Abstract
Rationale
Patients with schizophrenia exhibit high comorbidity for substance abuse, but the biological underpinnings of this dual diagnosis condition are still unclear. Previous studies have shown that rats with a neonatal ventral hippocampal lesion (NVHL), a widely used developmental animal model of schizophrenia, exhibit increased cocaine and methamphetamine self-administration, and cocaine-induced reinstatement.
Objective
Here, we assessed whether a NVHL would also potentiate cue-induced reinstatement of cocaine seeking and the time-dependent increases in cue-induced cocaine seeking after withdrawal (incubation of cocaine craving) in adult rats.
Methods
Rats were trained to self-administer cocaine (3 or 6 h/day, 0.75 mg/kg/infusion paired with a tone-light cue) for 10 days, followed by extinction training (3 h/day), and cue-induced reinstatement of cocaine seeking. Other rats were tested for incubation of cocaine craving, assessed in extinction tests 1 and 30 days after the last self-administration session.
Results
Although there was no significant difference in cocaine intake between NVHL and sham controls, NVHL rats took significantly longer to reach an a priori set extinction criterion and exhibited enhanced cue-induced reinstatement. However, while cue-induced cocaine seeking was higher after 30 days than after 1 day of withdrawal (incubation of cocaine craving), the NVHL had no effect on this incubation.
Conclusion
These data confirm previous reports on enhanced resistance to extinction after NVHL and demonstrate that NVHL rats exhibit enhanced cue-induced reinstatement of cocaine seeking after extinction, a measure of drug relapse.
Keywords: addiction, schizophrenia, dual diagnosis, relapse, animal model, cocaine self-administration
INTRODUCTION
Patients with schizophrenia are often addicted to abused drugs, with close to half of the patient population having such dual diagnosis (Buckley et al. 2009). Dual-diagnosis patients have a worse course of psychiatric illness than non-addicted schizophrenia patients, including more frequent relapses and re-hospitalizations, as well as poor treatment outcomes. While it has been proposed that drug use may represent an attempt to self-medicate, schizophrenia and drug addiction may share a common pathophysiology (Kapur 2003). One of the most comprehensively studied developmental animal models of schizophrenia is the neonatal ventral hippocampal lesion (NVHL) (Tseng et al. 2009). Adult rats with a NVHL exhibit behavioral, anatomical, and neurochemical anomalies resembling phenomena observed in schizophrenia, which emerge during late adolescence (O’Donnell 2011), reproducing the developmental trajectory of schizophrenia. The behavioral and electrophysiological anomalies in adult rats with an NVHL are primarily due to altered developmental trajectory in prefrontal cortical circuits as a consequence of the lesion (O’Donnell 2011).
Previous studies demonstrate that NVHL rats show increased cocaine self-administration (Chambers and Self 2002), ethanol intake (Berg et al. 2011), and higher breakpoint on a progressive-ratio reinforcement schedule for methamphetamine (Brady et al. 2008), a measure of the drug rewarding effect (Richardson and Roberts 1996). In addition, a study using the extinction-reinstatement model (de Wit and Stewart 1981; Self and Nestler 1998; Shaham et al. 2003) showed increased resistance to extinction and enhanced cocaine-induced reinstatement of drug seeking in NVHL rats (Chambers and Self 2002).
A critical problem in the treatment of drug addiction is the high rate of relapse to drug intake during abstinence; this relapse is often precipitated by exposure to drug-associated cues that provoke craving (O’Brien et al. 1992). Here, we used two established rodent behavioral procedures to study cue-induced reinstatement of drug seeking after extinction (See 2002; 2005) and incubation of drug craving (time-dependent increases in cue-induced drug seeking in extinction tests after withdrawal) (Grimm et al. 2001; Neisewander et al. 2000; Pickens et al. 2011) to determine whether the NVHL increases vulnerability to cue-induced relapse to drug seeking.
MATERIAL AND METHODS
Subjects
Timed pregnant Long-Evans rats were obtained at gestational day 13–15 from Charles River (Wilmington, MA) and were individually housed with free access to food and water in a temperature- and humidity-controlled environment with a 12-h:12-h light dark cycle (lights on at 7:00 am). Pups were left undisturbed until postnatal day (PD) 5–6 when female pups were culled, and at PD 7–9 healthy male offspring were randomly separated and received either NVHL or sham surgery. At PD 22–23, pups were weaned and housed in groups of 2–3, counterbalanced across lesion status. At this time, they were group-housed in a room with reversed light cycle (lights off at 8:00 am). After reaching adulthood (PD 56–90), rats were implanted with intravenous catheters and housed in individual cages. The rats had free access to food and water at all time. All experimental procedures were approved by University of Maryland School of Medicine Institutional Animal Care and Use Committee and were conducted according to the United States Public Health Service Guide for Care and Use of Laboratory
Animals
Neonatal ventral hippocampal lesion
Between PD7 and PD9, male pups (15–20 g) received either an excitotoxic lesion of the ventral hippocampus (NVHL) or sham procedure as previously described (Chambers and Lipska 2011). In short, pups were anesthetized with hypothermia and secured to a Styrofoam platform attached to a stereotaxic frame (David Kopf Instruments, Tujunga, CA). NVHL rats received a bilateral infusion of 0.3 μl per side of ibotenic acid (10 μg/μl in artificial cerebrospinal fluid [CSF]; ToCris, Minneapolis, MN) into the ventral hippocampus (3 mm rostral to bregma; 3.5 mm lateral to midline; 5 mm from surface) at a rate of 0.15 μl/min. Sham surgeries were done in exactly the same fashion but without any liquid infusion. After the procedure, wounds were clipped and when pups activity level had returned to normal they were returned to their dams and remained undisturbed, except for husbandry, until the wound clips were removed and animals weaned at PD22–23.
Intravenous surgery
Intravenous surgeries on NVHL and sham rats (weighing 350–400 g) were performed as previously described (REF) with the exception that the chronic indwelling jugular catheters, which were constructed in house using back-mount cannula (Plastics One, Roanoke, VA), silastic tubing (Dow Corning, Midland, MI), and cranioplastic cement (Lang, Wheeling, IL). One end of the catheter was inserted into the right jugular vein with the other end of the tubing run subcutaneously and exited the back between the scapulae. Wounds were sutured closed, and rats were treated with topical lidocaine/prilocaine (6% EMLA; Medic Roller, UK), antibiotic wound cream (Johnson & Johnson, Skillman, NJ) and 0.1 ml (0.1 mg/ml) of the opiate analgesic buprenorphine (Sigma Aldrich, Allentown, PA). At the completion of surgery and post-operative care, the rats were housed individually and given 7–11 days to recover prior to behavioral testing, during which their catheters were flushed every other day with 0.1–0.2 ml of gentamicin/saline solution (5 mg/ml in saline; Hospira, Lake Forest, IL).
Behavioral Procedures
Apparatus
Rats were tested in self-administration chambers (Med Associates, St. Albans, VT) housed within sound attenuating enclosures. Each chamber was equipped with two levers located 9 cm above the floor, a houselight, a tone cue, and a light cue. Presses on one (active, retractable) lever resulted in the activation of the infusion pump, the illumination of a light cue, and activation of a tone. Presses on the other (inactive, stationary) had no programmed outcome; however, each press on this lever was recorded.
Cue-induced reinstatement
The cue-induced reinstatement procedure is based on previous reports (Meil and See 1996; 1997). Each session started with the back-mount catheters being connected to the infusion pump via plastic tubing. The plastic tubing was encased in metal springs with brass collars that securely attached to the back-mount. Cocaine-HCl (National Institute of Drug Abuse, Baltimore, MD) was dissolved in sterile saline and self-administered at a dose of 0.75 mg/kg/infusion (0.1 ml/infusion over 3.5 sec). Active lever responses activated the infusion pump and led to the delivery of a 5-sec tone-light compound cue. Each infusion was followed by a 20-sec timeout period during which presses were recorded but did not have programmed consequences. A 100 infusion limit was used throughout testing to prevent overdose. The rats underwent cocaine self-administration training 3 h/day for 10 days with 1 off day in the middle. Before and after each self-administration session, the rats’ catheters were flushed with0.1 ml of Heparin Lock-Flush solution (10 USP/ml; Tyco Healthcare, Mansfield, MA). After the end of each self-administration session, rats were returned to the animal facility.
During extinction training, the rats were placed into the self-administration chambers and attached to the infusion pump in the same manner as previously described. During this phase of testing, neither active nor inactive lever press produced any consequence (i.e., no infusion, light, or tone cues). The rats continued extinction training, 3 h/session for a minimum of 10 days. After the rats reached extinction criterion (less than 15 active lever presses per 3 h for 2 consecutive days), they were given reinstatement testing. The rats were returned to the self-administration chamber and attached to the inactivated infusion pump. During the 3-hour reinstatement testing, an active-lever press resulted in the activation of the tone and light cues, but no drug delivery.
Incubation of cocaine craving
The ‘incubation of cocaine craving’ procedure is based on previous publications (Grimm et al. 2001; Lu et al. 2004; Lu et al. 2007; Lu et al. 2009). Briefly, separate groups of rats were trained in the same manner described above, except that the duration of the daily self-administration training session was 6 h/day (Grimm et al. 2001). The number of infusions per h was limited to 20 to prevent overdose. The day after the last self-administration session (day 1), the rats were subjected to an extinction test where they were placed into the self-administration chambers and attached to the infusion pump as described above. The extinction test consisted of a 1-hour session, to minimize carry-over effect of extinction responding from day 1 to day 30. At this time active-lever press resulted in the activation of the tone and light cues, but no drug delivery. The number of active and inactive lever presses was recorded. Subsequently, the rats were subjected to another extinction test (within-subject assessment) one month after last self-administration session (withdrawal day 30).
Histology
After completion of behavioral testing, the rats were euthanized and the brains removed and postfixed in 4% paraformaldehyde for at least 24 h. Brains were then transferred to 30% sucrose in 0.1 M phosphate buffer for cryoprotection. The dorsal and ventral hippocampus were sectioned (40 μm) using a freezing microtome, and sections were mounted on glass slides and Nissl stained. The hippocampus was examined microscopically for evidence of bilateral damage, which typically included cell loss, thinning, gliosis, cellular disorganization, and enlarged ventricles (Chambers and Lipska 2011).
Statistical analysis
Data were analyzed using Statistica 9.0 (Statsoft, Tulsa, OK, USA). Comparisons were made with analysis of variance, followed by Tukey post hoc-tests where appropriate.
RESULTS
Lesions
Nissl-stained hippocampal sections from NVHL rats showed some extent of cell loss, enlarged ventricles and cellular disorganization. Rats with any evidence of bilateral ventral hippocampal damage were included in the lesion group. Rats with no indication of lesion, unilateral lesion, or extension of damage to adjacent structures were excluded. A representative photomicrograph from an NVHL rat with significant cell loss and ventricle enlargement is shown in Figure 1A. Sham-operated rats showed no evidence of damage (Figure 1B). The final sample sizes for each experiment was as follows: reinstatement, n=33 (15 sham, 18 NVHL); incubation of cocaine craving, n=15 (7 sham; 8 NVHL).
Figure 1. Representative hippocampus from a control and NVHL rat.
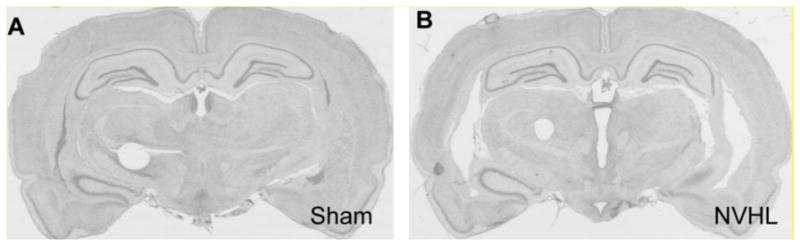
Photomicrographs from Nissl-stained brains from sham-treated (A) and NVHL (B) rats.
Cue-induced reinstatement of cocaine seeking after extinction
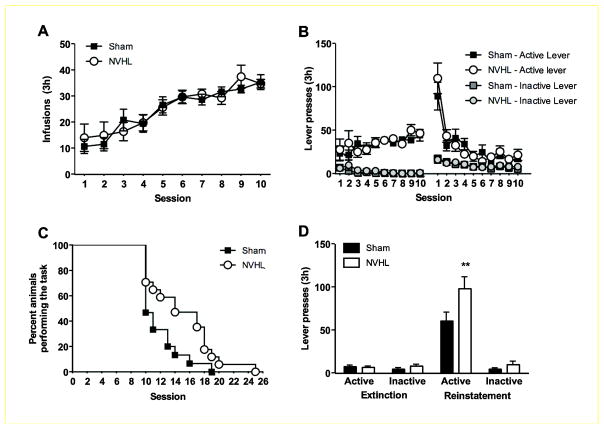
Rats from both groups acquired cocaine self-administration at a similar rate and showed similar levels of lever pressing. Statistical analyses of number of infusions and active-lever presses demonstrated a significant effect of session (F9.261=20.7 and F9,261=2.6, P<0.01) but no effect of lesion or lesion x session interaction (Figure 2A). During the daily extinction training without the cocaine-associated cues, a high initial level of lever pressing was followed by a daily decline in responding on the active lever (Figure 2B). Although there was a significant effect of session (F9,261=33.1, P<0.001), there was no significant effect of lesion or lesion x session interaction. The number of inactive lever presses remained low throughout cocaine self-administration and extinction training. While there was no significant difference in active or inactive lever presses during the first 10 days of extinction training, a survival analysis revealed that NVHL rats required significantly more days to reach the extinction criterion (χ2=5.1, df=1, P<0.05; Figure 2C). Thus, although NVHL rats acquired and maintained cocaine self-administration at a rate similar to sham-operated rats, they demonstrated a modest increase in resistance to extinction, a finding in agreement with a previous report (Chambers and Self 2002).
Figure 2. Enhanced cue-induced reinstatement of cocaine seeking after extinction in NVHL rats.
(A) Histogram depicting number of infusions in 3-hour sessions in sham (filled squares) and NVHL (open circles) rats. (B) Plot of numbers of active and inactive lever presses during acquisition and the initial part of extinction training for sham and NVHL rats. (C) Histogram showing the rate at which rats in both groups achieve the extinction criterion. The data are illustrated as percent of rats that have not achieved the criterion in consecutive sessions. (D) Mean ± SEM number of presses on the active and inactive levers during extinction and reinstatement of cocaine seeking in NVHL and sham rats. **P<0.01 compared to sham, n=15–16 per group.
Once rats reached the extinction criterion, they were tested for cue-induced reinstatement of cocaine seeking. Reinstatement was analyzed with lesion status as the between-subjects factor, and two hierarchical within-subjects factors: session (extinction and reinstatement) and lever (active and inactive) within the respective session. There were significant effects of lesion status (F1,29=4.9, P<0.05), session (F1,29=79.3, P<0.001), lever (F1,29=61.7, P<0.001) and lesion x session x lever interaction (F1,29=5.6, P<0.05). Post-hoc analysis revealed that the NVHL rats had a significantly higher number of active lever presses during the reinstatement session than the sham rats (Figure 2D). There was no significant difference in inactive lever presses during extinction or reinstatement. These data indicate that NVHL rats exhibit higher cue-induced reinstatement of cocaine seeking than sham rats.
Incubation of cocaine craving
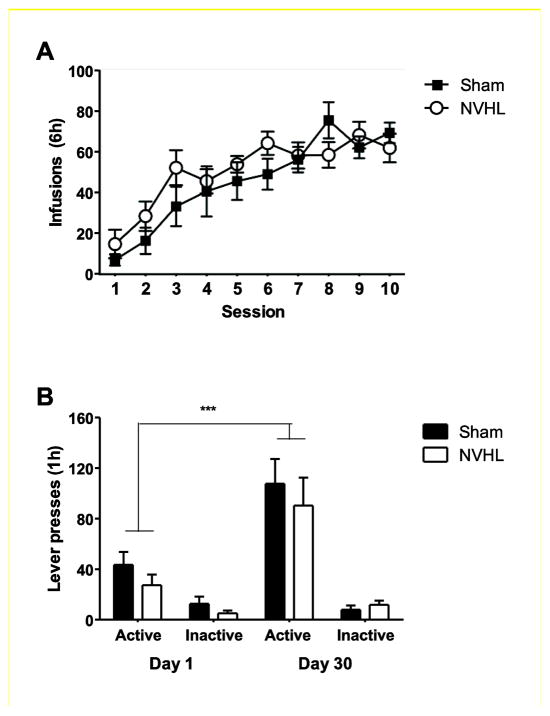
Similar to the 3-hour cocaine self-administration training condition, there was no significant difference between the NVHL rats and the sham rats trained to self-administer cocaine for 6 h per day. Both groups showed stable responding to cocaine reinforcement (overall mean for SHAM: 49.0 ± 2.3 infusions/session, NVHL: 50.7 ± 1.2). The analysis of ‘incubation of cocaine craving’ was performed with lesion as a between-subjects factor and two hierarchical within-subjects factors, test day (day 1 and day 30) and lever (active and inactive) within the respective session. There was a significant effect of test day (F1,13=11.6, P<0.01), lever (F1,13=52.1, P<0.001) and test day x lever interaction effect (F1,13=14.5, P<0.01). Post-hoc analysis demonstrated a significant increase in active lever presses on test day 30 (Figure 3A). However, there were no significant effects of lesion or interactions with lesion status, suggesting that both NVHL and sham rats exhibited similar time-dependent increases in cue-induced cocaine seeking in the extinction tests, the operational measure of incubation of cocaine craving.
Figure 3. Time-dependent changes in cue-induced cocaine seeking after withdrawal (incubation of cocaine craving) in NVHL and sham rats.
(A) Number of infusions in both NVHL and sham rats during the cocaine self-administration phase. (B). Mean ± SEM number of presses on the active and inactive levers one day after the last self-administration session (Day 1) and 30 days after the last self-administration session (Day 30). ***P<0.001 compared to Day 1, n=7–8 per group.
DISCUSSION
The main finding in this report is that NVHL rats demonstrated enhanced cue-induced reinstatement of cocaine seeking, but no enhancement of time-dependent cue-induced cocaine seeking after withdrawal (incubation of cocaine craving). Under our experimental conditions, NHVL rats also showed a modest increase in resistance to extinction, confirming results from a previous report (Chambers and Self 2002). Although rats were transferred from a group-housing condition to a single-housing condition after catheter implantation, this is not likely to play a role in differences between NVHL and sham rats, as rats from both groups were housed under the same experimental conditions. Finally, initiation and maintenance of cocaine self-administration did not differ between NVHL and sham-operated rats; these data are different from those of Chambers and Self (2002), who demonstrated increased cocaine self-administration in NVHL rats.
Our study extends previous work on in the effect of NHVL on drug self-administration, reinstatement, and locomotor sensitization. Earlier studies showed increased cocaine and alcohol self-administration (Berg et al. 2011; Chambers and Self 2002), cocaine-induced reinstatement (Chambers and Self 2002), as well as enhanced locomotor sensitization to cocaine, alcohol, and nicotine (Berg and Chambers 2008; Chambers and Taylor 2004; Conroy et al. 2007) in NVHL rats. In contrast to a previous report (Chambers and Self 2002), we have not observed an effect of NHVL on initiation and maintenance of cocaine self-administration. This difference is likely due to the different doses used in these studies. Lower cocaine doses that were used by Chambers and Self (Chambers and Self 2002) are known be more sensitive to environmental and pharmacological manipulations (Lu et al. 2003; Marinelli and Piazza 2002; Piazza and Le Moal 1996). In contrast, effects of stressors and developmental manipulations on psychostimulant self-administration are rarely observed when high unit drug dose and low response requirement fixed-ratio reinforcement schedules are used (Hall 1998; Lu et al. 2003). In this regard, we previously reported that the NHVL had no effect on methamphetamine self-administration under an FR-1 reinforcement schedule, but increased drug-reinforced responding under a progressive ratio reinforcement schedule (Brady et al. 2008). Thus, it is possible that psychostimulant self-administration under a fixed- ratio reinforcement schedule is not controlled by brain circuits affected by NVHL. In contrast, a more demanding cocaine self-administration reinforcement schedule such as a progressive ratio schedule may be controlled by circuits affected by this developmental manipulation.
A question that arises from our study is how to account for the different effect of NHVL on cue-induced reinstatement versus incubation of cocaine craving, as assessed in extinction tests in the presence of the cocaine-associated cues. This pattern of results is in agreement with previous studies suggesting that different brain circuits underlie cue-induced reinstatement after extinction versus initial extinction responding in the presence of cues. For example, cue-induced reinstatement of cocaine seeking after extinction training is blocked by reversible pharmacological inactivation of the dorsal but not ventral medial PFC (McLaughlin and See 2003; Peters et al. 2008). In contrast, when rats were subjected to a period of forced abstinence in their home cage, inactivation of the ventral but not dorsal medial PFC decreased initial extinction responding in the presence of the cocaine-associated cues (Fuchs et al. 2006; Koya et al. 2009). An alternative interpretation of the data is that the lesion had no effect on spontaneous recovery; however, this is not likely because this term refers to resumption of responding after completing extinction training (Bouton and Swartzentruber 1991), which was not the case in our study that only included 1-h extinction experience on withdrawal day 1 prior to the day 30 extinction test. Instead we think these data suggest that prelimbic (dorsal) PFC activity is critical for cue-induced reinstatement of drug seeking while the infralimbic (ventral) PFC activity is critical for incubation of drug craving (Koya et al. 2009). Additional correlative evidence for this idea is that cue-induced reinstatement after extinction was accompanied by more pronounced increase in Arc (an immediate early gene and a marker of neuronal activity) expression in the dorsal (cingulate and prelimbic) than in the ventral (infralimbic) medial PFC (Zavala et al. 2008). In contrast, the expression of incubation of craving during late withdrawal was associated with stronger neuronal activation (as assessed by ERK phosphorylation or ERK activity) in the ventral medial PFC than the dorsal medial PFC (Koya et al. 2009).
Thus, the ventral versus dorsal medial PFC dissociation for controlling cue-induced reinstatement after extinction versus incubation of cocaine craving, as assessed in extinction tests, could explain the differential effect of NVHL on the two measures. In this regard, in vitro recordings in the prelimbic (dorsal) medial PFC revealed that pyramidal neurons are hyperexcitable and interneurons cannot be activated by DA in slices obtained from NVHL rats (Tseng et al. 2006; Tseng et al. 2008). As a consequence, excitation-inhibition balance in the prelimbic PFC is altered in the NVHL model (Gruber et al. 2010). This was recently shown to play a significant role when rats are subjected to a reward-discounting choice task that activates the DA system (Roesch et al. 2007). NVHL rats demonstrated impaired cognitive flexibility in this task, and prelimbic PFC firing was exaggerated during epochs that activate ventral tegmental area (VTA) DA cell firing (Gruber et al. 2010). These data suggest that the NVHL rat presents a prelimbic PFC disinhibition that affects information processing and this altered neuronal responding may potentially influence reinstatement of cocaine seeking after extinction.
As discussed above, several brain circuits could be responsible for the enhanced cue-induced reinstatement in NVHL rats. This behavioral outcome is unlikely to arise from damage to the hippocampus itself, as lesions to the adult hippocampus or subiculum inhibit drug self-administration (Caine et al. 2001; Sun and Rebec 2003) and inactivation of the ventral hippocampus attenuates cue-induced reinstatement (Lasseter et al. 2010). Instead, this behavior is more likely a result of the impact of early postnatal hippocampal inactivation on the development of the mesocorticolimbic dopamine (DA) system, which is involved in the behavioral effects of cocaine (Pierce and Kumaresan 2006; Wise 1978), as well as in cue-induced cocaine seeking (Bossert et al. 2006; Kalivas and McFarland 2003; See 2005). The NVHL model entails interrupting ventral hippocampal activity at a period critical for the development of areas targeted by the hippocampus, such as the mPFC, causing long-term consequences on their neural circuits (O’Donnell 2011). For example, mPFC responses to DA afferent activation are abnormal in adult rats with a NVHL. Electrical stimulation of the VTA, the source of DA innervation to the mPFC, suppresses pyramidal cell firing in normal rats, but evokes a dramatic increase in firing in NVHL rats (O’Donnell et al. 2002). These abnormal responses are only observed in adulthood, indicating that the periadolescent maturation of PFC circuits may be affected by the absence of proper hippocampal innervation at early developmental stages.
In conclusion, NVHL rats have demonstrated exaggerated cue-induced reinstatement of cocaine seeking and are also more resistant to extinction than sham rats, but have normal incubation of cocaine craving. These findings complement previous reports on exaggerated cocaine-seeking behaviors in the NVHL model (Chambers and Self 2002), extending the features of this animal model of dual-diagnosis schizophrenia to a measure relevant to relapse and craving for cocaine in humans. Understanding the neurobiological underpinnings of the increased vulnerability for addictive behaviors in the NVHL model may aid in determining the involvement of brain circuits in schizophrenia-addiction comorbidity and perhaps inform novel therapeutic approaches for the treatment of this dual diagnosis.
Acknowledgments
The work was supported by a NIH grant (R01 DA014020; PO’D), by the Swedish Research Council (524-2009-621) to RMK, and by NIDA intramural research program to YS.
References
- Berg SA, Chambers RA. Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharmacology. 2008;54:1201–7. doi: 10.1016/j.neuropharm.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg SA, Czachowski CL, Chambers RA. Alcohol seeking and consumption in the NVHL neurodevelopmental rat model of schizophrenia. Behav Brain Res. 2011;218:346–9. doi: 10.1016/j.bbr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AM, McCallum SE, Glick SD, O’Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Psychopharmacology (Berl) 2008;200:205–15. doi: 10.1007/s00213-008-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clin Psychol Rev. 1991;11:123–140. [Google Scholar]
- Caine SB, Humby T, Robbins TW, Everitt BJ. Behavioral effects of psychomotor stimulants in rats with dorsal or ventral subiculum lesions: locomotion, cocaine self-administration, and prepulse inhibition of startle. Behav Neurosci. 2001;115:880–94. doi: 10.1037//0735-7044.115.4.880. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Lipska BK. A Method to the Madness: Producing the Neonatal Ventral Hippocampal Lesion Rat Model of Schizophrenia. In: O’Donnell P, editor. Animal Models of Schizophrenia (Neuromethods) Humana Press; 2011. pp. 1–24. [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biol Psychiatry. 2004;56:308–16. doi: 10.1016/j.biopsych.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Conroy SK, Rodd Z, Chambers RA. Ethanol sensitization in a neurodevelopmental lesion model of schizophrenia in rats. Pharmacol Biochem Behav. 2007;86:386–94. doi: 10.1016/j.pbb.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–8. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–10. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–62. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–85. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010;171:830–9. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004;176:101–8. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–91. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–8. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, Harvey BK, Hoffer BJ, Shaham Y. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–45. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–94. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–48. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–15. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–92. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–82. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–78. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–20. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci. 2007;10:1615–24. doi: 10.1038/nn2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–29. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–6. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–64. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Amin F, Lewis BL, O’Donnell P. Altered prefrontal cortical metabolic response to mesocortical activation in adult animals with a neonatal ventral hippocampal lesion. Biol Psychiatry. 2006;60:585–90. doi: 10.1016/j.biopsych.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O’Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–9. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Catecholamine theories of reward: a critical review. Brain Res. 1978;152:215–47. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008;62:421–31. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]