthritis among individuals with ACL rupture. Potentially important loading features include contact forces, shear forces, arthrokinematics and tissue stresses. Characterizing the loading environment of the knee after acute injury will promote a better understanding of the mechanical factors that may be involved in the initiation of osteoarthritis in these patients. Contact forces are a fundamental feature of joint loading; however they remain largely unknown for the ACL-deficient knee.
The importance of neuromuscular compensations for ACL injury to the initiation and progression of knee osteoarthritis is not well understood. Many of the estimated 200,000 individuals who sustain ACL injuries annually in the US3 experience a considerable degree of instability with daily activities (noncopers4). Their early compensation strategy consists of altered sagittal knee excursions and moments as well as elevated muscular co-activation.5–7 One or more of these components may contribute to altered knee contact forces, posing a potential insult to articular cartilage. Estimates of joint contact forces in ACL-injured individuals will further characterize the potential impact of their aberrant movement strategies on long-term joint health.
The knee adduction moment serves as an estimate of the load distribution between the medial and lateral compartments and correlates with the severity and progression of knee osteoarthritis.8 Nonetheless, the relationship between net moment and joint loading is not straightforward,9 particularly when agonist/antagonist muscle groups are co-activated, as in ACL-deficiency.5,6 Muscle forces are important contributors to total joint contact force,10 and consequently, altered muscle activity can substantially affect the loading of the articular surface. Since noninvasive measurement of muscle forces in vivo is impractical, a computational model must be used to estimate forces indirectly. Evaluation of joint loading for individuals with ACL injury—a population that exhibits neuromuscular compensation for injury— requires a modeling approach which can incorporate individual muscle activation strategies in its estimation of muscle forces. An EMG-driven model is well-suited for this purpose and has been implemented with uninjured athletes10,11 and patients with knee osteoarthritis.12
The purpose of this study was to evaluate knee joint contact forces for a group of acutely injured patients with ACL deficiency using an EMG-driven modeling approach. To control for potentially confounding effects of inflammation and conscious pain avoidance on movement patterns, patients were tested only after pain, effusion, range of motion and gait impairments were resolved. Based on previous work13 showing decreased injured limb muscle forces in patients with ACL-deficiency, we hypothesized that medial and lateral compartment contact forces for the injured knee would be lower than for the uninjured knee.
METHODS
Subjects
Patients who demonstrated characteristic, dynamic knee instability after ACL injury (noncopers14) were recruited for this study. These individuals are the largest fraction of the acute ACL-injured population (>60%).15 Further inclusion criteria were: 13 to 55 years of age, regular pre-injury participation in level I or II activities16 and complete ACL rupture within the past 7 months. Exclusion criteria were: bilateral knee involvement, presence of repairable meniscus tear, symptomatic grade III injury to other knee ligaments, full-thickness articular cartilage defect greater than 1 cm2, or presence of any other injuries which prevented completion of the screening exam.14 Knee range of motion, effusion, pain and obvious gait impairments were treated and resolved according to the protocol described by Hurd et al.17 prior to testing. This work was approved by the institutional Human Subjects Review Board, and all participants provided informed consent.
Testing
Patients were asked to walk at their self-selected, intentional speed and were required to maintain that speed (± 5%) throughout the testing session. Gait speed was monitored using two photoelectric beams which spanned the collection volume. Passive retro-reflective markers were used to define anatomical coordinate systems and track segment motion. Stance phase kinematics and ground reactions were recorded using an 8-camera video system (sampling rate 120 Hz) (VICON, Oxford Metrics Ltd., London, UK) and force platform (sampling rate 1080 Hz) (Bertec Corporation, Worthington, OH). Joint angles and moments were calculated using inverse dynamics, performed with commercial software (Visual3D, C-Motion, Germantown, MD).
Surface electromyography (EMG) was recorded using an MA-300 EMG System (sampling rate 1080 Hz) (Motion Lab Systems, Baton Rouge, LA) for a total of 14 lower extremity muscles: (bilaterally) the medial and lateral vasti, rectus femoris, medial and lateral gastrocnemeii, semitendinosus and long head of biceps femoris. Subjects performed unilateral squats and isolated maximum voluntary isometric contractions for normalization of EMG amplitude. Raw data were high-pass filtered (2nd order Butterworth, cutoff 30 Hz), rectified and subsequently low-pass filtered (2nd order Butterworth, cutoff 6 Hz) to create a linear envelope. Linear envelopes were normalized to the maximum value found during isometric, unilateral squat or walking trials. EMG for vastus intermedius was equal to be the average of the medial and lateral vasti linear envelopes. EMG for semimembranosus and short head of biceps femoris were equal to linear envelopes for semitendinosus and long head of biceps femoris, respectively.11
EMG-Driven Modeling
Muscle forces were estimated using a patient-specific EMG-driven model that is described in detail elsewhere.18 This modeling approach has been used to estimate muscle and joint contact forces during dynamic movements for healthy individuals10,11 and in patients with knee osteoarthritis.12 Joint kinematics and EMGs were used as input to estimate muscle forces and muscle moments during gait. Analysis steps included: 1) anatomical scaling, 2) EMG-driven model calibration, 3) muscle force prediction, and 4) contact force calculation.
Anatomical Scaling
Anatomical dimensions were captured for each patient during quiet standing, using the video system. These data were used to scale the pelvis, femur, tibia and foot segments of the anatomical model (SIMM 4.0.2, Musculographics, Inc. Chicago, IL). Patient-specific muscle-tendon lengths and muscle moment arms were calculated for the stance phase of each gait trial.
Model Calibration
During model calibration, muscle parameters which characterize the EMG-to-force conversion (six parameters per muscle, plus two global strength coefficents) were allowed to vary within previously-described limits.13 The six modifiable parameters were optimal fiber length, tendon slack length, electromechanical delay, nonlinear shape factor and two recursive filter coefficients. An expanded discussion of modifiable muscle parameters can be found in the original model description.18 Throughout the calibration process, the governing assumption was that the sum of the individual muscle moments (muscle forces × moment arms) obtained from EMG-driven forward methods should equal the net joint moment calculated from inverse dynamics. Muscle parameters were iteratively adjusted so that the sagittal plane moments from forward and inverse methods were in best agreement. A simulated annealing search strategy was used to find an optimal solution in which the squared difference between the two moment curves was minimized. The average coefficient of determination (R2) between the inverse dynamics moment and the calibrated model moment was 0.83. The mean relative RMSE of the inverse dynamics moment curve from the calibrated model moment curve was 10.0%.
Muscle Force Prediction
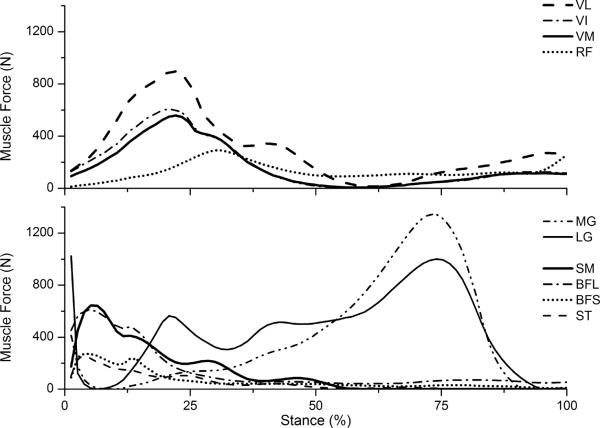
Upon calibration, the solution set of muscle parameters was fixed within the model. When provided a set of EMGs for a novel movement trial, the model predicted muscle forces and muscle moments for that movement. Each predicted knee joint moment (the sum of all predicted muscle forces times their moment arms) was compared to the measured moment from motion analysis in order to ensure that the modeled moment converged to the measured joint moment from inverse dynamics. A typical set of predicted muscle forces is shown in Figure 1.
Figure 1.
Representative set of muscle forces for the stance phase of gait. Muscles are semimembranosus (SM); semitendinosus (ST); long/short head of biceps femoris (BFL, BFS); rectus femoris (RF), vastus medialis, lateralis and intermedius (VM, VL, VI); medial/lateral gastrocnemius (MG, LG). The hamstrings and quadriceps are majorly active during loading response, with VL carrying the most force. Gastrocnemeii generate force primarily in the propulsive phase, with peak MG force being greater than that of LG.
Contact Force Calculation
A frontal plane moment balancing algorithm10 was used to compute medial and lateral compartment contact forces. (Moments in the transverse plane were neglected under the assumption that only forces acting along the long axis of the tibia or those which generate an adduction moment about the knee contribute to contact force.) Medially and laterally offset segmental coordinate systems were constructed so that the knee adduction moment could be expressed about the contact point within each compartment. Contact points were fixed within subjects but varied between subjects according to individual anthropometry (25% of tibial plateau width, measured medially and laterally from the joint center19). The knee adduction moment calculated from inverse methods was expressed about each contact point. To calculate the medial compartment contact force, a static equilibrium was assumed about the lateral contact point. Therefore, the net external knee adduction moment about the lateral contact point must be balanced by muscle forces and by the contact force acting at the medial contact point. Hence, contact force for the medial compartment was the only unknown and could be determined at every point of stance. To calculate the lateral compartment contact force, equilibrium was assumed about the medial contact point. Tibiofemoral contact force was the sum of the medial and lateral contact forces.
Variables of Interest
Contact forces for each gait trial were normalized to bodyweight (BW) and time-normalized to 100 samples. Three trials per subject were averaged in this manner for each limb (in 10 limbs, 2 trials were averaged). Statistical analyses were performed with PASSW 18.0 software (SPSS Inc, Chicago, IL). Paired t-tests were used to compare loading variables of interest between injured and uninjured limbs: peak contact forces and mean contact forces during stance (for tibiofemoral, medial and lateral compartments), mean percentage of tibiofemoral force borne by the medial compartment throughout stance, and percentage of tibiofemoral force borne by the medial compartment at peak knee flexion. Statistical significance was set at 0.05.
RESULTS
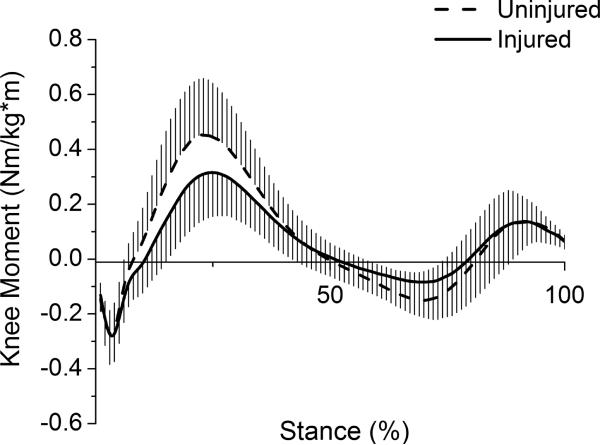
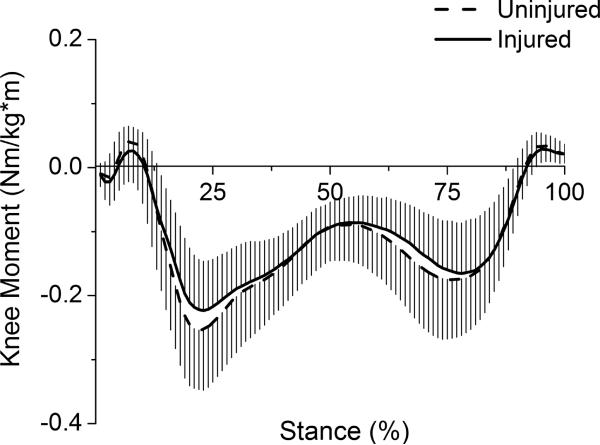
Thirty athletes with acute, unilateral ACL rupture (17 men, 13 women) participated in this study (mean ± SD; age=27.9 ± 10.5 yrs, time from injury to testing=7.9 ± 7.4 wks, BMI=27.0 ± 4.2 kg/m2, gait speed=1.53 ± 0.11 m/s). Sagittal and frontal plane moments for the stance phase of gait were normalized to body mass and height (Figs 2–3). Peak external knee flexion moment was significantly lower for the injured limb (I=0.328 ± 0.156 Nm/kg*m, U=0.464 ± 0.197 Nm/kg*m; t(29)=−4.02, p<.001). Peak external knee adduction moment was not significantly different between limbs (I=−0.237 ± 0.076 Nm/kg*m, U=−0.267 ± 0.092 Nm/kg*m; t(29)=1.92, p=.064).
Figure 2.
Sagittal plane knee moments for the stance phase of gait. Positive values represent external knee flexion moments. Whiskers are standard deviations.
Figure 3.
Frontal plane knee moments for the stance phase of gait. Negative values represent external knee adduction moments. Whiskers are standard deviations.
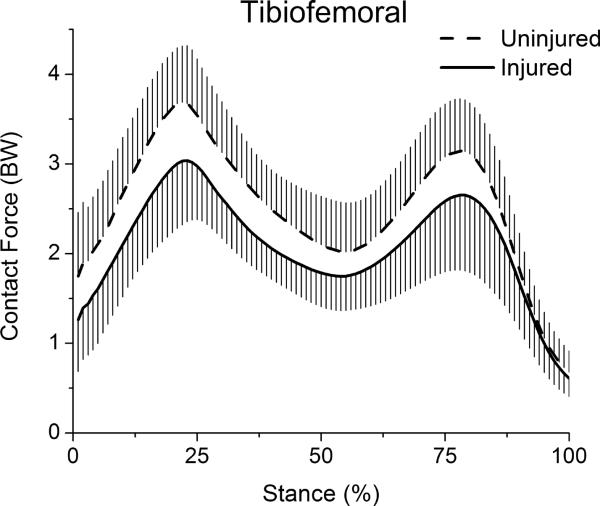
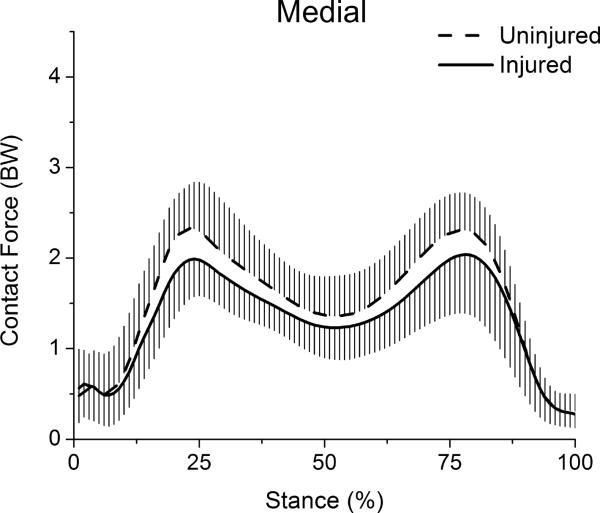
Tibiofemoral contact force for the stance phase of gait was biphasic in shape, with the largest peak occurring near the end of weight acceptance phase and a second, lesser peak occurring in late stance (>Table, Fig 4). Both peak tibiofemoral force (t(29)=−5.40, p<.001) and mean tibiofemoral force during stance (t(29)=−6.54, p<.001) were significantly lower for the injured limb.
Table.
Peak contact forces, the point at which peak forces occurred during stance phase and mean contact forces throughout stance phase. Values represent means ± standard deviations. Although the tibiofemoral contact force was the sum of the medial and lateral contact forces, since the medial and lateral contact forces each reached a peak at different times, the sum of their peaks does not equal the peak tibiofemoral contact force.
| Limb | Peak Force (BW) | Peak Force (Newtons) | Peak Occurrence (% Stance) | Mean Force (BW) | |
|---|---|---|---|---|---|
| Tibiofemoral | Inj. | 3.11 ± 0.69 | 2488 ± 603 | 23.5 ± 3.1 | 2.10 ± 0.42 |
| Un. | 3.81 ± 0.69 | 3051 ± 706 | 22.2 ± 3.5 | 2.51 ± 0.37 | |
|
| |||||
| Medial | Inj. | 2.07 ± 0.40 | 1648 ± 357 | 24.7 ± 4.0 | 1.35 ± 0.32 |
| Un. | 2.42 ± 0.55 | 1929 ± 488 | 23.3 ± 2.5 | 1.53 ± 0.30 | |
|
| |||||
| Lateral | Inj. | 1.58 ± 0.53 | 1250 ± 428 | 11.5 ± 3.6 | 0.73 ± 0.30 |
| Un. | 2.11 ± 0.75 | 1668 ± 569 | 11.3 ± 5.1 | 0.97 ± 0.32 | |
Figure 4.
Tibiofemoral contact forces for the stance phase of gait. Whiskers are standard deviations.
Medial compartment contact force was also biphasic in shape. Peak medial force occurred early in stance and was significantly lower for the injured limb (t(29)=−3.20, p=.003) (Table, Fig 5). Mean medial force during stance was likewise significantly lower for the injured limb (t(29)=−2.48, p=.019).
Figure 5.
Medial compartment contact forces for the stance phase of gait. Whiskers are standard deviations.
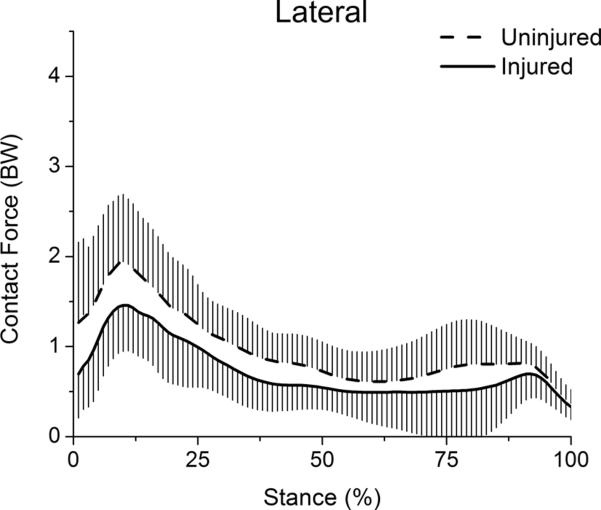
The lateral compartment exhibited only one consistent loading peak, which occurred early in stance. Peak lateral force was significantly lower for the injured limb (t(29)=−4.38, p<.001) (Table, Fig 6). Mean lateral force during stance was likewise significantly lower for the injured limb (t(29)=−3.72, p=.001).
Figure 6.
Lateral compartment contact forces for the stance phase of gait. Whiskers are standard deviations.
The percentage of force borne by the medial compartment at peak knee flexion angle was 67.6 ± 10.5% for the injured knee and 62.2 ± 18.5% for the uninjured knee; this difference was not significant (t(29)=1.59, p=.123). The mean percentage of tibiofemoral force borne by the medial compartment during stance phase was 63.1 ± 9.1% for the injured limb and 59.8 ± 9.1% for the uninjured limb; this difference was also not significant (t(29)=1.48, p=.150).
DISCUSSION
The purpose of this study was to evaluate articular loading for a group of acutely injured patients with ACL deficiency using an EMG-driven modeling approach. Our major finding was that joint loading was altered in the injured limb after acute ACL injury; patients walked with decreased force on their injured knee compared to their uninjured knee. Both medial and lateral compartment forces were reduced without a significant change in the distribution of tibiofemoral load between compartments. This is the first study to estimate medial and lateral compartment contact forces in patients with acute ACL rupture using an approach which is sensitive to individual muscle activation patterns.
The significantly lower peak external knee flexion moment for the injured limb among patients in this study is consistent with studies reporting gait biomechanics for individuals with ACL-deficiency,5,7,20 as is the lack of significant difference between limbs for peak knee adduction moment.21 The knee adduction moment provides a useful estimate of the load distribution between the medial and lateral compartments, and it is worthwhile to note that the present model-derived load-sharing results agree with the conclusion that would be reached by interpreting the adduction moment alone. There was no significant difference between limbs in either the distribution of forces between compartments or peak adduction moment. An apparent contradiction arises with our finding of lower injured limb medial compartment force without a significant decrease in the knee adduction moment. However, medial compartment force is not only related to the knee adduction moment; it is also influenced by the knee flexion moment.9 In this study, the decreased external knee flexion moment corresponded to the decreased tibiofemoral contact force for the injured knee. The reduction in medial and lateral force occurred without a change in the load distribution between compartments. Tibiofemoral contact force in the present study was higher than values reported by Alkjaer et al.21 for both control and injured limbs. However, the modeling approach used by the authors21 did not allow for muscle co-activation and consequently greater contact forces derived using an EMG-driven approach may be expected.
The shapes of the contact force patterns for medial and lateral compartments in this study were qualitatively similar to internal contact force measurements for an elderly individual with an instrumented total knee replacement.22 Medial loading curves had two peaks occurring near 25% and 80% of stance, respectively, with approximately 60% of the tibiofemoral load on the medial compartment during stance. Estimates of peak contact force for both limbs were higher in the present study than those obtained from the instrumented prosthesis.22 It is difficult to determine the effects of our subjects' youth, activity level (muscle mass) and faster self-selected walking speed on joint loading. Additionally, loading of the natural knee may be different than the loading of a prosthesis. Given these differences between athletes in this study and the individual with an instrumented total knee replacement, findings of higher joint contact forces for the patients in the present study are not unreasonable.
The relative decreased loading of the injured knee which was observed in this study prompts the question of whether the prevalence of OA following ACL injury may be related to the acute unloading of the injured knee. Cartilage, like other musculoskeletal tissues exhibits a unique structure-function relationship and appears to be conditioned to the loads it regularly experiences.23 In animal models, atrophic articular cartilage changes occur in response to repetitive over-loading,24 immobilization25 as well as limb unloading.23 Atrophic adaptations to decreased joint loads signify cartilage de-conditioning, and the reversibility of them with the return of normal loading is uncertain.26
Decreased joint loading in the ACL-deficient knee has been suggested previously. Unloading appears two weeks following ACL-transection in the cat hindlimb27 and precedes the onset of radiographic OA. Other studies report decreased ground reaction forces28–31 in animals after ACL-transection.28–31 Degenerative changes are also observed early after transection, suggesting that in animals, the initiation of OA after ACL disruption is associated with early decreased (rather than excessive) joint loads.
It is not known whether the decreased loading observed in these patients after injury may be significant (in duration or magnitude) enough to cause cartilage deconditioning. However, if decreased loads were persistent and articular cartilage became deconditioned, the potential for mechanical insult with the resumption of normal or excessive loading would increase. Greater peak knee adduction moments have been reported in ACL-reconstructed knees (ranging approximately 1–10 years post-surgery) compared to matched, uninjured knees.32 These higher moments suggest that injured limb loading may increase in the years following ACL injury and reconstruction. Investigation of the longitudinal loading profile of the ACL-injured knee is needed to clarify the impact of altered loading on the development of OA in these patients.
An important strength of this study was the use of a musculoskeletal model which is sensitive to neuromuscular adaptations to ACL injury. We also strove to capture gait strategies in our patients in the acute phase after injury while minimizing potentially confounding impact of trauma-related joint afferents on their movement strategy.
A potential limitation of this study was the exclusion of ligaments from our model. Consequently, we expect that joint contact forces reported here may be under-estimates of the loading which occurs in vivo. The fixation of medial and lateral tibiofemoral joint centers as discrete points throughout stance is a simplification incorporated into our model. The average excursion of these points in the mediolateral direction over 0–30 degrees of knee flexion is small (1–2 mm).33 However, in the ACL-deficient knee, medial and lateral contact points may be translated 3 mm laterally when compared to contact points in the intact knee over the range of motion used during walking,33 and therefore we examined the sensitivity of peak contact forces to the location of contact points. A lateral translation of both contact points by 5% of tibial plateau width (approximately 5 mm) resulted in a 3.7% increase in peak tibiofemoral force, a 4.9% increase in peak medial force and a 2.6% increase in peak lateral force. The mean interlimb differences in peak force for this study (tibiofemoral 20%; medial 15%; lateral 27%) were greater than the estimated potential errors due to contact point translation in the ACL-deficient knee.
It is also possible that loading features other than joint contact force may be more pertinent mechanical factors in the development of OA after ACL injury. Nonetheless, joint contact force is a fundamental feature of joint loading and provides an important basis for characterizing the mechanical environment in the ACL-injured knee.
Patients with ACL-deficiency walked with decreased loads on their injured knee in the acute period following injury. This finding prompts the question of whether early decreased forces in the injured knee may be related to the development of OA after ACL injury. Future work should investigate whether altered contact forces normalize with pre-operative rehabilitation or ligament reconstruction, and whether the duration or magnitude of decreased contact force plays a role in the development of OA among individuals after ACL injury.
ACKNOWLEDGEMENTS
We would like to acknowledge Drs. Daniel Ramsey, Wendy Hurd, Erin Hartigan and Stephanie Di Stasi for their assistance with data collection and Kristen Stump for her assistance with data processing. This work was supported by the National Institute of Health (RR016458, AR46386 & AR48212, S10RR022396). The authors have no potential conflicts of interest to declare.
REFERENCES
- 1.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries - Osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 2.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 3.Miyasaka K, Daniel D, Stone M, Hirschman P. The incidence of knee ligament injuries in the general population. Am J Knee Surg. 1991;4:43–48. [Google Scholar]
- 4.Chmielewski TL, Rudolph KS, Fitzgerald GK, Axe MJ, Snyder-Mackler L. Biomechanical evidence supporting a differential response to acute ACL injury. Clin Biomech. 2001;16:586–591. doi: 10.1016/s0268-0033(01)00050-x. [DOI] [PubMed] [Google Scholar]
- 5.Hurd WJ, Snyder-Mackler L. Knee instability after acute ACL rupture affects movement patterns during the mid-stance phase of gait. J Orthop Res. 2007;25:1369–1377. doi: 10.1002/jor.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 2001;9:62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph KS, Eastlack ME, Axe MJ, Snyder-Mackler L. 1998 Basmajian Student Award Paper: Movement patterns after anterior cruciate ligament injury: a comparison of patients who compensate well for the injury and those who require operative stabilization. J Electromyogr Kinesiol. 1998;8:349–362. doi: 10.1016/s1050-6411(97)00042-4. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20:101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- 9.Walter JP, D'Lima DD, Colwell CW, Fregly BJ. Decreased Knee Adduction Moment Does Not Guarantee Decreased Medial Contact Force during Gait. J Orthop Res. 2010;28:1348–1354. doi: 10.1002/jor.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009;42:2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd DG, Besier TF. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech. 2003;36:765–776. doi: 10.1016/s0021-9290(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 12.Kumar D, Rudolph K, Manal K. EMG-driven modelin approach to muscle force and joint load estimations: Case study in knee osteoarthritis. J Orthop Res. 2011 doi: 10.1002/jor.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardinier ES, Manal K, Buchanan TS, Snyder-Mackler L. Gait and neuromuscular asymmetries after acute anterior cruciate ligament rupture. Med Sci Sports Exerc. 2012 doi: 10.1249/MSS.0b013e31824d2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald GK, Axe MJ, Snyder-Mackler L. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2000;8:76–82. doi: 10.1007/s001670050190. [DOI] [PubMed] [Google Scholar]
- 15.Moksnes H, Snyder-Mackler L, Risberg MA. Individuals with an anterior cruciate ligament-deficient knee classified as noncopers may be candidates for nonsurgical rehabilitation. J Orthop Sports Phys Ther. 2008;38:586–595. doi: 10.2519/jospt.2008.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1:226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 17.Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: Part 1, outcomes. Am J Sports Med. 2008;36:40–47. doi: 10.1177/0363546507308190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: Estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004;20:367–395. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schipplein OD, Andriacchi TP. Interaction between Active and Passive Knee Stabilizers during Levelwalking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 20.Hart JM, Ko JWK, Konold T, Pietrosimione B. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: A systematic review. Clin Biomech. 2010;25:277–283. doi: 10.1016/j.clinbiomech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Alkjaer T, Henriksen M, Simonsen EB. Different knee joint loading patterns in ACL deficient copers and non-copers during walking. Knee Surgery Sports Traumatology Arthroscopy. 2011;19:615–621. doi: 10.1007/s00167-010-1302-2. [DOI] [PubMed] [Google Scholar]
- 22.Zhao D, Banks SA, D'Lima DD, Colwell CW, Jr., Fregly BJ. In vivo medial and lateral tibial loads during dynamic and high flexion activities. J Orthop Res. 2007;25:593–602. doi: 10.1002/jor.20362. [DOI] [PubMed] [Google Scholar]
- 23.Arokoski JPA, Jurvelin JS, Vaatainen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10:186–198. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- 24.Radin EL, Burr DB, Caterson B, Fyhrie D, Brown TD, Boyd RD. Mechanical determinants of osteoarthrosis. Semin Arthritis Rheum. 1991;21:12–21. doi: 10.1016/0049-0172(91)90036-y. [DOI] [PubMed] [Google Scholar]
- 25.Vanwanseele B, Lucchinetti E, Stussi E. The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthritis Cartilage. 2002;10:408–419. doi: 10.1053/joca.2002.0529. [DOI] [PubMed] [Google Scholar]
- 26.Haapala J, Arokoski JPA, Hyttinen MM, Lammi M, Tammi M, Kovanen V, Helminen HJ, Kiviranta I. Remobilization does not fully restore immobilization induced articular cartilage atrophy. Clin Orthop Relat Res. 1999:218–229. [PubMed] [Google Scholar]
- 27.Hasler EM, Herzog W, Leonard TR, Stano A, Nguyen H. In vivo knee joint loading and kinematics before and after ACL transection in an animal model. J Biomech. 1998;31:253–262. doi: 10.1016/s0021-9290(97)00119-x. [DOI] [PubMed] [Google Scholar]
- 28.Brandt KD, Braunstein EM, Visco DM, Oconnor B, Heck D, Albrecht M. Anterior (cranial) cruciate ligament transection in the dog - A bona-fide model of osteoarthritis, not merely of cartilage injury and repair. J Rheumatol. 1991;18:436–446. [PubMed] [Google Scholar]
- 29.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic Changes in Canine Articular-Cartilage, Subchondral Bone, and Synovium 54 Months after Transection of the Anterior Cruciate Ligament. Arthritis Rheum. 1991;34:1560–1570. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- 30.Suter E, Herzog W, Leonard TR, Nguyen H. One-year changes in hind limb kinematics, ground reaction forces and knee stability in an experimental model of osteoarthritis. J Biomech. 1998;31:511–517. doi: 10.1016/s0021-9290(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 31.Vilensky JA, O'Connor BL, Brandt KD, Dunn EA, Rogers PI, DeLong CA. Serial kinematic analysis of the unstable knee after transection of the anterior cruciate ligament: temporal and angular changes in a canine model of osteoarthritis. J Orthop Res. 1994;12:229–237. doi: 10.1002/jor.1100120212. [DOI] [PubMed] [Google Scholar]
- 32.Ferber R, Osternig LR, Woollacott MH, Wasielewski NJ, Lee JH. Bilateral accommodations to anterior cruciate ligament deficiency and surgery. Clin Biomech. 2004;19:136–144. doi: 10.1016/j.clinbiomech.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Guoan L, Moses J, Papannagari R, Pathare N, DeFrate L, Gill T. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. Journal of Bone and Joint Surgery. 2006;88-A:1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]