Abstract
Introduction
The commercially available Navigator system© (Esaote, Italy) allows easy 3D reconstruction of a single 2D acquisition of contrast-enhanced US (CEUS) imaging of the whole liver (with volumetric correction provided by the electromagnetic device of the Navigator©). The aim of our study was to compare the efficacy of this panoramic technique (Nav 3D CEUS) with that of conventional US and spiral CT in the detection of new hepatic lesions in patients treated for hepatocellular carcinoma (HCC).
Materials and methods
From November 2006 to May 2007, we performed conventional US, Nav 3D CEUS, and spiral CT on 72 cirrhotic patients previously treated for 1 or more HCCs (M/F: 38/34; all HCV-positive; Child: A/B 58/14) (1 examination: 48 patients; 2 examinations: 20 patients; 3 examinations: 4 patients). Nav 3D CEUS was performed with SonoVue© (Bracco, Milan, Italy) as a contrast agent and Technos MPX© scanner (Esaote, Genoa, Italy). Sensitivity, specificity, diagnostic accuracy, and positive and negative predictive values (PPV and NPV, respectively) were evaluated. Differences between the techniques were assessed with the chi-square test (SPSS release-15).
Results
Definitive diagnoses (based on spiral CT and additional follow-up) were: 6 cases of local recurrence (LocRecs) in 4 patients, 49 new nodules >2 cm from a treated nodule (NewNods) in 34 patients, and 10 cases of multinodular recurrence consisting of 4 or more nodules (NewMulti). The remaining 24 patients (22 treated for 1–3 nodules, 2 treated for >3 nodules) remained recurrence-free. Conventional US correctly detected 29/49 NewNods, 9/10 NewMultis, and 3/6 LocRecs (sensitivity: 59.2%; specificity: 100%; diagnostic accuracy: 73.6%; PPV: 100%; NPV: 70.1%). Spiral CT detected 42/49 NewNods plus 1 that was a false positive, 9/10 NewMultis, and all 6 LocRecs (sensitivity: 85.7%; specificity: 95.7%; diagnostic accuracy: 90.9%; PPV: 97.7%; NPV: 75.9%). 3D NAV results were: 46N (+9 multinodularN and 6 LR), 3 false-negatives, and one false-positive (sensitivity: 93.9; specificity: 97.9%; diagnostic accuracy: 95.6; PPV: 97.9; NPV: 93.9).
Conclusions
3D Nav CEUS is significantly better than US and very similar to spiral CT for detection of new HCCs. This technique revealed the presence of lesions that could not be visualized with spiral CT.
Keywords: Hepatocellular carcinoma, Liver, Detection, SonoVue, Three-dimensional ultrasound, Contrast-enhanced ultrasound, Spiral CT
Sommario
Introduzione
Il sistema “Navigator©” di Esaote consente di ottenere ricostruzioni 3-D di tutto il fegato (corrette volumetricamente da un sistema di guida) mediante singola acquisizione con CEUS (mediante scansione perpendicolare all'asse lungo del fegato, per una completa acquisizione 2-D del suo asse corto) e sovrappone tali ricostruzioni 3-D con quelle ottenute con la TC.
Scopo
valutare la capacità di tale sistema di diagnosticare nuovi HCC rispetto all'US e alla TC in una popolazione di HCC su cirrosi precedentemente trattati con varie metodiche.
Materiali e metodi
Settantadue cirrotici con pregressi HCC (M/F: 38/34; tutti HCV +vi, Child A/B: 58/14, con detection di 49 nuovi noduli (N) in 34 pazienti; 10 nuovi HCC multinodulari (NMulti); 6 riprese locali di malattia (Ri) in 4 pazienti (3 riprese singole, in un paziente tre noduli con ripresa di malattia); 47 HCC trattati efficacemente (neg) in 22 pazienti + 2 pazienti con HCC multinodulare senza segni di ripresa (neg-Multi) sono stati sottoposti a 100 esami (1 esame: 48 pazienti; 2 esami: 20 pazienti; 3 esami: 4 pazienti) dal 1 novembre 2006 al novembre 2007. La Nav 3D CEUS è stata eseguita con SonoVue© (BR1; Bracco) e con l'ecografo Esaote MPX© collegato a un sistema “Navigator©” con software di ricostruzione 3-D dedicato. La TC spirale di controllo è stata eseguita entro 30 giorni dall'esecuzione di Nav 3D CEUS. Sono stati valutati sensibilità, specificità, accuratezza diagnostica (ODA), valore predittivo positivo (PPV) e negativo (NPV).
Risultati
La diagnosi finale fu: 34 pazienti con 49 nuove lesioni (N), 10 con HCC multiN e 6 recidive loco-regionali in 4 pazienti; 47 noduli in 24 pazienti senza nuove lesioni durante il follow-up. Gli US hanno ottenuto: 29 N (+5 multinodularN e 3 LR), 20 falsi negativi (+5 Nmulti e 3 LR) (sensibilità: 59,2, specificità: 100%; accuratezza diagnostica: 73;6; VPP: 100; VPN: 70, 1); la TC spirale ha ottenuto: 42 N (+9 multinodularN e 7 LR), 7 falsi negativi (+1 Nmulti), un falso positivo (sensibilità: 85, 7, specificità: 95,7%, ODA: 90,9, PPV: 97,7; NPV: 75,9); Nav 3D CEUS ha ottenuto: 46 N (+9 multinodularN e 7 LR), 3 falsi negativi, 1 falso positivo (sensibilità: 93,9; specificità: 97,9%; accuratezza diagnostica: 95,6; PPV: 97,9; NPV: 93,9)
Conclusioni
La Nav 3D CEUS è significativamente migliore dell'ecografia convenzionale ed è simile alla TC spirale nella ricerca di nuovi HCC. Questa tecnica, in particolare, è stata capace di evidenziare lesioni non viste neanche dalla TC.
Introduction
Sensitive, reliable detection and precise localization and characterization of small hepatic lesions are the goals of modern radiological imaging [1] in patients with cirrhosis [2] or cancer [3,4]. Recent technological improvements, particularly in the field of magnetic resonance imaging (MRI), have allowed spatial reconstruction of the liver [2–4].
Three-dimensional (3D) imaging offers several recognized advantages, including its ability to determine the exact location of a lesion and its relations to neighboring structures and organs. (Assessment of the lesion's continuity with these structures is particularly important for the study of infiltrating neoplastic lesions.) The 3D approach also provides more accurate measurements of lesion volume, which is helpful for assessing expanding liver tumors before and after treatment (percutaneous ethanol injection, injection of antitumor agents, radio-frequency ablation, shock-wave therapy, etc.) [5].
Spiral computed tomography (CT) [6] and MRI [2–4] are used for this purpose. These techniques provide contrast-enhancement (capable of revealing the arterial, portal, and late phases); high temporal resolution (associated with short acquisition times); high spatial resolution (use of thin layers between two-dimensional (2D) imaging sections, which helps reduce volume artifacts); and panoramic views (reconstruction of the whole liver in a breath-hold period) [2,3].
Three-dimensional ultrasonography (3D US) is a technique based on the reconstruction of consecutive 2D US scans in a three-dimensional space. It involves the acquisition of a series of 2D images and the creation of a volume data set, which can be interactively manipulated to display the region of interest from user-specified viewpoints and provide direct 3D images [7–10].
Our previous experience [11] showed that 3D US is useful for the detection of hepatocellular carcinoma (HCC). We demonstrated that it was possible to detect HCC using an US scanner (TECHNOS MPX©, Esaote, Italy) equipped with 3D software and a free-hand technique. The limitation was that the 3D reconstruction of the liver was dependent on the type of scan; in other words, the acquired volume was not corrected in the three-dimensional space. To eliminate this problem, we used an adapted form of the Navigator system© (Esaote, Italy) to obtain a continuous scan of the entire liver. This image-fusion system was originally developed to provide real-time guidance for electrode insertion during radiofrequency thermal ablation (RFTA) of liver lesions based on a combination of US and CT information [12]. This scanning technique can be used with conventional and contrast-enhanced US (CEUS) (Navigator 3D CEUS©). The post-processing fusion technique consistently demonstrated precise overlap between the CT and US 3D reconstructions of the liver.
The aim of our study was to evaluate the performance of Navigator 3D CEUS© in the follow-up in cirrhotic patients who had undergone nonsurgical treatment for HCCs and to compare the results with those obtained with spiral CT (with multiple techniques).
Materials and methods
Patients
From 1 November 2006 through 30 April 2007, we followed 72 patients with HCV-related cirrhosis (38 males [mean age ± SD: 70.7 ± 10 years] and 34 females [74 ± 10 years]) who had been treated for HCC (1 nodule in 43 cases, 2 nodules in 6 cases, 3 nodules in 11 cases, and 4 or more nodules in the remaining 12 cases). According to the BCLC classification [13], 35 patients had “early” disease and 37 were classified as “intermediate.” Sixty-eight (94.4%) of the patients had been treated within the 6 months preceding enrolment (27 with RFTA, including 17 with early and 10 with intermediate-stage tumors; 16 with percutaneous ethanol injection [PEI] [10 early, 14 intermediate]; and 16 with transarterial chemoembolization [TACE] [6 early, 10 intermediate]). The other 5 (6.9%) had been treated ≤12 months before enrolment (all 5 with RFTA). During the interval between treatment and enrolment in our study, all of the patients had been followed by our staff, and none had shown signs of local recurrence, new HCCs, or distant metastases. At the time of treatment, 64 patients were in Child class A (A5 in 48 cases, A6 in 8), and 8 were in Child class B (B7 in 6 cases, B8 in 2). During the 6-month follow-up, 58 patients were in Child class A (class A-5 in 32, class A-6 in 18), and 14 were in Child class B (B-7 in 8 cases, B-8 in 4, B-9 in 2).
Methods
Navigator 3D CEUS© (Nav 3D CEUS) was performed using a suspension of sulfur hexafluoride microbubbles in sterile saline (SonoVue©, Bracco, Italy) as the contrast agent. SonoVue© combines improved stability with favorable resonance behavior at low acoustic pressures. It allows minimally disruptive contrast-specific imaging at low mechanical indexes (MI) and effective investigations over several minutes with real-time visualization of the dynamic enhancement pattern [14].
Adapted software was used with the Navigator system© (Esaote, Genoa, Italy) to continuously scan the entire liver. The Navigator system© (Fig. 1) includes a computer with touch-screen monitor and a PCIBird-type tracking system (Ascension technology – Degrees of freedom: Six [position and orientation]; Translation range, any direction: Standard transmitter 0–30 (76.2 cm); Angular range: All attitudes; Static accuracy standard sensor: 0.040 (1.0 mm) RMS position 0.15 degree RMS orientation). The system was coupled to an ultrasound scanner (Technos MPX©; Esaote, Genoa, Italy). The PCI bird is composed of an active sensor attached to the US probe and a passive sensor placed near the patient. The active sensor signals its spatial position and movements with reference to the passive sensor. Probe movements are recorded, and the 3D software inside the Navigator system© corrects the static 3D reconstruction obtained from the US scanning in comparison to probe movements (Fig. 2). The final result is an accurate spatial reconstruction of the volume that is independent of the type of 2D acquisition (Fig. 3).
Fig. 1.

The Navigator system© (from left to right): ultrasound scanner, TECHNOS MPX. Navigator System© (with touch-screen monitor) connected to the navigation system, which consists of a passive electromechanical sensor (near the patient) and an active sensor attached to the ultrasound probe.
Fig. 2.

Draft of 3D reconstruction: Left: bi-dimensional continuous scanning of the liver is performed by scanning its short axis through its long axis. Center: Bi-dimensional data are collected and processed by a specific software inside the Navigator System©. Right: planes captured along the liver are rendered in a three-dimensional space.
Fig. 3.
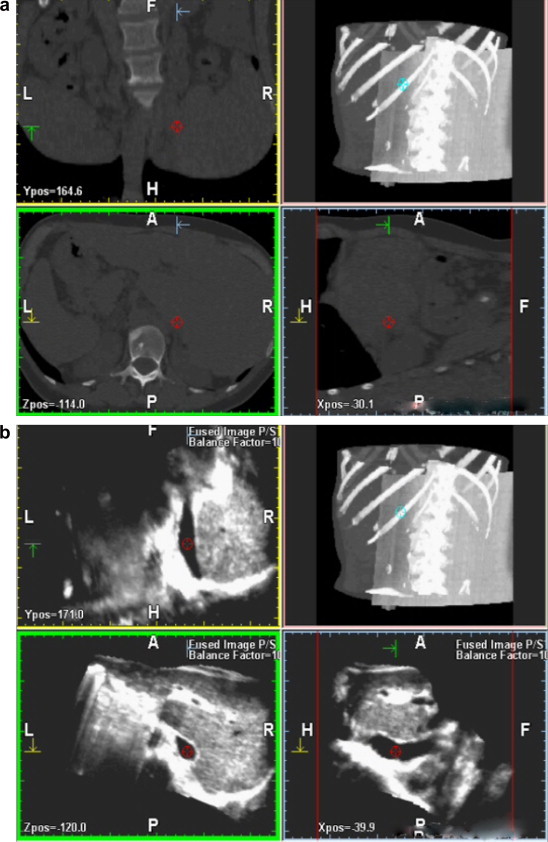

3D reconstruction of bi-dimensional spiral CT sequences (a) and bi-dimensional US sequences (b); the fusion technique allows superimposition of the two volumes, which display overlap precisely (c).
This type of scanning can be used with both conventional and contrast-enhanced US techniques. The Navigator system© can also provide a volumetric reconstruction of the liver with CT scanning and compare US- and CT-reconstructed volumes using the fusion technique (Fig. 5). This technique involves superimposing each resliced layer of the US and CT volumes and displaying both on the monitor. The post-processing fusion technique has always demonstrated precise overlap between the CT and US 3D reconstructions of the liver (Fig. 4).
Fig. 5.

Nav 3D CEUS reconstruction with parenchymal rendered volume. Two new HCCs in different liver segments are evident.
Fig. 4.
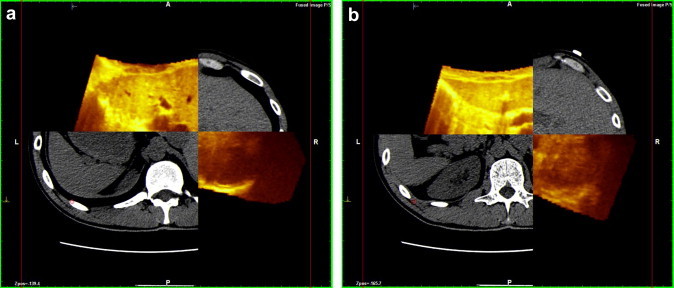
Fusion technique with Navigator system©: two different planes of reconstruction [(a) and (b)] of CT and US show perfect overlap. This system is able to separate the two planes (yellow for US, gray for CT), showing perfect correspondence between the margins of each.
To facilitate 3D reconstruction of 2D CEUS images of the whole liver, the 2D acquisition is performed in real-time perpendicular to the long-axis of the liver, for a complete 2D image of its short axis (Fig. 3). Subsequent reconstructions of these planes show a rendered volume with a “parenchymal” aspect (Fig. 5) or with a “vascular” map (Fig. 6) of all the hepatic segments with an acquisition in the early arterial and portal phases.
Fig. 6.
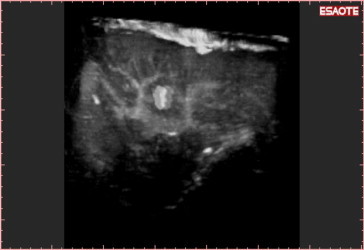
Nav 3D CEUS reconstruction with vascular rendered volume of the same acquisition shown in Fig. 7. The software can “translate” the volumetric data into different kinds of visualization. In Fig. 7 the new HCCs are inserted in the parenchymal volume, while here the main vascularization of liver can be appreciated in a “one-shot vision.”
3D Nav CEUS was performed on the same days as spiral CT/MRI. Follow-up was based on assessment of AFP levels and conventional US every three months and spiral CT and Nav 3D CEUS every six months. If AFP levels increased and/or US findings suggested local recurrence or new HCCs, Nav 3D CEUS was repeated after conventional US. During the 6-month follow-up, the number of Nav 3D CEUS examinations performed per patient was 3 (in 4 patients), 2 (in 20 patients), and 1 (in 48 patients). A total of 100 examinations with this new technique were performed from November 2006 to May 2007.
Spiral CT was considered the gold-standard imaging technique; MRI was performed in 11 cases in which spiral CT and Nav 3D CEUS results were discordant. Definitive diagnoses were based on spiral CT/MRI findings together with follow-up findings recorded during the 6 months following the study. Sensitivity, specificity, diagnostic accuracy, and positive and negative predictive values (PPV and NPV, respectively) were evaluated. Differences between the techniques were evaluated with the chi-square (SPSS software, version 15).
The protocol for this study was approved by the Medical Research Ethics Committee of our hospital, and informed consent was obtained from all patients.
Results
Table 1 summarizes the findings obtained with the three imaging methods considered in this study. During the 6-month follow-up, 24 (33.3%) of the 72 patients (8 treated for one nodule, 3 treated for two nodules, 11 treated for three nodules, and 2 treated for four or more nodules) remained disease-free. 4 (5.5%) patients (3 treated for 1 HCC nodule, 1 treated for 3 nodules) developed local recurrence (LocRec) at the site of all treated tumors (total no. LocRecs = 6). 34 (47.2%) patients (29 treated for 1 HCC nodule, 5 treated for 2 HCC nodules) presented 1–3 new nodules located 2 cm or more from treated lesions (NewNods) (total: 49) during follow-up, and 10 (13.9%) others (each treated for >3 nodules) developed 4 or more new nodules (new multinodular disease, NewMulti).
Table 1.
Detection of new and recurrent HCCs by conventional US, spiral CT, and 3D NAV CEUS.
| True positive | True negative* | False positive | False-negative | |
|---|---|---|---|---|
| Definitive diagnosisa |
|
|
NA | NA |
| Conventional US |
|
|
– |
|
| Nav 3D CEUSb |
|
|
1 NewNod (yes) |
|
| Spiral CT |
|
|
1 NewNod (yes) |
|
Abbreviations: NewNod, number of new HCC nodules arising 2 cm or more from the margins of a treated lesion (calculated only for patients who developed ≤3 new nodules during follow-up); NewMulti, number of patients who developed new multinodular disease (4 or more nodules) outside the originally involved liver segments. (The total number of new nodules was not calculated for this category.); LocRec, number of local recurrences observed at the site of a treated tumor; TrtNod, number of treated nodules; TrtMulti, number of patients treated for multinodular HCC (4 or more nodules).
* True negatives are expressed in terms of the number of treated nodules (TrtNod) that remained negative for local recurrence or new HCC during follow-up for patients treated for 1–3 HCCs; for patients treated for 4 or more tumors, results are expressed as the number of patients treated who remained negative during follow-up for local recurrence or new HCC.
Definitive diagnoses were based on spiral CT (plus MRI in some cases) and the results of 6 additional months of follow-up based on imaging and AFP levels.
The diagnostic accuracy of Nav 3D CEUS was significantly better than that of conventional US (p < 0.001) but not significantly different from that of spiral CT (p = ns).
Conventional US successfully detected 3/6 (50%) LocRecs, 29/49 (59.1%) NewNods, and 5/10 (50%) cases of NewMulti. In 4/5 cases, the NewMulti involved nodules less than 2 cm in diameter; the fifth patient had multiple nodules measuring more than 2 cm. The 3 LocRecs that were missed were located in segments II, IV, and VIII, and all measured less than 2 cm. The 20 NewNods that were not detected included 17 smaller than 2 cm and 3 with diameters between 2.0 and 2.5 cm. They were located in segment VIII (6 cases), segment IV (4 cases), segment V (3 cases), segment II (2 cases), or multiple segments (5 cases).
Spiral CT correctly detected all 6 LocRecs, 42/49 (85.7%) of the NewNods, and 9/10 (90%) cases of NewMulti (including one case characterized by the presence of small active nodules suspected solely on the basis of elevated AFP levels and diagnosed only by MRI). The 7 false-negative CT examinations involved 6 NewNods less than 2 cm in diameter and 1 measuring 3 cm. They were located in segment VIII (4 nodules) or segments IV, VI, or VII (one nodule each). 4 of the NewNods were located in segments different from those harboring the previously treated tumor; the other 3 were in the same segment as the original tumor. Spiral CT failed to detect one case of NewMulti characterized by nodules that were all less than 2 cm, and it erroneously diagnosed one arteriovenous malformation as a new HCC (this finding was confirmed to be a false positive by MRI findings and subsequent follow-up data).
3D Nav CEUS correctly detected all the LocRecs, 46/49 (93.87%) of the NewNods (Fig. 7), and 9/10 (90%) cases of NewMulti (Figs. 8 and 9), (the tenth case of NewMultiwas the same one with false-negative findings on spiral CT). The 4 NewNods missed by 3D NAV CEUS included 3 that were less than 2 cm and one measuring 3 cm. Their segmental distribution was as follows: 2 nodules in segment IV and 2 nodules in segment VI. The single false-positive result recorded for Nav 3D CEUS involved the arteriovenous malformation mentioned above, which was also identified as a new HCC by spiral CT (as noted, this finding was confirmed to be a false positive by MRI and follow-up data). Twenty NewNods were visible on spiral CT but not on conventional US. Seventeen of these (85%) were detected by Nav 3D CEUS (Fig. 10).
Fig. 7.
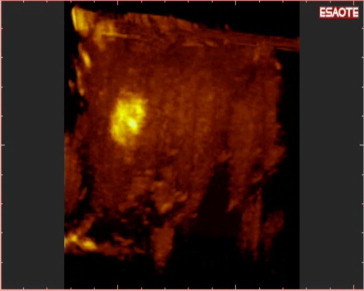
Detection of a new HCC during follow-up: Nav 3D CEUS was able to show the exact position of the nodule in a three-dimensional space.
Fig. 8.

The liver volume can be studied plane by plane with the reslicing technique. This approach shows (in the same patient) a previously treated HCC in segment VII…
Fig. 9.
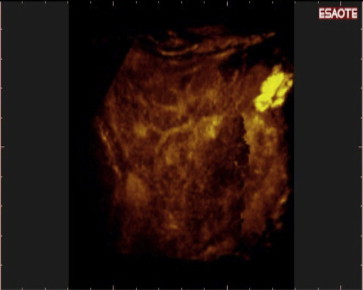
Examination with 3-D NAV CEUS was performed because of elevated AFP levels: New multinodular HCCs were detected in segment IV (and in other segments too). No nodules were visible with conventional US.
Fig. 10.

(a) Bi-dimensional acquisition plane of a female patient with elevated AFP levels and no evidence of new nodules on conventional US. Spiral CT revealed a small HCC in segment VIII. Nav 3D CEUS also detected the nodule. (b) The 3D reconstruction clearly revealed the nodule in the three different planes (red cross) and on the volumetric reconstruction. After PEI, the AFP levels normalized, and no new nodules were detected over the next six months.
As shown in Table 2, conventional US displayed low sensitivity (59.2%), high specificity (100%), and overall diagnostic accuracy of 73.6%. Spiral CT displayed better sensitivity (85.7%) and diagnostic accuracy (90.9%) that were, however, inferior to those observed with Nav 3D CEUS (sensitivity 93.9%, diagnostic accuracy 95.6%). Differences between conventional US and Nav 3D CEUS were statistically significant (p < 0.001), but the differences between spiral CT and Nav 3D CEUS were not (p = ns).
Table 2.
Performance of the three imaging modalities.
| Sensitivity | Specificity | Diagnostic accuracy | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|---|
| Conventional US | 59.2 | 100 | 73.6 | 100 | 70.1 |
| Nav 3D CEUS | 93.9 | 97.9 | 95.6 | 97.9 | 93.9 |
| Spiral CT | 85.7 | 95.7 | 90.9 | 97.7 | 75.9 |
As shown in Table 2, conventional US displayed low sensitivity (59.2%), high specificity (100%), and overall diagnostic accuracy of 73.6%. Spiral CT displayed better sensitivity (85.7%) and diagnostic accuracy (90.9%) that were, however, inferior to those observed with 3-D NAV CEUS (sensitivity 93.9%, diagnostic accuracy 95.6%). Differences between conventional US and 3-D NAV CEUS were statistically significant (p < 0.001), but the differences between spiral CT and 3-D NAV CEUS were not (p = ns).
Discussion
The detection of HCC in patients with cirrhosis is generally based on conventional US and CT performed according to EASL [15] and AASLD guidelines [16]. The Nav 3D CEUS technique described here may be used instead of conventional US, and this approach may reduce the need for spiral CT during the post-treatment follow-up of HCC in cirrhotic livers.
3D US is a relatively new technique with demonstrated accuracy in volumetric assessment of the liver [7]. It does not provide real-time reconstruction, but it is capable of revealing a three-dimensional space similar to those provided by CT and MRI [7]. Thanks to these characteristics, 3D US [9,17] and 4D US [18,19] (that is real-time 3D US) have proved to be effective in US-guided biopsy of hepatic [9,17,18] and superficial [19] masses. Three-dimensional sonography has also been found to be useful during percutaneous ablation of liver cancers for visualization of expandable radio-frequency electrodes and the position of the applicator placement with respect to adjacent critical structures [20].
Conventional 3D US has three important disadvantages: the lack of contrast-enhanced imaging, the absence of panoramic visualization, and substantial operator-dependency. New US systems are now able to perform 3D reconstruction using contrast-enhanced imaging. This means that CEUS 3D reconstructions are based on the macro-and microcirculations. This makes it possible to detect the enhanced vascularity of an HCC during the arterial phase and the typical late wash-out that are seen with spiral CT/MRI. However, 3D reconstruction of US images is somewhat more complex since the various 2D slices are obtained manually (rather than in an automated manner) using conventional US. Image acquisition thus depends on the operator's skills. In addition, the number of slices that can be obtained is infinite – one more slice is always feasible. Finally, the acquired volume has to be corrected in relation to the type of scanning.
To render the US scan more “panoramic” (it was impossible to perform an US scan of the entire liver in a few seconds during the arterial phase), we decided to perform single acquisitions of the liver parenchyma visualizing its “short” axis. The superimposition of the images acquired by scanning the liver through its long-axis allowed us to image the entire liver parenchyma during the arterial phase, in a manner similar to that offered by spiral CT.
New fusion (US and CT or MRI) and navigation systems for US have been developed for correcting US reconstruction of the liver in the three-dimensional space. They offer real-time guidance during RFTA of liver targets that cannot be visualized on US. Ohto and colleagues [21] showed that contrast-enhanced 3D fusion US makes it possible to display images combining the plane-shift and opacity-control modes, which reveal even minimal levels of blood flow within hepatic tumors in a 3D perspective and can be used to identify tumor-specific vascular flow patterns.
Virtual US (VUS) is a system that generates 3D images using thin sections obtained with multi-detector row CT and computer software, simulating images obtained using conventional US. Hirooka [22] first demonstrated that this approach is useful for visualization of HCC nodules that cannot be seen with conventional US. Virtual US was also a useful tool for US-guided treatment of HCC. The same author confirmed this experience in vitro and in vivo [23]. The same group combined CEUS with VUS to assess the responses of HCCs to RFA, and this approach reduced the need for spiral CT [24].
Chopra et al. developed a navigation system for soft-tissue tumor resection (Optoelectronic navigation with section-mode visualization in 2 orthogonal planes) that was based on 3D US imaging and optical tracking [25]. This system was validated [26] and employed [27] for accurate ultrasound-based navigation of liver-tumor resections.
Recently, Crocetti et al. tested the “Navigator system©” used in our study to correct the 3D reconstruction and concluded that it is both feasible and accurate [12]. With the navigator system, our group was able to reconstruct the whole liver during the arterial phase (typically 15–20 s) [14] from a single scan carried out along the long axis (from right to left). This tomographic approach is similar to that used in spiral CT, with the difference that the CT scans are carried out along the short axis of the organ (in a cephalocaudal direction).
We demonstrated that the Nav 3D CEUS connected to the navigation system is capable of detecting and characterizing new HCCs in a similar manner to spiral CT. This is the first demonstration that CEUS is able to detect HCCs during follow-up in patients with cirrhosis although CEUS detection of HCC is currently considered difficult because full surveillance of the whole liver is impossible [14].
Conclusions
3DNavCEUS can be used to detect and characterize HCC recurrence in cirrhotic patients in a manner similar to CT. This approach may reduce or even eliminate the need for spiral CT during the follow-up of these patients.
Conflict of interest statement
The authors have no conflict of interest.
Footnotes
SIUMB 2007 – SIUMB Award for the best oral communication presented at the XIX National Congress of the SIUMB.
References
- 1.Elsayes K.M., Narra V.R., Yin Y., Mukundan G., Lammle M., Brown J.J. Focal hepatic lesions: diagnostic value of enhancement pattern approach with contrast-enhanced 3D gradient-echo MR imaging. Radiographics. 2005;25:1299–1320. doi: 10.1148/rg.255045180. [DOI] [PubMed] [Google Scholar]
- 2.Burrel M., Llovet J.M., Ayuso C., Iglesias C., Sala M., Miquel R. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 3.Lee V.S., Lavelle M.T., Rofsky N.M., Laub G., Thomasson D.M., Krinsky G.A. Hepatic MR imaging with a dynamic contrast-enhanced isotropic volumetric interpolated breath-hold examination: feasibility, reproducibility, and technical quality. Radiology. 2000;215:365–372. doi: 10.1148/radiology.215.2.r00ma16365. [DOI] [PubMed] [Google Scholar]
- 4.Ward J., Robinson P.J., Guthrie J.A., Downing S., Wilson D., Lodge J.P. Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology. 2005;237:170–180. doi: 10.1148/radiol.2371041444. [DOI] [PubMed] [Google Scholar]
- 5.Munoz Agel F., Varas Lorenzo M.J. Tridimensional (3D) ultrasonography. Rev Esp Enferm Dig. 2005;97:125–134. doi: 10.4321/s1130-01082005000200007. [DOI] [PubMed] [Google Scholar]
- 6.Ueda T., Mori K., Minami M., Motoori K., Ito H. Trends in oncological CT imaging: clinical application of multidetector-row CT and 3D-CT imaging. Int J Clin Oncol. 2006;11:268–277. doi: 10.1007/s10147-006-0586-1. [DOI] [PubMed] [Google Scholar]
- 7.Wong J., Gerscovich E.O., Cronan M.S., Seibert J.A. Accuracy and precision of in vitro volumetric measurements by three-dimensional sonography. Invest Radiol. 1996;31:26–29. doi: 10.1097/00004424-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Rose S.C., Pretorius D.H., Nelson T.R., Kinney T.B., Huynh T.V., Roberts A.C. Adjunctive 3D US for achieving portal vein access during transjugular intrahepatic portosystemic shunt procedures. J Vasc Interv Radiol. 2000;11:611–621. doi: 10.1016/s1051-0443(07)61614-5. [DOI] [PubMed] [Google Scholar]
- 9.Rose S.C., Hassanein T.I., Easter D.W., Gamagami R.A., Bouvet M., Pretorius D.H. Value of three-dimensional US for optimizing guidance for ablating focal liver tumors. J Vasc Interv Radiol. 2001;12:507–515. doi: 10.1016/s1051-0443(07)61892-2. [DOI] [PubMed] [Google Scholar]
- 10.Rose S.C., Behling C., Roberts A.C., Pretorius D.H., Nelson T.R., Kinney T.B. Main portal vein access in transjugular intrahepatic portosystemic shunt procedures: use of three-dimensional ultrasound to ensure safety. J Vasc Interv Radiol. 2002;13:267–273. doi: 10.1016/s1051-0443(07)61719-9. [DOI] [PubMed] [Google Scholar]
- 11.Giangregorio F., Marinone M.G., Di Stasi M., Sbolli G., Aragona G., Tansini P. A new tridimensional (3D) contrast-enhanced US og the whole liver (panoramic 3D CEUS) in monitoring treated HCCs and in the detection of new lesions. A one year experience. Gut. 2006;55:a27. [Google Scholar]
- 12.Crocetti L., Lencioni R., Debeni S., See T.C., Pina C.D., Bartolozzi C. Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Invest Radiol. 2008;43:33–39. doi: 10.1097/RLI.0b013e31815597dc. [DOI] [PubMed] [Google Scholar]
- 13.Llovet J.M., Bru C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 14.Claudon M., Cosgrove D., Albrecht T., Bolondi L., Bosio M., Calliada F. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) – update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J., Sherman M., Llovet J.M., Beaugrand M., Lencioni R., Burroughs A.K. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J., Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 17.Sjolie E., Lango T., Ystgaard B., Tangen G.A., Nagelhus Hernes T.A., Marvik R. 3D ultrasound-based navigation for radiofrequency thermal ablation in the treatment of liver malignancies. Surg Endosc. 2003;17:933–938. doi: 10.1007/s00464-002-9116-z. [DOI] [PubMed] [Google Scholar]
- 18.Won H.J., Han J.K., Do K.H., Lee K.H., Kim K.W., Kim S.H. Value of four-dimensional ultrasonography in ultrasonographically guided biopsy of hepatic masses. J Ultrasound Med. 2003;22:215–220. doi: 10.7863/jum.2003.22.2.215. [DOI] [PubMed] [Google Scholar]
- 19.Chang C.Y., Wang H.K., Chiou H.J., Chou Y.H., Chen T.H., Chiou S.Y. Interventional procedures in superficial lesions: the value of 2D with additional coronal reformatted 4D ultrasonography guidance. Korean J Radiol. 2006;7:28–34. doi: 10.3348/kjr.2006.7.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H.X., Yin X.Y., Lu M.D., Xie X.Y., Xu Z.F., Liu G.J. Usefulness of three-dimensional sonography in procedures of ablation for liver cancers: initial experience. J Ultrasound Med. 2003;22:1239–1247. doi: 10.7863/jum.2003.22.11.1239. [DOI] [PubMed] [Google Scholar]
- 21.Ohto M., Kato H., Tsujii H., Maruyama H., Matsutani S., Yamagata H. Vascular flow patterns of hepatic tumors in contrast-enhanced 3-dimensional fusion ultrasonography using plane shift and opacity control modes. J Ultrasound Med. 2005;24:49–57. doi: 10.7863/jum.2005.24.1.49. [DOI] [PubMed] [Google Scholar]
- 22.Hirooka M., Iuchi H., Kurose K., Kumagi T., Horiike N., Onji M. Abdominal virtual ultrasonographic images reconstructed by multi-detector row helical computed tomography. Eur J Radiol. 2005;53:312–317. doi: 10.1016/j.ejrad.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Hirooka M., Iuchi H., Kumagi T., Shigematsu S., Hiraoka A., Uehara T. Virtual sonographic radiofrequency ablation of hepatocellular carcinoma visualized on CT but not on conventional sonography. Am J Roentgenol. 2006;186:S255–S260. doi: 10.2214/AJR.04.1252. [DOI] [PubMed] [Google Scholar]
- 24.Kisaka Y., Hirooka M., Kumagi T., Uehara T., Hiasa Y., Kumano S. Usefulness of contrast-enhanced ultrasonography with abdominal virtual ultrasonography in assessing therapeutic response in hepatocellular carcinoma treated with radiofrequency ablation. Liver Int. 2006;26:1241–1247. doi: 10.1111/j.1478-3231.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 25.Chopra S.S., Hunerbein M., Eulenstein S., Lange T., Schlag P.M., Beller S. Development and validation of a three dimensional ultrasound based navigation system for tumor resection. Eur J Surg Oncol. 2008 Apr;34(4):456–461. doi: 10.1016/j.ejso.2007.07.011. [Epub 2007 Sep 4] [DOI] [PubMed] [Google Scholar]
- 26.Beller S., Hunerbein M., Eulenstein S., Lange T., Schlag P.M., Gebauer B. Feasibility of navigated resection of liver tumors using multiplanar visualization of intraoperative 3-dimensional ultrasound data image-guided surgery of liver metastases by three-dimensional ultrasound-based optoelectronic navigation. Ann Surg. 2007;246:288–294. doi: 10.1097/01.sla.0000264233.48306.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beller S., Hunerbein M., Lange T., Eulenstein S., Gebauer B., Schlag P.M. Image-guided surgery of liver metastases by three-dimensional ultrasound-based optoelectronic navigation. Br J Surg. 2007;94:866–875. doi: 10.1002/bjs.5712. [DOI] [PubMed] [Google Scholar]


