Abstract
In a widely cited study, Mattay et al. (2003) reported that amphetamine (0.25 mg/kg oral, or 17mg for a 68kg individual) impaired behavioral and brain indices of executive functioning, measured using the Wisconsin Card Sorting Task (WCST) and N-Back working memory task, in 6 individuals homozygous for the met allele of the val158met polymorphism in the catechol-O-methyltransferase (COMT) gene, whereas it improved executive functioning in 10 individuals homozygous for the more active val allele. We attempted to replicate their behavioral findings in a larger sample, using similar executive functioning tasks and a broader range of amphetamine doses. Over four sessions, n = 200 healthy normal adults received oral placebo, d-amphetamine 5mg, 10mg, and 20mg (average of 0.07, 0.15 and 0.29 mg/kg), under counterbalanced double-blind conditions, and completed WCST and N-back tests of executive functioning. Amphetamine had typical effects on blood pressure and processing speed but did not affect executive functioning. COMT genotype (val158met) was not related to executive functioning under placebo or amphetamine conditions, even when we compared only the homozygous val/val and met/met genotypes at the highest dose of amphetamine (20 mg). Thus, we were not able to replicate the behavioral interaction between COMT and amphetamine seen in Mattay et al. (Mattay et al., 2003). We discuss possible differences between the studies and the implications of our findings for the use of COMT genotyping to predict clinical responses to dopaminergic drugs, and the use of intermediate phenotypes in genetic research.
Keywords: COMT genotype, executive functioning, working memory, d-amphetamine, genetics, stimulant response
Introduction
Stimulants like d-amphetamine are used to treat disorders of executive functioning (e.g. attention-deficit/hyperactivity). Executive functioning is often used as an intermediate phenotype, which is a laboratory based measure of normal behavior that may be informative about the genetics of diseases characterized by executive dysfunction, such as ADHD and schizophrenia (Goldberg et al, 2003). Thus, genetic variants that modulate the effect of stimulants on executive functioning have important implications. In a widely cited study Mattay and colleagues (2003) found the val158met polymorphism of the catechol-O-methyltransferase (COMT) gene modulated d-amphetamine’s effects on executive functioning. In a within-subjects design, Mattay and colleagues administered oral placebo or 0.25 mg/kg d-amphetamine to healthy adults who completed the N-back test of working memory during fMRI scanning, and the Wisconsin Card Sorting Test (WCST) of set-shifting. Amphetamine worsened performance of met/met individuals, while mildly improving that of val/val individuals. Further, amphetamine reduced brain activity in working memory regions in val/val individuals (indicating increased cognitive efficiency), but increased activity for met/met individuals. This data were interpreted as suggesting that d-amphetamine improved executive functioning in val/val individuals, but impaired met/met individuals. However, that study was small (n=27) and has never been replicated. The current study provides an opportunity to replicate the behavioral results of Mattay et al. (2003) in a larger sample and a wider range of amphetamine doses.
Results of Mattay et al. (2003) were interpreted as being consistent with the inverted U hypothesis of pre-frontal cortex dopamine (DA), which posits executive functioning is optimal in a limited DA range, but poorer above and below those levels (Robbins & Arnsten, 2009). COMT enzymatically degrades DA, with the met allele resulting in lower COMT activity, and higher DA levels (Chen et al., 2004). Thus, COMT might be expected to affect baseline executive functioning (with met/met individuals showing better functioning than val/val individuals), and the effect of DA stimulants (with val/val individuals benefiting, while met/met individuals are adversely impacted). The relationship of COMT to executive functioning and stimulant responses has been supported in transgenic mice (Papaleo et al., 2008). However, findings in healthy humans are mixed. Although early studies suggested associations between COMT and executive functioning (Egan et al., 2001), a meta-analysis found no significant association in healthy adults (Barnett et al., 2008, 2011). We recently conducted the largest analysis to date (n = 2,659) of COMT and working memory in healthy adults, and found no relationship (Wardle et al., under review). However, DA challenges might reveal differences not evident at baseline, as exampled by the amphetamine by COMT interaction in Mattay et al. (2003). Thus, the current study examined both: 1. COMT and baseline executive functioning (during placebo), and 2. COMT and executive functioning at different amphetamine doses, in the first attempt to replicate Mattay et al. (2003).
In our study, 200 healthy adults completed the WCST and N-back after placebo, 5mg, 10mg and 20mg d-amphetamine (average of 0.07, 0.15 and 0.29 mg/kg -- under the Mattay protocol, 17mg would be the average dose for our participants), and were genotyped for COMT. Based on the meta-analyses of Barnett et al. (2008, 2011), we did not expect COMT to affect baseline executive functioning. However, following Mattay et al. (2003), we predicted amphetamine (20mg) would improve executive functioning in val/val individuals but impair met/met individuals.
Material and Methods
Study Design
Participants attended four sessions at which they received placebo, 5mg, 10mg or 20mg d-amphetamine in counterbalanced order under double-blind conditions, and completed the WCST and the N-Back. They were genotyped for the val158met polymorphism and responses to amphetamine were compared across genotypic groups. The WCST and N-Back were administered to a subset (n = 200) of participants in a larger study (n =400) examining acute responses to amphetamine. To date, the full sample has been used to conduct a genome-wide association study of genetic associations with the subjective and cardiovascular effects of amphetamine (Hart et al., 2012), and for replications of our own prior findings on candidate genes associated with acute responses to amphetamine (Hart et al., in press). The WCST and N-Back data from this sub-sample have not been previously reported.
Participants
We recruited 200 healthy Caucasian adults (101 male, 4% identifying as Hispanic ethnicity), ages 18–35, through advertisements and word-of-mouth. Screening consisted of physical examination, electrocardiogram, modified Structured Clinical Interview for DSM-IV (SCID; First et al., 1996), and self-reported health and drug use history. Inclusion criteria were: Body Mass Index 18–26, no current prescription drug use, no medical conditions/contraindications to amphetamine, not pregnant, nursing, or trying to become pregnant, no past year DSM-IV Axis I Disorders or lifetime history of drug dependence, mania or psychosis, some previous recreational drug use, no previous adverse amphetamine reactions, smoking <10 cigarettes per week and drinking <3 cups of coffee per day, English speaking, at least high school education, not currently performing night shift work. Women not on hormonal birth control were scheduled only during the follicular phase of the menstrual cycle (White et al., 2002), and all female participants provided urine samples for pregnancy tests before each session. Participants were primarily in their twenties (M = 23.3, SD = 3.3) with at least a college degree (64%) and light to moderate recreational drug use (see Table 1 for demographic characteristics). Participants were paid $200 for completing all study procedures.
Table 1.
Demographics and drug use history for whole sample (N = 193) by genotype.
| M(SD) or n(%) by genotype
|
ANOVA or χ2 Test | M(SD) or n(%)total sample (N = 193) | |||
|---|---|---|---|---|---|
| Val/val | Val/met | Met/met | |||
| Demographics | |||||
| Age | 23.3 (3.5) | 23.1 (3.3) | 23.8 (3.1) | F(2, 190) = .70, p = .50 | 23.3 (3.3) |
| BMI | 22.7 (2.0) | 22.5 (2.3) | 22.5 (1.9) | F(2, 190) = .16, p = .85 | 22.6 (2.1) |
| Gender | |||||
| Male | 34 (61%) | 45 (51%) | 22 (46%) | χ2(1, 193) = 2.50, p = .29 | 101 (52%) |
| Female | 22 (39%) | 44 (49%) | 26 (54%) | 93 (48%) | |
| Education | |||||
| HS/GED | 2 (4%) | 1 (1%) | 1 (2%) | χ2(6, 193) = 3.62, p = .73 | 4 (2%) |
| Some Coll. | 19 (34%) | 31 (35%) | 15 (31%) | 65 (34%) | |
| BA/BS | 29 (52%) | 47 (53%) | 30 (63%) | 106 (55%) | |
| Graduate | 6 (11%) | 10 (11%) | 2 (4%) | 18 (9%) | |
| Current Drug Use | |||||
| Alcoholic drinks/week | 5.4 (4.7) | 6.0 (5.0) | 5.0 (5.3) | F(2, 190) = .43, p = .65 | 5.7 (4.8) |
| Cigarettes/week | 1.1 (2.6) | 1.1 (2.2) | 0.9 (2.2) | F(2, 190) = 1.8, p = .84 | 1.0 (2.3) |
| Cannabis uses/month | 2.1 (5.8) | 1.9 (4.8) | 1.3 (3.8) | F(2, 190) = .37, p = .70 | 1.8 (4.9) |
| Lifetime Drug Use | |||||
| Cannabis | |||||
| Never | 11 (20%) | 16 (18%) | 12 (25%) | χ2(8, 193) = 11.33, p = .18 | 39 (20%) |
| 1–10x | 25 (45%) | 26 (29%) | 15 (31%) | 66 (34%) | |
| 11–50x | 6 (11%) | 25 (28%) | 11 (23%) | 42 (22%) | |
| 51–100x | 4 (7%) | 2 (2%) | 3 (6%) | 9 (5%) | |
| >100x | 10 (18%) | 20 (23%) | 7 (15%) | 37 (19%) | |
| Tranquilizers | |||||
| Never | 51 (93%) | 79 (89%) | 43 (90%) | χ2(4, 193) = 3.89, p = .42 | 173 (90%) |
| 1–10x | 4 (7%) | 7 (8%) | 5 (10%) | 16 (8%) | |
| 11–50x | 0 (0%) | 3 (3%) | 0 (0%) | 3 (2%) | |
| Stimulants | |||||
| Never | 41 (73%) | 60 (67%) | 36 (75%) | χ2(8, 193) = 13.14, p = .11 | 137 (71%) |
| 1–10x | 10 (18%) | 23 (26%) | 7 (15%) | 40 (21%) | |
| 11–50x | 4 (7%) | 5 (6%) | 1 (2%) | 10 (5%) | |
| 51–100x | 0 (0%) | 0 (0%) | 3 (6%) | 3 (2%) | |
| >100x | 1 (2%) | 1 (1%) | 1 (2%) | 3 (2%) | |
| Opiates | |||||
| Never | 49(88%) | 64 (72%) | 40 (83%) | χ2(6, 193) = 8.36, p = .21 | 153 (79%) |
| 1–10x | 5(9%) | 19 (21%) | 5 (10%) | 29 (15%) | |
| 11–50x | 2(4%) | 4 (5%) | 3 (6%) | 9 (5%) | |
| 51–100x | 0(0%) | 2 (2%) | 0 (0%) | 2 (1%) | |
| Hallucinogens | |||||
| Never | 40 (73%) | 50 (56%) | 35 (73%) | χ2(6, 193) = 7.29, p = .30 | 125 (65%) |
| 1–10x | 13 (24%) | 34 (38%) | 10 (21%) | 57 (30%) | |
| 11–50x | 2 (4%) | 4 (5%) | 2 (4%) | 8 (4%) | |
| 51–100x | 0 (0%) | 1 (1%) | 1 (2%) | 2 (1%) | |
| Other Drugs | |||||
| Never | 49 (89%) | 81 (91%) | 43 (90%) | χ2(4, 193) = 3.12, p = .54 | 173 (90%) |
| 1–10x | 4 (7%) | 8 (9%) | 4 (8%) | 16 (8%) | |
| 11–50x | 2 (4%) | 0 (0%) | 1 (2%) | 3 (2%) | |
Procedure
Participants first attended an orientation during which they gave informed consent, practiced cognitive tasks, and gave a blood sample for genotyping. To enhance blinding, participants were told they might receive a stimulant, sedative, alcohol, or placebo during experimental sessions. All procedures were approved by The University of Chicago Institutional Review Board, and carried out in accordance with the Declaration of Helsinki.
The four individually-run experimental sessions were conducted from 9am to 1pm and separated by at least 48 hours. Participants were asked to maintain normal caffeine and nicotine intake 24 hours before and 12 hours after sessions, fast for 12 hours before sessions, and refrain from recreational and over-the-counter drugs for 24 hours before and 12 hours after sessions. Compliance was verified using breath alcohol (Alcosensor III, Intoximeters Inc., St. Louis, MO) and urine tests (ToxCup, Branan Medical Corporation, Irvine, CA) upon participant arrival. After verification, participants received a standard light breakfast, and completed baseline measures of mood, subjective and cognitive drug effects. At 9:30am, participants ingested an opaque capsule containing either d-amphetamine tablets (Mallinkrodt, MO, USA) totaling 5, 10 or 20 mg, with dextrose filler, or dextrose only (placebo). Additional measures (mood, subjective drug effects, cardiovascular, and cognitive) were obtained throughout, but we report only cardiovascular variables for comparison with Mattay et al. (Mattay et al., 2003), and a processing speed task, the Digit-Symbol Substitution Task (DSST), which measures the typical cognitive effects of amphetamine. Ninety minutes following the capsule, during peak drug effect, participants completed the WCST and N-back, in counterbalanced order. At 1pm participants left the laboratory. After all four sessions, participants were debriefed and paid.
Genotyping
DNA was extracted from blood at the General Clinical Research Center at the University of Chicago. Genotyping of rs4680 was performed according to the procedures described previously in Hart et al. (2012). We verified each individual’s self-reported sex and ancestry against our assessment based on genotyping; ancestry was assessed using the SmartPCA component of EIGENSOFT (Patterson et al., 2006). All individuals included in the final sample were verified to be Caucasian. Seven individuals did not pass quality control procedures, leaving 193 individuals with genotypic data suitable for analysis.
Measures
Digit-Symbol Substitution Task (DSST)
The DSST is a pencil and paper test in which participants are required to substitute a series of symbols for a series of numbers, based on a key (Wechsler, 1997). Participants are given 90s to finish as many items as they can. Although this task may involve working memory slightly, it is primarily dependent upon processing speed (Joy et al., 2004). One of the most reliable cognitive effects of amphetamine is an improvement in speed of processing (Silber et al., 2006). This task was not included in Mattay et al., but is included here to demonstrate the robust cognitive effects of amphetamine in our protocol. We have already reported that in the larger sample from which this subset is drawn, COMT did not alter DSST performance, either at baseline or in response to amphetamine (Hart et al., in press). Thus we do not further address the relationship between COMT and the DSST in this report, but only use the DSST to confirm that the typical cognitive effects of amphetamine are present in this sub-sample.
The DSST was given once 20min prior to administration of the capsule, then at 30, 60, 90, 150 and 180min after capsule administration. We used eight alternate forms in mixed order to reduce practice effects. To provide a single summary score for the effect of drug on the DSST at each session, we calculated the area under the curve (AUC) for DSST scores each session relative to that session’s baseline (e.g. Hamidovic et al., 2010). One individual was missing DSST data, leaving n = 192 individuals for analysis.
Cardiovascular
We measured blood pressure and heart rate using portable monitors (Life Source, A&D Company, Tokyo, Japan) 20min before the capsule and 30, 60, 90, 150 and 180min after the capsule. For consistency with Mattay et al. (Mattay et al., 2003), we report change from baseline (−20min) to the 60min time point.
(Berg) Wisconsin Card Sorting Task (b-WCST)
We used a computerized variant of the Wisconsin Card Sorting Task in the Psychology Experiment Building Language (PEBL; Piper et al., 2012). In this task, participants view a computer screen displaying four key “cards.” On each trial a fifth card must be matched to one of the four key cards on the basis of a rule (color, shape, or number of shapes on the card). After the participant has made five correct matches, the rule changes. Participants are not informed what the rules are or when they change, but must determine these by trial and error. Trials continue until the participant completes 128 trials or nine correct series. Consistent with Mattay et al. (Mattay et al., 2003), primary outcome was percentage of “perseverative errors”: the number of trials on which the participant matched based on the immediately previous (rather than current) rule, divided by total number of trials. Fourteen individuals with genotypic data were missing b-WCST data, and were removed from analysis. Two had outlier values (>3 SD from the mean on number of trials required to complete the task), and were also removed, leaving n = 177 participants for b-WCST analyses.
N-back
Participants performed 0-, 1-, 2-, and 3-back versions of the N-back working memory task implemented in PEBL (Piper et al., 2012). This task consisted of 120 trials on which a number between 1 and 4 was presented randomly in one corner of a large diamond-shaped square. Participants responded by pressing a key, as follows: For n = 0 (i.e., 0-back), they pressed the key corresponding to the number on the screen. For n = 1 (i.e., 1-back), participants pressed the key corresponding to the number presented on the trial before the current one, and for n =2, two trials before the current one, and 3-back three trials before the current one, producing a working memory test of increasing difficulty. Twenty-item blocks of 1-, 2-, and 3-back were presented in randomized order, separated by 0-back blocks to decrease participant fatigue. Following Mattay et al. (2003), primary N-back outcome was percentage of correct responses, and we only analyzed 3-back responses, as only this most demanding condition showed COMT effects in the previous paper. Twenty-four individuals were missing N-back data, and were removed from analysis. Careful inspection indicated that 70 other participants did not perform the task as required: instead of responding to each stimulus by indicating the value of the nth stimulus before, these subjects waited for n stimuli to pass before making another response. This was evident in the data: each response is interspersed with n timeouts, where n corresponds to the n on the N-back. Given that this pattern appears for these participants on even the relatively undemanding 1-back block, this likely indicates a misunderstanding of the directions. Data from these participants were excluded from all analyses, leaving n = 99 participants for N-back analyses.
Statistical Analyses
Met/met, met/val and val/val carriers were first compared on demographic measures using ANOVA for continuous and chi-square for categorical measures to test for possible covariates. We then confirmed the effect of amphetamine on AUC DSST scores, blood pressure and heart rate using within-subject ANOVA with dose as an independent variable, to establish that our doses effectively induced amphetamine-typical cognitive and cardiovascular changes. We give additional detail on blood pressure and heart rate at the 60 min. post capsule time point, for comparability with Mattay et al. (2003). We then conducted our primary analyses testing the effect of amphetamine and genotype on executive functioning. Our primary analyses first examined whether COMT genotype related to performance under placebo conditions (similar to previous baseline functioning studies), and second whether COMT genotype moderated response to amphetamine (similar to the Mattay study). All analyses used a regression approach to ANOVA, in which contrast coding is used to examine specific hypotheses (see Chapter 11 of Judd et al., 2009). All analyses of genotype used an additive model of the contribution of met alleles to cognitive functioning, i.e. a linear val/val < val/met < met/met contrast. For analyses of the placebo session, we used placebo session scores as the dependent variable in this regression. For analyses involving dose-response curves we tested a complete set of orthogonal polynomial dose contrasts, entering each contrast (linear, quadratic and cubic) as the dependent variable in the regression in turn (this produces identical results to within-subject contrast analyses in a classic within-subject ANOVA). We examined a complete set of dose contrasts because we hypothesized val/val individuals would show a monotonic improvement with increasing doses (a linear effect), while lower doses might produce improvement in met/met individuals, but higher doses would produce impairments (a quadratic effect). Our secondary analyses more closely followed Mattay et al. (2003), contrasting only the homozygous groups (val/val and met/met), and using difference scores contrasting the 20mg amphetamine dose, which most closely resembled the dose used in the previous study, to placebo as the dependent variables in the regression. All analyses examined possible sex differences, but there were no sex differences in the effects of amphetamine, and sex did not significantly moderate the effects of COMT, so these analyses are reported only in the Supporting Information for completeness. Effect sizes are reported as unstandardized estimates (B) with standard errors (SE). Of note, there were effects of repeated assessment on both the WCST and N-Back, with performance improving significantly over sessions. We performed additional analyses in which we regressed out the effect of session, by saving the residuals from an ANOVA with session as the independent variable, and then performing our primary analyses on those residuals. The results did not differ meaningfully from those obtained by analyzing the original metric, and so for ease of interpretation and comparison, we report the results in the original metrics.
Results
Genotype frequencies and associations
The sample as a whole had 56 val/val (29%), 89 val/met (46%) and 48 met/met (25%) individuals. The sub-samples of individuals with complete data for the WCST and N-back respectively had 53 val/val (30%), 79 val/met (45%), and 45 met/met (25%) and 31 val/val (31%), 44 val/met (45%), and 24 met/met (24%). Each of these was in Hardy-Weinberg equilibrium. The allele frequencies are comparable to those observed in the HapMap CEU panel (val allele 47.8% in CEU, 47.9% in our sample; met allele 52.2% in CEU, 52.1% in our sample). The genotypic groups did not differ on any demographic measures (whole sample analyses are reported in Table 1, but the sub-samples used for each task also showed no significant associations between genotype and demographics).
Typical amphetamine effects
Amphetamine robustly and dose-dependently increased DSST AUC scores, indicating a cognitive effect of enhanced processing speed; see Fig. 1a; linear drug effect B = 50.04, SE = 7.08, t(191) = 7.07, p < .001. Further, Fig. 1b illustrates the large dose dependent effect of amphetamine on systolic blood pressure over the time course of the study (results for other cardiovascular variables were effectively similar). Of note, our window for executive functioning testing (as shown in Fig. 1b) was similar to Mattay et al. (2003), who administered the WCST at 90 min., and the N-back at 120 min. post drug administration. Following Mattay et al. (2003), we also specifically examined amphetamine effects on blood pressure and heart rate at 60 min. vs. baseline.
Fig. 1.
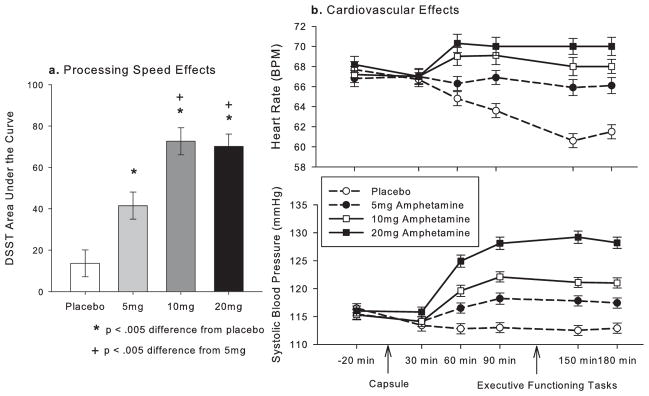
Amphetamine had typical dose dependent effects on (a) processing speed, shown here as area under the curve of Digit-Symbol Substitution Task (DSST) scores (SEM) and (b) Heart rate, shown here in the top panel as beats per minute (BPM) and blood pressure, shown here as systolic blood pressure in mmHg at each time-point (SEM).
Amphetamine increased systolic blood pressure at 60 min.; significant linear effect of drug dose, B = 10.28, SE = 11.71, t(192) = 12.19, p < .001. For comparison with Mattay et al., we provide the mean mmHg at 60 min. for the highest dose vs. placebo: 20mg = 124 (SD = 14.96), PL = 112 (SD = 12.01). The drug also increased diastolic blood pressure and heart rate at 60 min.; significant linear effect of drug on diastolic blood pressure, B = 6.62, SE = 8.75, t(192) = 10.50, p < .001 (mean mmHG at 60 min: 20mg = 79.39 [SD = 9.42], PL = 72.56, [SD = 7.65]), and heart rate: B = 4.28, SE = 8.35, t(192) = 7.12, p < .001 (mean BPM at 60 min: 20mg = 70.10 [SD = 11.61], PL = 64.61 [SD = 9.39]).
Executive functioning
Performance on the b-WCST and N-back was correlated, with fewer perseverative errors on the b-WCST predicting more correct answers on the N-back (r = −0.25 p = 0.01). This correlation was not large, but this might be expected given that these measures are intended to tap different aspects of executive functioning, i.e. cognitive flexibility vs. working memory.
b-WCST
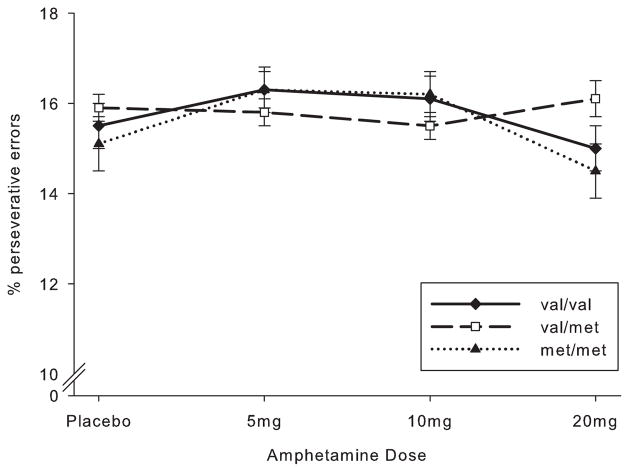
Examining overall drug effects on executive functioning, the 5mg and 10mg doses slightly increased perseverative errors compared to the placebo and 20mg dose (quadratic drug effect, B = .005, SE = .002, t[175] = 2.17, p = 0.03). However, follow up t-tests comparing the 5mg and 10mg amphetamine doses to placebo were not significant, making this quadratic effect difficult to interpret. Examining genotype, consistent with the previous meta-analysis of COMT and baseline WCST performance (Barnett et al., 2008, Barnett et al., 2011), the three genotypic groups did not differ on b-WCST performance under placebo conditions; B = −.004, SE = .007, t(175) = .56, p = .58. Further, COMT genotype did not modulate the effects of amphetamine on performance (linear or quadratic; see Fig. 2). In a separate analysis, we compared the effects at the highest dose of d-amphetamine (20 mg vs. placebo) only in the val/val and met/met groups, providing a more direct comparison with Mattay et al. (2003), but again no genotype-drug interactions were observed; B < .001, SE = .01, t(97) = 0.02, p = .99.
Fig. 2.
Amphetamine did not alter percentage of perseverative errors (SEM) on the Wisconsin Card Sorting Task (WCST), and COMT genotypes did not differ either under placebo conditions or in response to the drug.
N-back
d-Amphetamine did not significantly alter 3-back performance overall. Examining genotype, consistent with the previous meta-analysis of COMT and baseline N-back performance (Barnett et al., 2008, Barnett et al., 2011), and our recent large study (Wardle et al., under review) genotypic groups did not differ on 3-back performance under placebo conditions; B = .04, SE = .06, t(98) = 0.70, p = .48. There were also no interactions between COMT genotype and linear responses to the drug on this measure (see Fig. 3). There was a nominally significant COMT genotype x quadratic drug interaction, suggesting val/val carriers might show greater improvements in performance than met/met carriers at the intermediate (5mg and 10mg) doses (see Figure 2); B = −.12, SE = .05, t(98) = 2.38, p = .02. However, this finding does not survive correction for multiple testing. Further, post-hoc tests examining the change between PL and 5mg and PL and 10mg showed no significant differences by genotype for either dose; B = −.12, SE = .07, t(98) = −1.66, p = .10 for 5mg and B = −.09, SE = .07, t(98) = 1.34, p = .18 for 10mg. In a separate analysis, we compared the effects at the highest dose of d-amphetamine (20 mg vs. placebo) only in the val/val and met/met groups, providing a more direct comparison with Mattay et al. (2003), but again no genotype-drug interactions were observed; B = .03, SE = .07, t(54) = 0.45, p = .66.
Fig. 3.
Amphetamine did not alter percentage of correct responses (SEM) on the 3-back condition of the N-back working memory task, and COMT genotypes did not differ either under placebo conditions or in response to the drug.
Discussion
The val158met polymorphism in COMT was not related to either baseline performance (under placebo conditions) or effects of d-amphetamine on two executive functioning tasks, the b-WCST and the N-back. These findings are not consistent with the expected effects of COMT based on the inverted U hypothesis (Robbins & Arnsten, 2009; Egan et al. 2001), i.e. that individuals with the COMT met/met genotype (associated with higher dopamine levels in the PFC) would exhibit better baseline performance on the cognitive tasks compared to val/val individuals, and that performance of met/met individuals would be worsened by amphetamine, while the performance of val/val individuals would be improved. Further, our results thus do not replicate those of Mattay et al. (2003), who found that amphetamine significantly worsened the executive functioning performance of met/met individuals, while improving that of val/val individuals. We failed to observe such an interaction between COMT and responses to d-amphetamine on the b-WCST or N-back in the present study, even though we tested many more subjects, included multiple doses of the drug compared to a single dose in the initial report by Mattay et al. (2003), and observed clear cardiovascular and cognitive effects of amphetamine in our sample.
We also did not observe an effect of COMT at baseline, or a main effect of amphetamine on executive functioning, but these results were less unexpected. The lack of an effect for COMT at baseline was consistent with a recent meta-analysis suggesting a smaller than expected, or even null effect of COMT on baseline executive functioning (Barnett et al., 2008, Barnett et al., 2011), and with our recent large population-based study of COMT and N-Back performance that detected no effect of COMT on working memory at baseline (Wardle et al., under review). The lack of a main effect of d-amphetamine on executive functioning measures is also not entirely unexpected, as the effects of amphetamine on executive functioning in healthy normal adults appear less robust than those on processing speed, and may be more dependent upon baseline performance and other factors (Barch, 2004). Indeed, in one review of amphetamine and executive functioning, only a minority of studies found a main effect of amphetamine (Smith & Farah, 2011).
There were minor differences between our study and Mattay et al. (2003) and we cannot rule out the possibility that they influenced the discrepancy in our results. First, there may have been subtle demographic differences in the subject samples, related to drug use history or other inclusion/exclusion criteria. For example, Mattay et al. excluded all smokers and any individuals with previous amphetamine experience, while our sample included some light smokers and individuals who had tried amphetamine (29% had tried some type of stimulant drug in their lives, but only 9% had tried a stimulant drug more than 10 times). However, both studies used essentially healthy young adults without extensive drug use histories or psychiatric problems. Further, when we examined the subset of non-smokers with no previous stimulant use in our data (n = 94 for b-WCST, n = 52 for N-back), we still did not replicate the pattern seen in the Mattay study (data not shown). Second, Mattay et al. (2003) administered a dose of amphetamine adjusted for body weight, whereas we used fixed doses of 5mg, 10mg and 20mg. However, the Mattay et al. dosing protocol would have administered 17mg d-amphetamine to a 68kg individual (the average weight in our study), which is comparable to the highest dose tested in our study (20mg). Third, there may have been differences in the tasks. For example, it may be more difficult to perform the tasks in an fMRI scanner than in a quiet room, or there may have been differences in task parameters. However, rate of errors in the two studies was fairly comparable. Thus, the initial behavioral effects seen with 6 met/met and 10 val/val participants were not replicable in our sample with 45 met/met and 53 val/val participants.
There are some limitations of the current study. One of the primary ones is the number of participants who apparently did not understand the N-Back instructions. This is especially surprising given that participants were trained on tasks at the orientation prior to the drug sessions. This phenomenon was only evident when the raw data was carefully inspected, and it is unclear whether it has been encountered in other studies (or whether individuals displaying this “skipping” pattern were simply assumed to have difficulty with the task, based on total correct scores). This problem would be unique to the “continuously updating” version of the N-Back used here (and also in Mattay et al, 2003). Of note, results were similar whether these participants were included (data not shown) or excluded, and even with these participants excluded our sample size was approximately three times as large as that examined in Mattay et al. Another potential limitation is the within-participant design. There were effects of session on performance, which may have reduced sensitivity to either the effects of amphetamine or of COMT. We did carefully counterbalanced dose orders and conducted additional analyses regressing out the effect of session that produced substantively similar results (data not shown). Although Mattay et al (2003) was also a within-subject design, participants did complete the tasks more times in our study (four times vs. twice).
Taken together our results suggest that the modulation by COMT of amphetamine’s behavioral effects on executive functioning seen in the initial Mattay study may have been a false positive. Alternative possibilities are that this effect is either smaller than expected based on the Mattay study, or only evident under fairly specific conditions or in fairly specific populations. Any of these possibilities suggest that COMT genotype is not likely to be useful for prediction of stimulant treatment outcomes on the behavioral measures typically used in clinical settings. Further, they suggest caution with regard to the use of intermediate phenotypes, and reinforce doubts about the effect sizes that can realistically be ascribed to individual loci (Hart et al 2012). It has suggested that intermediate phenotypes may be more tractable than disease states, producing larger genetic associations and having simpler genetic bases (Goldman & Ducci, 2007). The usefulness of intermediate phenotypes will be determined by the weight of evidence across many studies. But this case provides an example of a careful examination of a biologically-based intermediate phenotype in a larger sample that failed to replicate original results. However, our results do not speak to the brain indices of executive functioning used in Mattay et al. (2003), which might still replicate in future studies. Additionally, some critiques of research in this area have noted that COMT may more strongly influence tasks with high manipulation demands, rather than memory storage demands, and that standard neuropsychological tasks like the N-Back involve components of both (Goldman et al., 2009). This may decrease their sensitivity to COMT variation. More sensitive techniques that combine fMRI with pharmacological challenges, or that focus more tightly on cognitive skills directly influenced by COMT, may still reveal differences in amphetamine’s effects on executive functioning that are attributable to COMT.
Supplementary Material
Acknowledgments
The National Institute on Drug Abuse supported this work through grant R01 DA021336 and R21 DA024845 to AAP and R01 DA02812 to HdW. MCW and ABH were supported by a National Institute on Drug Abuse Training grant, T32 DA007255.
References
- Barch DM. Pharmacological manipulation of human working memory. Psychopharmacology (Berl) 2004;174:126–135. doi: 10.1007/s00213-003-1732-3. [DOI] [PubMed] [Google Scholar]
- Barnett J, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Barnett J, Scoriels L, Munafò MR. Erratum. Biol Psychiatry. 2011;69:389. [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences. 2001;98:6917. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Strutured clinical interview for DSM-IV axis I disorders. Biometrics Research Department; New York: 1996. [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana B, Goldman D, Weinberger DR. Executive subprocesses in working memory: Relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldman D, Ducci F. Deconstruction of vulnerability to complex diseases: enhanced effect sizes and power of intermediate phenotypes. ScientificWorldJournal. 2007;7:124–130. doi: 10.1100/tsw.2007.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Weinberger DR, Malhotra AK, Goldberg TE. The role of COMT Val158Met in cognition. Biol Psychiatry. 2009;65:e1–e2. doi: 10.1016/j.biopsych.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Palmer AA, de Wit H. Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine. Psychiatr Genet. 2010;20:85–92. doi: 10.1097/YPG.0b013e32833a1f3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, de Wit H, Palmer AA. Candidate gene studies of a promising intermediate phenotype: Failure to replicate. Neuropsychopharmacology. doi: 10.1038/npp.2012.245. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AB, Engelhardt BE, Wardle MC, Sokoloff G, Stephens M, de Wit H, Palmer AA. Genome-wide association study of d-amphetamine response in healthy volunteers identifies putative associations, including cadherin 13 (CDH13) PLoS ONE. 2012;7:e42646. doi: 10.1371/journal.pone.0042646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy S, Kaplan E, Fein D. Speed and memory in the WAIS-III Digit Symbol-Coding subtest across the adult lifespan. Archives of Clinical Neuropsychology. 2004;19:759–767. doi: 10.1016/j.acn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Judd CM, McClelland GH, Ryan CS. Data analysis: A model comparison approach. 2. Routledge/Taylor & Francis Group; New York, NY, US: 2009. [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol-O-methyltransferase Val158Met genotype and individual variation in the brain response to amphetamine. Proceedings of the National Academy of Sciences. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of Catechol-O-Methyltransferase in cognition and stress reactivity in mice. The Journal of Neuroscience. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS genetics. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper B, Li V, Eiwaz M, Kobel Y, Benice T, Chu A, Olsen R, Rice D, Gray H, Mueller S. Executive function on the Psychology Experiment Building Language tests. Behavior Research Methods. 2012;44:110–123. doi: 10.3758/s13428-011-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber BY, Croft RJ, Papafotiou K, Stough C. The acute effects of d-amphetamine and methamphetamine on attention and psychomotor performance. Psychopharmacology (Berl) 2006;187:154–169. doi: 10.1007/s00213-006-0410-7. [DOI] [PubMed] [Google Scholar]
- Smith ME, Farah MJ. Are prescription stimulants “smart pills”? The epidemiology and cognitive neuroscience of prescription stimulant use by normal healthy individuals. Psychol Bull. 2011;137:717–741. doi: 10.1037/a0023825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, de Wit H, Penton-Voak IS, Lewis G, Munafò MR. Lack of association between COMT and working memory in a population-based cohort of healthy young adults. doi: 10.1038/npp.2013.24. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. Harcourt Assessment; San Antonio, TX: 1997. (WAIS-III) [Google Scholar]
- White TL, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.