Abstract
Aims
Solid pseudopapillary neoplasm of the pancreas (SPN) is a rare low-grade malignant neoplasm. SPN with prominent atypical multinucleated giant tumor cells (MNGTCs) has not been reported.
Methods and results
We identified four cases of SPN with prominent atypical MNGTCs in a cohort of 62 cases of SPN (6.5%). The MNGTCs contained multiple enlarged, hyperchromatic, irregular nuclei with ample eosinophilic cytoplasm, typically present in the solid area of the tumor. The MNGTCs had a typical immunohistochemical profile of the conventional SPN and were positive for vimentin, β-catenin, CD10, and progesterone receptor, but were negative for pan-cytokeratin chromogranin, synaptophysin, trypsin, Ki-67 and CD68 in all four cases. Patients of SPN with prominent MNGTCs were older than those with conventional SPN (p=0.01), tumors were incidentally discovered by imaging studies for an unrelated disease in all four cases, and with a female to male ratio of 1:1. The proliferation index (Ki-67) was <1% in all four cases. None of the three patients, whose follow up information was available, developed recurrence during follow-up of 2.7, 3.8 and 5.0 years.
Conclusions
The presence of MNGTCs in SPN most likely represents degenerative change of the tumor cells and does not seem to affect the prognosis.
Keywords: solid pseudopapillary neoplasm, multinucleated giant cells, prognosis
Introduction
Solid pseudopapillary neoplasm of the pancreas (SPN) is a rare low-grade malignant neoplasm that accounts for about 1% to 2% of all pancreatic tumors 1. The clinicopathologic features of SPN are distinctive from other pancreatic neoplasms 1–3. SPN occurs mainly in young women in their 20s and 30s of age with a median age of 28 years 3. The tumor sizes are often very large at the time of presentation with a heterogeneous cut surface consisting of solid brown lobulated areas, hemorrhage, and cystic spaces containing necrotic debris 4. Tumors are confined to the pancreas in 85% of patients. Patients with SPN have excellent prognosis after complete surgical resection with a five-year survival rate over 85%. Even the 10% to 15% of patients who developed recurrent SPN, liver or peritoneal metastases commonly enjoy long-term survival5–8. Although there are no pathological factors that can reliably predict the clinical outcome of patients with SPN, Tang et al recently reported two rare cases of clinically aggressive SPN that were associated with diffuse growth pattern, extensive tumor necrosis, significant nuclear atypia, and high mitotic count. Both of their patients died of disease at 6 and 16 months after presentation 9.
Despite numerous studies using immunohistochemistry markers, electron microscopy and molecular pathology, the cell origin of SPN remains unclear. Characteristically, SPNs almost always harbor β-catenin mutation and have nuclear localization of β-catenin by immunohistochemistry due to lack of degradation of mutated protein, but lack K-ras mutation that is a hallmark of pancreatic ductal adenocarcinomas. β-catenin immunohistochemistry has been widely used as one of the diagnostic markers for SPN 10, 11. The typical histologic pattern and cytologic features of SPN are characterized by numerous delicate blood vessels surrounded by variable amount of hyalinized stroma and relative uniform, non-cohesive polygonal cells forming pseudopapillae. Intra-cytoplasmic PAS-positive diastase resistant eosinophilic globules, foamy histiocytes, cholesterol crystals with foreign body giant cell reaction are commonly present in SPN. Rare cases of clear cell variant of SPN had also been reported. The clinical features, gross characteristics, and immunohistochemical staining profile of clear cell SPN are similar to those patients with conventional SPNs 12–14. Recently, we encountered an unusual case of SPN represented by numerous atypical multinucleated giant tumor cells (MNGTCs). This feature, to our knowledge, has not been previously reported. The purpose of this study is to investigate the frequency of SPN with MNGTCs and to describe the clinical and histologic features of four cases of SPN with MNGTCs and compared these to 58 patients with conventional SPNs. The significance of MNGTCs in SPN and the differential diagnoses are discussed.
Materials and Methods
Four patients of SPN with MNGTCs and 58 patients with conventional SPN (control group) identified from the pathology file at the University of Texas MD Anderson Cancer Center from 1994 to 2011 were included in this study. The hematoxylin-eosin (H&E) stained slides from the pancreatectomy specimens of all patients were reviewed and the diagnosis of SPN was confirmed in all cases. Clinical and pathologic information, including patient age, gender, type of specimen, tumor size, tumor location in the pancreas, was collected from computerized patient medical record. The study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center.
All four patients of SPN with prominent MNGTCs were evaluated by immunohistochemistry for vimentin (clone V9, 1:900, Dako, Carpinteria, CA), CD10 (clone 56C6, 1:50, Novocastra, Buffalo Grove, IL), β-catenin (clone 14, 1:500, BD Biosciences, San Jose, CA), progesterone receptor (PR, clone 1294, 1:200, Dako), chromogranin (clone LK2H10, 1:4000, Chemicon), synaptophysin (clone 27G12, 1:600, Novocastra), trypsin (1:130,000, Chemicon, Billerica, MA), Ki-67 (clone MIB-1, 1:100, Dako), and CD68 (clone KP-1, 1:900, Dako). The pancytokeratin cocktail consisted of a mixture of four different antibodies against cytokeratins: AE1/AE3 (1:50), CAM5.2 (1:50), MNF116 (1:50) and 8/18 (1:25). Standard immunohistochemical staining techniques were used as described previously with appropriate positive and negative controls 15.
Patient follow-up information through April of 2012 was extracted from patient medical record and if necessary, by review of the U.S. Social Security Index. The overall survival was calculated as the time from the date of diagnosis to the date of death or the date of last follow-up if death did not occur. The Fisher’s exact tests were used to compare categorical data. Survival curves were constructed using the Kaplan-Meier method, and the log-rank test was used to evaluate the statistical significance of differences. Statistical analysis was performed using Statistical Package for Social Sciences software (for Windows 12.0, SPSS Inc., Chicago, IL). A two-sided significance levelof 0.05 was used for all statistical analyses.
Results
Prevalence and clinicopathologic features of SPN with multinucleated giant tumor cells
We reviewed the H&E slides from 62 pancreatectomy specimens with the diagnosis of SPN in the pathology file at our institution for the presence of multinucleated giant tumor cells (MNGTCs). We found 4 cases (6.5%) of SPN with prominent MNGTCs. In all four cases, many MNGTCs were present either in the solid area of the tumor or in area with conventional SPN histology. The clinicopathologic features of the four patients of SPN with prominent MNGTCs and their comparison with the patients with conventional SPN are shown in Table 1 and Table 2 respectively. The tumor was incidentally identified by computed tomography (CT) during work up for other unrelated diseases in all four cases (Table 1). All four patients had serum CA19–9 levels within the normal range. Fifty percent (2/4) patients of SPN with prominent MNGTCs were male compared to 13.8% (8/58) of male patients with conventional SPN (P=0.06, Table 1). Patients with SPN and MNGTCs had a mean age of 51.3 years (range 36 to 59 years) at the time of diagnosis and were older than those with conventional SPN, whose mean age was 32.1 years (range: 9.4 to 62.2 years, p=0.01).
Table 1.
Clinicopathologic features of solid pseudopapillary neoplasm with prominent MNGTCs.
| Case number | Age at diagnosis | Gender | Clinical presentation | CA19.9 (unit/ml) | Type of surgery | Location | Tumor Size | Percentage of tumor with MNGTCs (%) | Follow-up (months) | Vital status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52.7 | Female | Incidental finding by CT during follow up for breast cancer | 10.7 | Pylorus-preserving pancreaticoduodenectomy | Head | 1.8 cm | 60 | 45.2 | Alive with no evidence of disease |
| 2 | 36.0 | Female | Pneumonia, incidental finding by CT | 15.2 | Distal pancreatectomy | Tail | 10.3 cm | 90 | 32.4 | Dead of abdominal abscess |
| 3 | 59.0 | Male | Kidney stone and lithotripsy, incidental finding by CT | 11.2 | Pancreaticoduodenectomy | Head | 5.0 cm | 30 | 60.0 | Alive with no evidence of disease |
| 4 | 57.2 | Male | Diverticulitis, incidental finding by CT | 16.6 | Pancreaticoduodenectomy | Head | 2.7 cm | 25 | 1.1 | Lost to follow-up |
Table 2.
Comparison of the clinicopathologic features of SPN with prominent MNGTCs with conventional SPN
| Clinicopathologic features | SPN with prominent MNGTCs | Conventional SPN | p value |
|---|---|---|---|
| Number of cases | 4 | 58 | |
| Female: male ratio | 1:1 | 4:25 | 0.06 |
| Mean age ± SD (years) | 51.3 ± 10.5 | 32.1 ± 14.1 | 0.01 |
| Location | 0.13 | ||
| Head | 3 | 16 | |
| Body | 0 | 12 | |
| Tail | 1 | 30 | |
| Mean tumor size ± SD (cm) | 5.0 ± 3.8 | 6.7 ± 4.2 | 0.41 |
| Liver metastasis or other organ involvement at the time of presentation | 0/4 | 5/58 | 1.00 |
| Recurrence | 0/3* | 10/57* | 0.42 |
Number of patients whose follow-up information is available.
Gross and morphologic features
SPNs with MNGTCs were located in the head of pancreas in 3 patients and in the tail of the pancreas in one patient. Grossly three tumors were well-circumscribed without capsule and with capsule in one remaining case (case #2). All four tumors showed tan, soft cut surfaces with areas of cystic degeneration and hemorrhage. The tumor size ranged from 1.8 cm to 10.3 cm (mean tumor size: 5.0 ± 3.8 cm), which was not significantly different from the mean tumor size of 6.7 ± 4.2 cm for the conventional SPN (p=0.41).
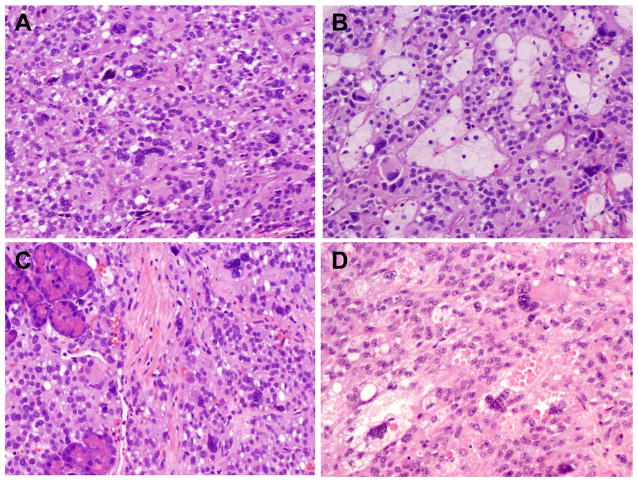
In all four cases, many atypical MNGTCs were seen in otherwise conventional SPN histology. However, MNGTCs were more prominent in the solid areas of the tumor (Figure 1). Most of the MNGTCs contained multiple enlarged, hyperchromatic, irregular nuclei with ample eosinophilic cytoplasm (Figure 1). While some of the MNGTCs had the nuclear features similar to the mononuclear tumor cells, most MNGTCs showed smudged chromatin, suggestive of degenerative changes. Intra-cytoplasmic eosinophilic hyaline globules were present in the MNGTCs in one case (Figure 1D). Areas of hemorrhage, collections of foamy histiocytes and foreign body multinucleated giant cells with cholesterol crystals were present in three of four cases. Focal calcification was identified in two cases. In all four cases, mitoses were rare and no mitoses were identified in the MNGTCs. Although the tumors were grossly well-circumscribed, microscopic tumor invasion into the adjacent normal pancreas (Figure 1C) was present in three cases and peripancreatic soft tissue in two cases. Perineural invasion was present in one case. Lymphovascular invasion, lymph node metastasis or positive resection margin was not present in any of the four cases.
Figure 1.
Representative micrographs showing many atypical MNGTCs in case #4 present in the solid area of the tumor (A), in area with conventional SPN histology and foamy histiocytes (B), and tumor invasion into the adjacent pancreatic parenchyma (C). D. Representative micrograph from Case #3 show atypical MNGTCs present in area with conventional SPN histology with intracytoplasmic globules (H&E stain, original magnification, 200x).
Immunohistochemistry
The immunohistochemical profile of the MNGTCs was similar to the mononuclear tumor cells for all four cases. Both mononuclear tumor cells and MNGTCs were diffusely positive for vimentin, beta-catenin (nuclear and cytoplasmic staining), CD10, and progesterone receptor (PR) in all four cases (Figure 2). Immunohistochemical stains for pancytokeratin and synaptophysin were focally positive in one case and were negative in the other three cases. All four cases were negative for chromogranin and trypsin. The MNGTCs were negative for CD68 in all four cases (Figure 2F). Thus the immunohistochemical staining profile suggests that the MNGTCs have similar origin as the mononuclear tumor cells, but not from the histiocytes that are commonly present in SPNs. Immunohistochemical stain for Ki-67 was performed on all four cases and all cases showed less than 1% nuclear staining for Ki-67 in the tumor cells, and no staining of MNGTCs (Figure 2E).
Figure 2.
Representative micrographs from Case #1 show immunohistochemical staining for pan-cytokeratin (A); β-catenin (B), CD10 (C), PR (D), Ki-67 (E) and CD68 (F). Normal pancreatic acinar cells as internal control were marked with dot line in A, B, and C. The atypical MNGTCs were marked with arrows in B–F. (Original magnification, 100x for A and E; 200x for B, C, D and F).
Clinical Outcome
The follow up information was available in three patients with the follow up time of 2.7, 3.8 and 5.0 years and one patient was lost to follow up one month after the surgery. During follow-up, one patient died of abdominal abscess at 2.7 years after the distal pancreatectomy. The other two patients were alive with no evidence of recurrence at 3.8 and 5.0 years after the surgery. Although there was no significant difference in overall survival compared to the 58 patients with conventional SPN, whose mean survival was 15.26 ± 0.72 years with a median follow up of 3.41 years (P=0.21), the number of patients with MNGTCs was too small to be meaningful.
Discussion
Multinucleated giant cells of histocytic origin are common findings in SPNs. They represent a reaction to the necrotic and degenerative changes that are commonly seen in these tumors. However, MNGTCs have not been reported in SPNs of the pancreas. In this study, we reported four cases of SPN with prominent MNGTCs in a cohort of 62 patients with SPN. The index case of SPN with prominent MNGTCs was found incidentally during routine histologic evaluation of an otherwise usual SPN. Three additional cases of SPN with prominent MNGTCs were found in 61 pancreatectomy specimens with the diagnosis of SPN after retrospective review. The prevalence of SPN with prominent MNGTCs was 6.5% in our study population. We demonstrated that the MNGTCs in all four cases had the same immunohistochemical profile as the mononuclear tumor cells of SPNs and were immunoreactive to vimentin, beta-catenin, CD10, and PR, but not to histiocytic marker CD68. The MNGTCs are also negative for pan-cytokeratin, chromogranin, synaptophysin and trypsin. These findings suggest that the MGNTCs are originated from the mononuclear tumor cells, instead of histotiocytic origin. Application of immunohistochemistry would help to differentiate SPN with prominent MNGTCs from other tumors with cells similar to those seen in our cases, including pleomorphic pancreatic neuroendocrine tumor 16, pancreatic acinar cell carcinoma, undifferentiated carcinoma of the pancreas, undifferentiated carcinoma of the pancreas with osteoclast-like giant cells, metastatic carcinoma or sarcoma.
The MNGTCs in our cases showed multiple enlarged, irregular or bizarre, hyperchromomatic nuclei with significant nuclear atypia. In addition, many MNGTCs were present in the solid areas of SPN in our cases. These findings raised the concern for an aggressive behavior of this tumor. Rare cases of clinically aggressive SPN of the pancreas that are associated with diffuse growth pattern, extensive tumor necrosis, significant nuclear atypia, and high mitotic count have been reported 9. However, all four patients of SPN with prominent MNGTCs in this study were identified incidentally by CT scan during work up for other unrelated diseases (three patients during work up for inflammatory conditions and one during follow-up for breast cancer after lumpectomy and cyclophosphamide/epirubicin/5-fluorouracil therapy) and two of the tumors were less than 3.0 cm in size. None our patients had tumor invasion to the other organs, lymphovascular invasion or lymph node metastasis. In addition, all four cases of SPN with MNGTCs showed very low proliferation index and all giant cells were negative for Ki-67 in this study. No recurrence was detected during the follow up of three patients whose follow up information were available, which was similar to those with conventional SPN. These findings argued against the possibility that the presence of prominent MNGTCs in SPN was associated with more aggressive behavior of SPN. Long term follow-up and more cases are needed to further confirm the indolent behavior of this tumor. The histologic appearance and clinical follow-up data in our study of SPN with prominent MNGTCs bear analogy to the degenerative, pleomorphic atypia seen in pleomorphic pancreatic neuroendocrine tumors 16, symplastic leiomyomas 17, symplastic glomus tumor 18, symplastic hemangioma 19, and bizarre giant cells of mammary fibroadenomas 20. The presence of atypical cells in these tumors has also been shown to have no significant effect on the prognosis, but the demographic trend appeared to be similar to their conventional counterparts.
In summary, we identified, for this first time, four cases (6.5%) of SPN with prominent MNGTCs of the pancreas in a cohort of 62 patients with SPN at our institution. The patients of SPN with prominent MNGTCs were older, slightly more frequent in males and located in the head of the pancreas compared to the patients with conventional SPN. The patients of SPN with prominent MNGTCs seem to have a good prognosis similar to those with conventional SPN. We speculate that the MNGTCs in our cases may represent degenerative changes in tumor cells.
Acknowledgments
Supported by the National Institutes of Health grant (1R21CA149544–01A1) and G. S. Hogan Gastrointestinal Cancer Research Fund at The University of Texas M.D. Anderson Cancer Center
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose.
References
- 1.Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: a typically cystic carcinoma of low malignant potential. Semin Diagn Pathol. 2000 Feb;17(1):66–80. [PubMed] [Google Scholar]
- 2.Adsay NV. Cystic neoplasia of the pancreas: pathology and biology. J Gastrointest Surg. 2008 Mar;12(3):401–404. doi: 10.1007/s11605-007-0348-z. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Pitman MB, Klimstra DS, editors. Tumors of the Pancreas. Washington DC: American Registry of Pathology; 2007. [Google Scholar]
- 4.Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002 Jan-Feb;9(1):35–40. doi: 10.1245/aso.2002.9.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Horisawa M, Niinomi N, Sato T, et al. Frantz’s tumor (solid and cystic tumor of the pancreas) with liver metastasis: successful treatment and long-term follow-up. J Pediatr Surg. 1995 May;30(5):724–726. doi: 10.1016/0022-3468(95)90701-7. [DOI] [PubMed] [Google Scholar]
- 6.Saiura A, Umekita N, Matsui Y, et al. Successful surgical resection of solid cystic tumor of the pancreas with multiple liver metastases and a tumor thrombus in the portal vein. Hepatogastroenterology. 2000 May-Jun;47(33):887–889. [PubMed] [Google Scholar]
- 7.Rebhandl W, Felberbauer FX, Puig S, et al. Solid-pseudopapillary tumor of the pancreas (Frantz tumor) in children: report of four cases and review of the literature. J Surg Oncol. 2001 Apr;76(4):289–296. doi: 10.1002/jso.1048. [DOI] [PubMed] [Google Scholar]
- 8.Seo HE, Lee MK, Lee YD, et al. Solid-pseudopapillary tumor of the pancreas. J Clin Gastroenterol. 2006 Nov-Dec;40(10):919–922. doi: 10.1097/01.mcg.0000225671.91722.10. [DOI] [PubMed] [Google Scholar]
- 9.Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005 Apr;29(4):512–519. doi: 10.1097/01.pas.0000155159.28530.88. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Kato K, Notohara K, et al. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001 Dec 1;61(23):8401–8404. [PubMed] [Google Scholar]
- 11.Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. The American journal of pathology. 2002 Apr;160(4):1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albores-Saavedra J, Simpson KW, Bilello SJ. The clear cell variant of solid pseudopapillary tumor of the pancreas: a previously unrecognized pancreatic neoplasm. Am J Surg Pathol. 2006 Oct;30(10):1237–1242. doi: 10.1097/01.pas.0000209849.97787.e0. [DOI] [PubMed] [Google Scholar]
- 13.Hav M, Lem D, Chhut SV, et al. Clear-cell variant of solid-pseudopapillary neoplasm of the pancreas: a case report and review of the literature. The Malaysian journal of pathology. 2009 Dec;31(2):137–141. [PubMed] [Google Scholar]
- 14.Tanino M, Kohsaka S, Kimura T, et al. A case of clear cell variant of solid-pseudopapillary tumor of the pancreas in an adult male patient. Annals of diagnostic pathology. Apr;16(2):134–140. doi: 10.1016/j.anndiagpath.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Hsu SM, Raine L, Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. American journal of clinical pathology. 1981 Jun;75(6):816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- 16.Zee SY, Hochwald SN, Conlon KC, Brennan MF, Klimstra DS. Pleomorphic pancreatic endocrine neoplasms: a variant commonly confused with adenocarcinoma. Am J Surg Pathol. 2005 Sep;29(9):1194–1200. doi: 10.1097/01.pas.0000164370.81132.25. [DOI] [PubMed] [Google Scholar]
- 17.Downes KA, Hart WR. Bizarre leiomyomas of the uterus: a comprehensive pathologic study of 24 cases with long-term follow-up. Am J Surg Pathol. 1997 Nov;21(11):1261–1270. doi: 10.1097/00000478-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Falleti J, Vita G, De Cecio R, et al. Symplastic glomus tumor: report of a challenging lesion with literature review. Pathology, research and practice. Jun 15;208(6):372–375. doi: 10.1016/j.prp.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Goh SG, Dayrit JF, Calonje E. Symplastic hemangioma: report of two cases. Journal of cutaneous pathology. 2006 Nov;33(11):735–740. doi: 10.1111/j.1600-0560.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 20.Berean K, Tron VA, Churg A, Clement PB. Mammary fibroadenoma with multinucleated stromal giant cells. Am J Surg Pathol. 1986 Nov;10(11):823–827. doi: 10.1097/00000478-198611000-00010. [DOI] [PubMed] [Google Scholar]