Abstract
Alzheimer's disease (AD) patients have reduced brain acetylcholine and reversing this deficit yields clinical benefits. In this study we explored how increased cholinergic tone impacts cell bioenergetics, which are also perturbed in AD. We treated SHSY5Y neuroblastoma cells with carbachol, a cholinergic agonist, and tested for bioenergetic flux and bioenergetic infrastructure changes. Carbachol rapidly increased both oxidative phosphorylation and glycolysis fluxes. ATP levels rose slightly, as did cell energy demand, and AMPK phosphorylation occurred. At least some of these effects depended on muscarinic receptor activation, ER calcium release, and ER calcium re-uptake. Our data show that increasing cholinergic signaling enhances cell bioenergetics, and reveal mechanisms that mediate this effect. Phenomena we observed could potentially explain why cholinesterase inhibitor therapy increases AD brain glucose utilization and N-acetyl aspartate levels. The question of whether cholinesterase inhibitors have a disease modifying effect in AD has long been debated; our data suggest a theoretical mechanism through which such an effect could potentially arise.
Keywords: Acetylcholine, Alzheimer's disease, bioenergetics, carbachol, glycolysis, mitochondria
INTRODUCTION
Acetylcholine levels are reduced in the brain of Alzheimer's disease (AD) patients [1–4]. For unknown reasons, certain anatomically defined cholinergic neuron subgroups excessively degenerate [5]. The activity of choline acetyltransferase, which combines acetyl coenzyme A with choline, is less than normal [6]. Although choline uptake by surviving neurons is not reduced, perturbed energy metabolism could affect the acetyl coenzyme A pool and through this effect indirectly slow acetylcholine synthesis [7, 8].
As an association between hypocholinergic states and encephalopathy has long been recognized [9, 10], investigators proposed several decades ago that increasing brain cholinergic tone might symptomatically benefit AD patients [11]. Targeting the cholinergic system was subsequently attempted using different strategies. Although adding choline to the diet did not show a clinical effect [12], cholinesterase inhibitors clearly and cholinergic agonists possibly improve cognitive performance [13–17]. Partly because of systemic side effects, no cholinergic agonist is currently approved for clinical use. Cholinesterase inhibitor side effects, on the other hand, show an acceptable profile and these agents now constitute the largest class of drugs used to treat AD patients. Cholinesterase inhibitors boost brain acetylcholine levels by blocking its enzymatic degradation within synapses. Four cholinesterase inhibitors have received regulatory approval for use in AD patients, and nutraceutical products that contain a cholinesterase inhibitor compound are available [18, 19].
While the logic of reversing the AD cholinergic deficit to treat AD symptoms is straightforward, many of this intervention's molecular consequences have not been extensively studied. Because relationships exist between cholinergic signaling and cell bioenergetics [8, 20], and brain bioenergetics are perturbed in AD [21], we examined how increasing cholinergic tone specifically affects neuronal cell bioenergetic fluxes and infrastructure.
METHODS
Undifferentiated SH-SY5Y cells, which express muscarinic and nicotinic cholinergic receptors [22, 23], were expanded at 37°C, 5% CO2 in pyruvate-free DMEM containing 25 mM glucose (catalogue number 11965-092, Invitrogen) and supplemented with 10% fetal bovine serum (catalogue number 12203 C, Sigma) and 1% of a penicillin-streptomycin stock (catalogue number 30-001-CI, Fisher Scientific). SH-SY5Y ρ0 cells were expanded at 37°C, 5% CO2 in SH-SY5Y growth medium that also contained 100 μg/ml sodium pyruvate and 50 μg/ml uridine [24].
For bioenergetic flux analyses, approximately 80,000 cells were used to seed the wells of XF cell culture microplates (Seahorse Bioscience, Billerica, MA). A standard manufacturer-recommended two-step seeding procedure was utilized. After achieving cell adherence, microplates were placed overnight in a 37°C, 5% CO2 incubator. On the analysis day, the incubation medium for each well was aspirated, the adherent cells were washed, and cells were placed in serum-free, pyruvate-free, buffer-free DMEM containing either 2.5 mM or 25 mM glucose. After the switch to unbuffered medium, the microplates were placed in a 37°C, non-CO2 incubator for 1 h and then transferred to the microplate stage of a Seahorse XF24 flux analyzer (Seahorse).
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) readings were taken using a 2 min mix, 1 min wait, and 2 min read cycling protocol. Carbachol, a cholinergic agonist that stimulates both muscarinic and nicotinic acetylcholine receptors, was injected into the wells to a final concentration of 100 μM in order to determine the effects of increased cholinergic tone on OCR and ECAR values. To provide further information into mechanisms that mediate these effects, additional compounds were injected at particular points. Atropine, a muscarinic antagonist, was used at a concentration of 100 μM. Mecamylamine, a nicotinic antagonist, was used at a concentration of 100 μM. 2-aminoethoxydiphenyl borate (2-APB), an inositol triphosphate (IP3) receptor (IP3 R) inhibitor, was used at a concentration of 200 μM. Thapsigargin, which facilitates endoplasmic reticulum (ER) calcium release and prevents the return of calcium to the ER by ATP-dependent smooth endoplasmic reticulum channel (SERCA) pumps, was used at concentrations of 0.3 μM and 3 μM [25–27].
In order to distinguish mitochondrial from cell non-mitochondrial oxygen consumption, we injected a mixture of rotenone and myxothiazol in order to achieve working concentrations of 1 μM rotenone and 2 μM myxothiazol. When specified, the non-mitochondrial OCR (the rate after rotenone/myxothiazol) was subtracted from the total OCR to provide the true mitochondrial OCR. In some experiments FCCP, an ionophore that depolarizes the mitochondrial membrane potential and induces a maximum OCR state, was used at a 0.3 μM concentration.
Protein lysates were prepared using the “mammalian protein extraction reagent” (M-PER) according to the manufacturer's instructions (Thermo Scientific, Rockford, IL). Activity of AMP kinase (AMPK), which regulates and is regulated by cell energy status [28], was estimated by measuring its phosphorylation status by western blot. A primary antibody to phospho-AMPK (1 : 1000 dilution; Cell Signaling Technology) was used. Primary antibody binding was revealed using a horseradish peroxidase-conjugated secondary antibody (1 : 2000 dilution; Cell Signaling Technology) and Supersignal West Femto Maximum Sensitivity Substrate (Thermo Scientific). Densitometry was performed using a ChemiDoc XRS with Quantity One software (BioRad, Hercules, CA). To ensure equivalent protein loading, blots were subsequently stained with a primary antibody to actin (1 : 200 dilution; Santa Cruz Biotechnology).
ATP levels from cell homogenates were measured using an ATP Bioluminescent Assay Kit (Sigma-Aldrich) according to the manufacturer's instructions. This is a luminescence-based assay; luminescence values were determined using a Tecan Infinite M200 plate reader.
OCR-ECAR data are presented as absolute rates and as percent changes from a stable baseline. For comparisons of data points between cells receiving active versus control treatments, group means were compared by two-way Student's t-tests (for comparisons of two groups), or by one-way ANOVA followed by Fisher's least significant difference post hoc testing (for comparisons of three groups) with p values less than 0.05 considered significant. For comparisons of the magnitude-of-rate change that occurred following injection of metabolism-altering compounds, the mean of the percent change for each group was determined and group means were compared by two-way Student's t-tests (for comparisons of two groups), or by one-way ANOVA followed by Fisher's least significant difference post hoc testing (for comparisons of three groups) with p values less than 0.05 considered significant. Where specified, paired Student's t-tests were used to compare group means for a parameter before and after a particular treatment was administered, and when applied p values less than 0.05 were considered significant. For the immunochemistry studies, relative density values for each protein analyzed were summarized by average and standard error. Means were compared by two-way Student's t-tests, with p values less than or equal to 0.05 considered significant. For ATP measurements, values from individual samples belonging to a group were summarized by average and standard error. Means were compared by two-way Student's t-tests, with p values less than 0.05 considered significant.
RESULTS
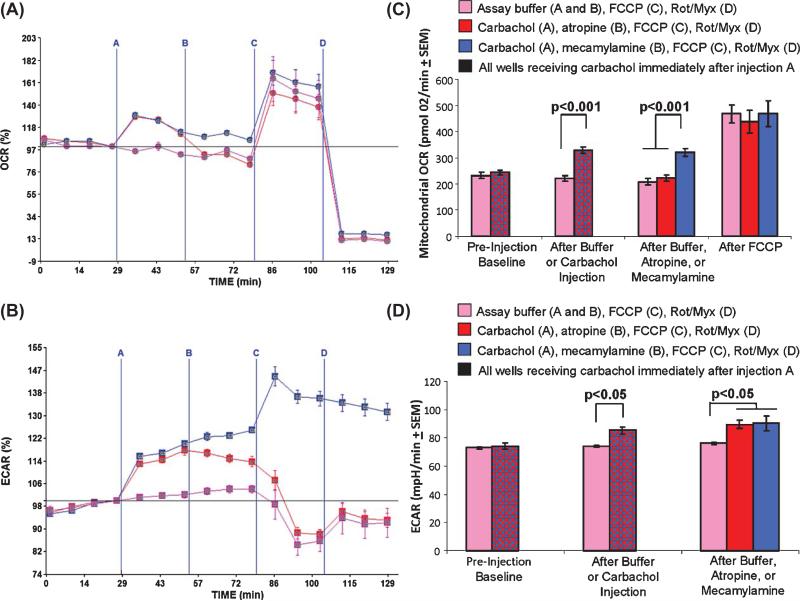
SH-SY5Y cells were transferred to a Seahorse XF 24 plate at a density of 80,000 cells per well. After adhering overnight, the standard growth medium (buffered DMEM containing 25 mM glucose and 10% fetal bovine serum) was replaced with an assay medium (unbuffered DMEM containing 2.5 mM glucose and no fetal bovine serum). No pyruvate was added to the assay medium. After a 60 min de-gassing period in which cells were maintained at 37°C in a non-CO2 incubator, the plate was transferred to the Seahorse platform for OCR and ECAR measurements. During the experiment four injections were administered. For the first injection, wells received either 100 μM carbachol in assay buffer or else just assay buffer. For the second injection, one group of wells received 100 μM atropine (a muscarinic antagonist) in assay buffer, one group of wells received 100 μM mecamylamine (a nicotinic antagonist) in assay buffer, and one group of wells received just assay buffer. For the third injection, all the wells received FCCP, and for the fourth injection all of the wells received a mixture of rotenone and myxothiazol. The results of this experiment are shown in Fig. 1. Carbachol induced an approximately 30% increase in the total OCR rate. When the non-mitochondrial OCR was subtracted from the total OCR, the carbachol-induced initial increase in the mitochondrial OCR was almost 50% (from 221 pmol O2/min to 329 pmol O2/min). Carbachol also caused an acute ECAR increase, from 74 mpH/min to 85 mpH/min (a 15% increase). Atropine rapidly reversed the carbachol-induced OCR increase, while mecamylamine did not. Neither atropine nor mecamylamine restored the ECAR to its pre-treatment level. These results indicate carbachol increases mitochondrial oxygen consumption through a muscarinic receptor-dependent pathway.
Fig. 1.
Carbachol effects SH-SY5Y cell oxygen consumption and extracellular acidification rates. For injection A, wells received either assay buffer (purple lines, purple bars) or 100 μM carbachol (red and blue lines, red and blue bars). For injection B, wells received assay buffer (purple lines and purple bars), 100 μM atropine (red lines and red bars), or 100 μM mecamylamine (blue lines and blue bars). For injection C, all wells received 0.3 μM FCCP. For injection D, all wells received 1 μM rotenone and 2 μM myxothiazol. A) OCR values shown as a percent change from the final baseline reading (point 4) ± SEM. Carbachol increases the OCR. Atropine, but not mecamylamine, terminates this effect. B) True mitochondrial OCRs, in which non-mitochondrial oxygen consumption has been subtracted out, after the different injections. C) ECAR values shown as a percent change from the final baseline reading (point 4) ± SEM. Carbachol increases the ECAR. Neither atropine nor mecamylamine terminate this effect. D) Absolute ECAR rates after the different injections. Rot, rotenone; Myx, myxothiazol.
Because atropine did not reverse the carbachol-induced ECAR increase, we pre-treated SH-SY5Y cells with atropine before adding carbachol. When the carbachol injection was preceded by the addition of atropine, carbachol failed to change either the OCR or ECAR (Fig. 2). This further suggests carbachol's main bioenergetic flux effects are mediated through muscarinic receptor activation. Preventing or interrupting muscarinic receptor activation is sufficient to prevent the carbachol-induced OCR change. Preventing muscarinic receptor activation also prevents the carbachol-induced ECAR change. However, as Fig. 1 illustrates, once this change is initiated, it is no longer solely dependent on continued muscarinic receptor activation.
Fig. 2.
Atropine pretreatment blocks carbachol-induced bioenergetic flux changes. When cells received atropine prior to carbachol, carbachol's OCR and ECAR effects were no longer observed.
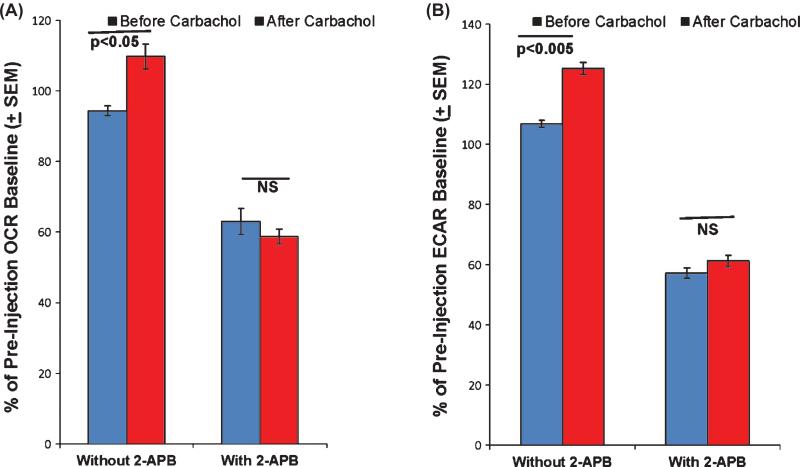
Muscarinic cholinergic receptor activation initiates the phospholipase C (PLC)-catalyzed formation of IP3 from membrane phospholipid. IP3 enters the cytosol, translocates to the ER, and binds IP3Rs [29, 30]. Binding of IP3 to ER IP3Rs triggers the release of ER calcium. It is known that even under basal conditions ER calcium release can, when properly targeted to mitochondria, enter the matrix, depolarize the mitochondrial membrane potential, and stimulate respiration [31]. Accordingly, we tested whether ER-dependent calcium release contributes to the carbachol-induced mitochondrial OCR increase by determining whether 2-APB, an IP3R inhibitor, can prevent this increase. Cells pre-treated with 200 μM 2-APB prior to carbachol did not show a carbachol-induced OCR increase, although it is important to note that the 2-APB pre-treatment itself did reduce the OCR to only 63% of its baseline value (Fig. 3). Despite 2-APB's induction of an OCR decline, this does suggest carbachol's effect on the mitochondrial OCR results from muscarinic receptor activation, IP3 translocation to the ER, and finally an IP3-dependent ER calcium release. Similarly, 2-APB pre-treatment prior to carbachol blocked the carbachol-induced ECAR increase, although it is important to note that the 2-APB treatment itself reduced the ECAR to only 57% of its pre-treatment baseline value (Fig. 3).
Fig. 3.
2-APB pretreatment blocks the carbachol-induced OCR and ECAR flux changes. Baseline OCR and ECAR values were first determined for SH-SY5Y cells. Cells then received either pre-treatment with 200 μM 2-APB or assay buffer with no 2-APB. Lastly, all cells were treated with 100 μM carbachol. A) Compared to the baseline OCR, carbachol increased the OCR in cells that did not receive the 2-APB pre-treatment (p < 0.05 by paired t-test). While the 2-APB pre-treatment did itself reduce the OCR, carbachol did not further change the OCR in the 2-APB pre-treated cells (p = 0.21 by paired t-test). B) Compared to the baseline ECAR, carbachol increased the ECAR in cells that did not receive the 2-APB pre-treatment (p < 0.005 by paired t-test). While the 2-APB pre-treatment did itself reduce the ECAR, carbachol did not further change the ECAR in the 2-APB pre-treated cells (p = 0.08 by paired t-test). NS, not significant.
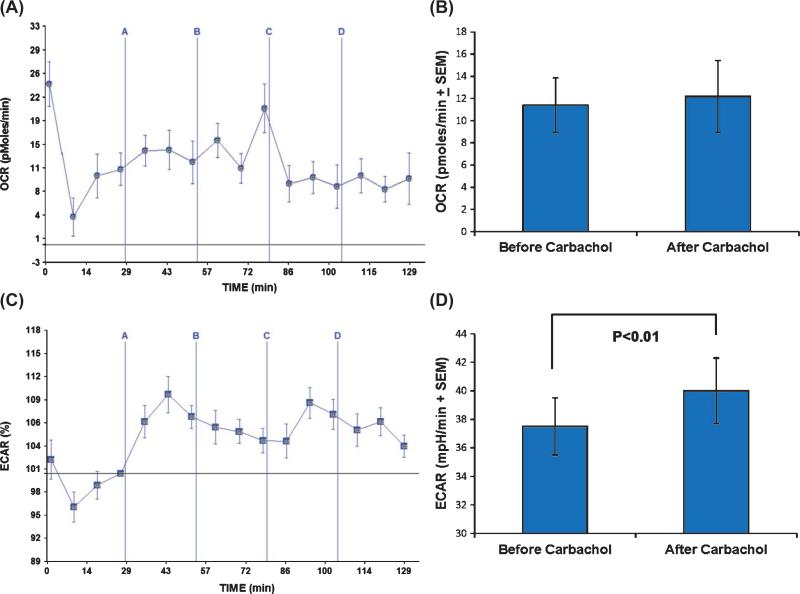
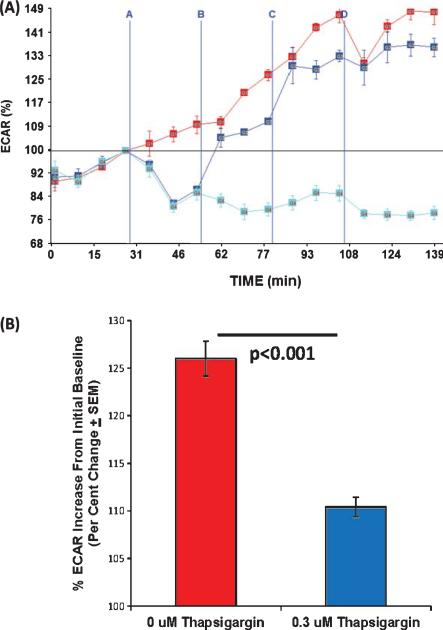
While the effects of ER calcium release on mitochondrial respiration have been considered in detail elsewhere [31], the relationship between ER calcium release and glycolytic flux has not been comprehensively addressed. To better understand this relationship, we first determined whether the or occurred independent of carbachol's mitochondrial effects. First, we found that carbachol induced a small but significant ECAR increase in SH-SY5Y ρ0 cells (Fig. 4). Since SH-SY5Y ρ0 cells lack functional mitochondrial respiratory chains and are unable to perform oxidative phosphorylation [24], the presence of an ECAR increase indicates mitochondrial respiration is not a necessary component of the carbachol-induced ECAR response. Next, we determined how thapsigar gin, which blocks the ATP-dependent return of calcium to the ER via SERCAs, affected the ECAR. We found that both low (0.3 μM) and high (3 μM) thapsigargin pre-treatments lowered the ECAR baseline. The lower thapsigargin concentration partially prevented, and the higher concentration completely prevented, the carbachol-induced ECAR increase (Fig. 5). Carbachol's effect on the ECAR, therefore, appears to be coupled to the activity of ATP-dependent SERCA pumps.
Fig. 4.
Carbachol affects the ECAR but not the OCR of SH-SY5Y ρ0 cells. SH-SY5Y ρ0 cells were exposed to carbachol in injection A, oligomycin in injection B, FCCP in injection C, and rotenone/myxothiazol in injection D. A) ρ0 cells have a very low OCR, and this rate shows very little variation when carbachol is added, ATP synthase is inhibited, or mitochondrial membranes are permeabilized. Lack of a clear rotenone/myxothiazol response indicates very little, if any, of the oxygen consumed by these cells is consumed by the mitochondrial electron transport chain. Variability at each measured time point is shown as SEM. B) Carbachol did not increase the OCR, even when before and after treatment readings were compared by a paired t-test approach. C) The ECAR, though, did change from its pre-carbachol baseline once carbachol was added. The vertical axis in this panel shows the post-carbachol ECAR percent change from the pre-carbachol ECAR baseline (point 4), as this view best illustrates the effect. Variability at each measured time point is shown as SEM. D) Although the magnitude of the carbachol-induced ECAR change was small, the pre and post carbachol treatment values were different when compared using a paired t-test approach.
Fig. 5.
Thapsigargin's effect on SH-SY5Y cell ECAR values. For injection A, SH-SY5Y cells received either 0 μM (red line and red bar), 0.3 μM (blue line and blue bar), or 3 μM (cyan line) thapsigargin. For injection B, all of the cells received 100 μM carbachol, for injection C all cells received 0.3 μM FCCP, and for injection D all cells received 1 μM rotenone and 2 μM myxothiazol. Point 4 represents the “baseline” value; the horizontal line drawn through point 4 depicts this value (100% of the baseline value) across the horizontal length of the graph. A) Both 0.3 μM and 3 μM thapsigargin reduced the ECAR below its baseline level. Carbachol subsequently increased the ECAR in the 0 μM thapsigargin and 0.3 μM thapsigargin groups, but carbachol did not increase the ECAR in the group that received 3 μM thapsigargin. B) In the 0.3 μM thapsigargin group carbachol treatment brought the ECAR back above the pre-thapsigargin baseline, but at time point 10 the percent increase over baseline (time point 4) was less (a 10% increase) than it was when no thapsigargin was present (a 26% increase).
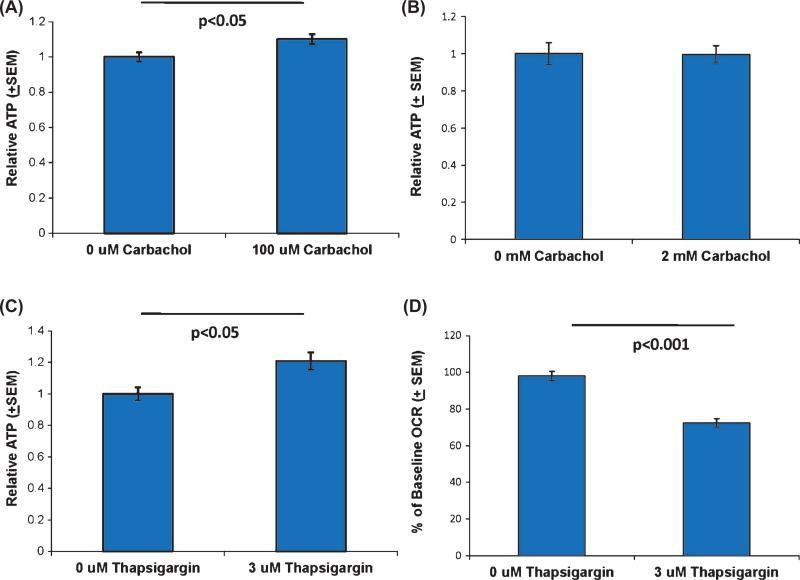
We determined how these OCR and ECAR flux manipulations affected cell ATP levels. In SH-SY5Y cells, the 100 μM carbachol treatment produced a small but significant increase in the ATP level, but this treatment produced no change in SH-SY5Y ρ0 cell ATP levels (Fig. 6). An ATP increase in the parent but not the ρ0 cell line suggests the carbachol- induced ATP surplus is a consequence of enhanced oxidative phosphorylation flux, and not enhanced glycolysis flux. 3 μM thapsigargin by itself also yielded a small but significant ATP increase in SH-SY5Y cells, despite the fact that 3 μM thapsigargin reduced the OCR (Fig. 6). Since 3 μM thapsigargin lowered the OCR and ECAR, the ATP surplus we observed most likely arises from decreased ATP consumption, and not from increased ATP production. Because thapsigar gin antagonizes ATP-dependent SERCA pumps, we presume decreased ATP consumption at least partly reflects decreased SERCA pump ATP consumption.
Fig. 6.
ATP levels in carbachol and thapsigargin-treated SH-SY5Y and SH-SY5Y ρ0 cells. A) SH-SY5Y cells treated with 100 μM carbachol showed a small but significant increase in their ATP level. B) 100 μM carbachol treatment did not change SY5Y ρ0 cell ATP levels. C) SH-SY5Y cells treated with 3 μM thapsigargin showed a small but significant increase in their ATP level. D) Even though ATP levels increased in SH-SY5Y cells treated with 3 μM thapsigargin, 3 μM thapsigargin reduced the OCR.
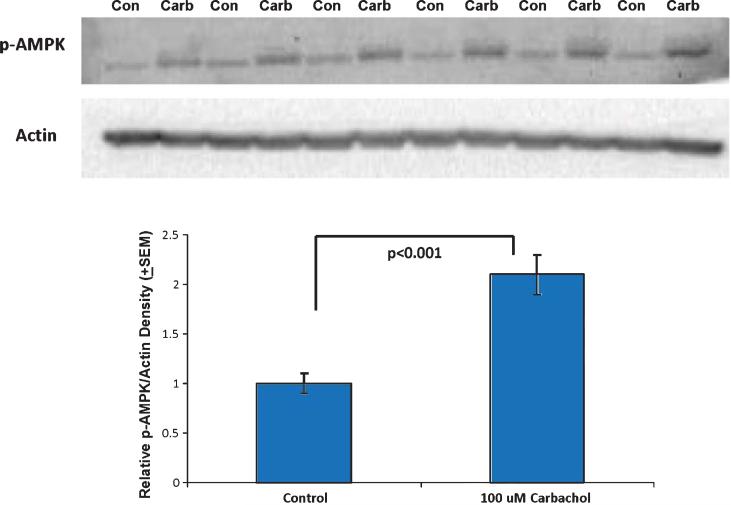
AMPK functions as a cell energy level sensor [32]. When energy levels are high, AMPK exists in a relatively unphosphorylated state. Under conditions of low energy, which are reflected by a rising AMP to ATP ratio, AMPK phosphorylates and this post-translational modification leads to AMPK activation. AMPK activity produces changes in cell energy infras tructure; in some tissues this manifests as an increase in mitochondrial mass [33]. Despite the fact that carbachol elevated total cell ATP levels, we found that carbachol also increased AMPK phosphorylation (Fig. 7).
Fig. 7.
Carbachol increased SH-SY5Y cell AMPK phosphorylation. After a 30 minute exposure to 100 μM carbachol, SH-SY5Y cell AMPK phosphorylation levels were increased over baseline. Con, control; carb, carbachol.
DISCUSSION
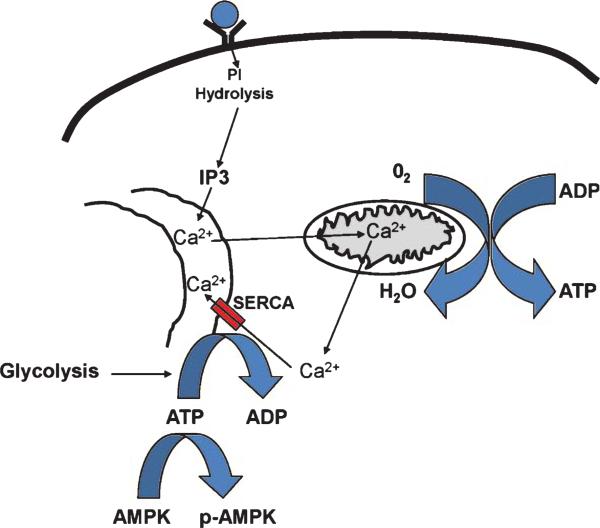
This study shows that cholinergic signaling affects both oxidative phosphorylation and glycolysis bioen ergetic fluxes, and illustrates a specific chain of events that unfolds to cause these effects. First, activation of muscarinic cholinergic receptors leads to PLC-mediated IP3 production. IP3 then moves through the cytoplasm and binds IP3Rs, which releases ER calcium. This ER calcium release impacts mitochondria in a way that increases oxidative phosphorylation; the OCR increase ceases when the cholinergic signal is itself interrupted. ER calcium release is followed by its return to the ER by SERCA pumps, which consumes ATP. The ATP used by SERCA pumps likely comes largely from glycolysis. Therefore, even though one result of the cholinergic signal is a net increase in total cell ATP, regional ATP levels are probably more variable. Mitochondrial ATP levels likely rise, but cytoplasmic ATP levels probably decline to sub-baseline levels. A decline in cytoplasmic ATP pre sumably drives a sustained increase in glycolysis and also AMPK phosphorylation. This scheme is illustrated in Fig. 8.
Fig. 8.
How a cholinergic agonist affects cell bioenergetics. Muscarinic receptor binding initiates an IP3-mediated release of ER calcium. Calcium released from the ER accesses mitochondrial matrices, depolarizes mitochondrial matrices, and enhances mitochondrial respiratory chain flux. This increases mitochondrial ATP production and oxygen consumption. Calcium released to the cytosol is returned to the ER by ATP-dependent SERCA pumps, which consumes cytosolic ATP, accelerates glycolysis, and promotes AMPK phosphorylation. PI, phosphoinositide.
Some of these steps were directly interrogated, while others were inferred. We did not show that PLC produces IP3 following muscarinic receptor stimulation, but rather assumed the known PLC activation that occurs when muscarinic receptors are engaged also occurred when we engaged muscarinic receptors in our experiments [29]. We did not show the mitochondrial OCR increase was due to ER calcium release, but recent studies clearly demonstrate that IP3-induced ER calcium release causes mitochondrial matrix depolarization and stimulates oxidative phosphorylation [31]. Although we did not measure ATP levels in different cytoplasmic compartments, several findings suggest mitochondrial ATP levels rose while cytoplasmic levels (at least in the region of SERCA pumps) fell. For example, prompt reversion of the OCR to baseline following signal interruption argues accelerated mito chondrial ATP consumption did not cause the OCR increase. In the cytoplasm, persistence of the glycol ysis response after signal termination, in conjunction with increased AMPK phosphorylation, argues a cytoplasmic ATP deficit did occur.
The relationship between IP3 and mitochondrial respiration, as well as the components that link them, is recognized [29–31, 34–37]. Within this context, it is not surprising that muscarinic receptor activation increases oxidative phosphorylation. Other findings we present, though, are relatively novel. To our knowledge, how a muscarinic receptor-initiated IP3 signal affects glycolysis flux has not been well-characterized. Also, it has previously been reported that inhibiting the IP3 pathway activates AMPK [30, 31]. Our finding, then, that increasing IP3 signaling also activates AMPK is of particular interest. Finally, it is perhaps surprising that disruption of normal bioenergetic relationships with thapsigargin caused an overall cell ATP increase, as opposed to decrease. This finding emphasizes the occasionally overlooked fact that ATP balance is not solely a function of ATP production, but also of ATP consumption.
Although our work establishes a link between muscarinic receptor-initiated IP3 signaling and glycolysis flux, we did not critically determine whether other carbachol-initiated molecular changes can also affect glycolysis flux. For example, while nicotinic receptor activation in the absence of muscarinic receptor activation did not significantly affect the ECAR in our experiments, this finding could reflect our use of SH-SY5Y cells or limitations in our ability to detect an ECAR change. Conceptually, we see no reason why nicotinic receptor-dependent ion currents should not affect ATP levels and, therefore, glycolysis. We also did not specifically assess the potential contribution of ryanodine receptors, which could add to an IP3R-mediated ER calcium release by establishing a “calcium-induced calcium release” positive feedback loop [38]. Finally, it should be noted that while adequate concentrations of 2-APB and thapsigargin ultimately abrogated the carbachol-induced ECAR change in SH-SY5Y cells, both compounds on their own also substantially affected the ECAR flux. It is possible that as a consequence of this, or perhaps as a consequence of other unknown compound effects, we created an environment in which IP3 or IP3R-independent relationships between cholinergic receptor activation and glycolysis flux were obscured. Regardless of such potential artifacts, though, the sequence described above (muscarinic receptor activation leads to an IP3 signal, ER calcium release, ATP consumption by SERCA pumps, and glycolysis activation) appears to play a key role in mediating carbachol's effect on glycolysis flux.
Our findings may help address the results of some cholinesterase inhibitor studies previously reported in the literature. Using magnetic resonance spectroscopy (MRS), investigators found donepezil treatment increased brain N-acetyl aspartate (NAA) levels in the brains of AD subjects [39–42]. Because NAA is found mostly in neurons, it is said to indicate neuron density. NAA, though, localizes to the mitochondrial compartment and it is therefore also felt to estimate mitochondrial mass [43–46]. Due to the short duration of these MRS studies, we postulate this NAA increase more likely reflects mitochondrial biogenesis than new neuron formation. Since AMPK activity in general increases mitochondrial mass [33], increased AMPK phosphorylation in our experiments could potentially explain this phenomenon.
Fluorodeoxyglucose positron emission tomography (FDG-PET) studies have also shown that cholinesterase inhibition increases or helps preserve over time brain glucose uptake in primates and human AD subjects [47–49]. Brain glucose uptake rates reflect brain glucose consumption, and a substantial amount of brain glucose is directly consumed by astrocyte glycolysis [50]. As astrocytes produce muscarinic receptors [51], we postulate that cholinesterase inhibitor-induced FDG-PET changes are due to an increase in astrocyte glycolysis that largely occurs through the muscarinic receptor-mediated cascade we have characterized.
The question of when to discontinue cholinesterase inhibitor therapy in AD patients is not straightforward. In late AD stages, a treatment-related symptomatic benefit is not always obvious, and treatment is, therefore, sometimes terminated [52]. In some of these cases, sub-acute clinical decline is nevertheless judged to occur [52]. Quantitative data also show that when cholinesterase inhibitor therapy is discontinued in patients with mild and moderate AD, a six week period of accelerated cognitive decline occurs [53, 54]. Since brain mitochondria, once generated, likely function for several weeks (rat brain mitochondria reportedly have a half-life of two to five weeks, depending on how it is assessed) [55–58], it is worth considering whether a secondary mitochondrial mass destabilization contributes to this phenomenon.
The question of when to initiate cholinesterase inhibitor therapy is also worth considering. In the United States, cholinesterase inhibitors are approved for use in mild AD, but whether to use these drugs in patients with mild cognitive impairment (MCI), which often reflects emerging AD, is controversial [59]. Part of this controversy is due to the fact that over the short term, cholinesterase inhibitor clinical trials show at most very limited symptomatic benefit [59–62]. An extended trial of donepezil in MCI subjects, however, did show clinical divergences between the active treatment and placebo groups (lasting for about one year in APOE4 non-carriers and at least three years in APOE4 carriers) that were driven by greater declines over time in the treatment group [63]. While a symptomatic benefit in the active treatment group could account for this, it is also worth considering whether the active treatment group's relative stabilization was at least partly due to a relative stabilization of brain mitochondrial mass.
This point is relevant to the long-debated question of whether cholinesterase inhibitors, in addition to providing a “symptomatic” benefit, also have a “disease modifying” effect in AD [64]. Data from pivotal cholinesterase inhibitor trials consistently show that when subjects randomized to placebo are switched to active drug their cognition improves, but not to the level of those who initially received the active treatment [65–67]. This has been argued to possibly constitute evidence of a disease modifying effect.
Our study does not address whether cholinesterase inhibitors do or do not actually have an AD-modifying effect, but since bioenergetic failure and reduced mitochondrial mass occur in AD [21, 68] and cholinergic signaling activates bioenergetic fluxes and mitochondrial biogenesis signaling, our study does define potential mechanisms through which cholinesterase inhibitors might confer such an effect. Despite the fact that human neuroblastoma cell line data may not extrapolate well to the human brain, this study still has important implications. If cholinesterase inhibitor efficacy derives at least partly through effects on cell bioenergetics, then these drugs could and should be considered a form of “mitochondrial medicine” [69, 70]. If increasing cholinergic tone confers, through bioenergetic manipulations, even a small AD-modifying effect, the development of more robust pro-cholinergic drugs would be justified. In particular, our data support the previously stated view that cholinergic muscarinic agonists, despite generating only very limited evidence of clinical efficacy to date, are still worth considering for the treatment of AD [71, 72]. Finally, considerable private and public resources are being used to determine whether classes of anti-amyloid therapies that failed in AD treatment trials might succeed in AD prevention trials. Based on our study, a cholinesterase inhibitor AD prevention trial also seems reasonable.
ACKNOWLEDGMENTS
This work was supported by the Mitochondrial Genomics and Metabolism Core and the Database Management and Statistics Core of the University of Kansas Alzheimer's Disease Center (P30AG035982), the Morgan Family Foundation, and the Frank and Evangeline Thompson Alzheimer's Treatment Program Fund.
Footnotes
Authors’ disclosures available online (http://www.jalz.com/disclosures/view.php?id=1541).
REFERENCES
- 1.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 2.Mesulam M. The cholinergic lesion of Alzheimer's disease: Pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- 3.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 4.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: Loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 5.Geula C, Mesulam MM. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer's disease. Cereb Cortex. 1996;6:165–177. doi: 10.1093/cercor/6.2.165. [DOI] [PubMed] [Google Scholar]
- 6.Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99:459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- 7.Slotkin TA, Seidler FJ, Crain BJ, Bell JM, Bissette G, Nemeroff CB. Regulatory changes in presynaptic cholinergic function assessed in rapid autopsy material from patients with Alzheimer disease: Implications for etiology and therapy. Proc Natl Acad Sci U S A. 1990;87:2452–2455. doi: 10.1073/pnas.87.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson GE, Jope R, Blass JP. Decreased synthesis of acetylcholine accompanying impaired oxidation of pyruvic acid in rat brain minces. Biochem J. 1975;148:17–23. doi: 10.1042/bj1480017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 10.Tune LE, Egeli S. Acetylcholine and delirium. Dement Geriatr Cogn Disord. 1999;10:342–344. doi: 10.1159/000017167. [DOI] [PubMed] [Google Scholar]
- 11.Roberts E. Potential therapies in aging and senile dementias. Ann N Y Acad Sci. 1982;396:165–178. doi: 10.1111/j.1749-6632.1982.tb26851.x. [DOI] [PubMed] [Google Scholar]
- 12.Perry EK, Perry RH, Tomlinson BE. Dietary lecithin supplements in dementia of Alzheimer type. Lancet. 1977;2:242–243. doi: 10.1016/s0140-6736(77)92852-5. [DOI] [PubMed] [Google Scholar]
- 13.Summers WK, Majovski LV, Marsh GM, Tachiki K, Kling A. Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med. 1986;315:1241–1245. doi: 10.1056/NEJM198611133152001. [DOI] [PubMed] [Google Scholar]
- 14.Davis KL, Mohs RC. Enhancement of memory processes in Alzheimer's disease with multiple-dose intravenous physostigmine. Am J Psychiatry. 1982;139:1421–1424. doi: 10.1176/ajp.139.11.1421. [DOI] [PubMed] [Google Scholar]
- 15.Fisher A. Cholinergic treatments with emphasis on m1 muscarinic agonists as potential disease-modifying agents for Alzheimer's disease. Neurotherapeutics. 2008;5:433–442. doi: 10.1016/j.nurt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veroff AE, Bodick NC, Offen WW, Sramek JJ, Cutler NR. Efficacy of xanomeline in Alzheimer disease: Cognitive improvement measured using the Computerized Neuropsychological Test Battery (CNTB). Alzheimer Dis Assoc Disord. 1998;12:304–312. doi: 10.1097/00002093-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol. 1997;54:465–473. doi: 10.1001/archneur.1997.00550160091022. [DOI] [PubMed] [Google Scholar]
- 18.Samanta MK, Wilson B, Santhi K, Kumar KP, Suresh B. Alzheimer disease and its management: A review. Am J Ther. 2006;13:516–526. doi: 10.1097/01.mjt.0000208274.80496.f1. [DOI] [PubMed] [Google Scholar]
- 19.Orhan IE, Orhan G, Gurkas E. An overview on natural cholinesterase inhibitors–a multi-targeted drug class–and their mass production. Mini Rev Med Chem. 2011;11:836–842. doi: 10.2174/138955711796575434. [DOI] [PubMed] [Google Scholar]
- 20.Hoyer S. Brain glucose and energy metabolism abnormalities in sporadic Alzheimer disease. Causes and consequences: An update. Exp Gerontol. 2000;35:1363–1372. doi: 10.1016/s0531-5565(00)00156-x. [DOI] [PubMed] [Google Scholar]
- 21.Swerdlow RH. Mitochondria and cell bioenergetics: Increasingly recognized components and a possible etiologic cause of Alzheimer's disease. Antioxid Redox Signal. 2012;16:1434–1455. doi: 10.1089/ars.2011.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adem A, Mattsson ME, Nordberg A, Pahlman S. Muscarinic receptors in human SH-SY5Y neuroblastoma cell line: Regulation by phorbol ester and retinoic acid-induced differentiation. Brain Res. 1987;430:235–242. doi: 10.1016/0165-3806(87)90156-8. [DOI] [PubMed] [Google Scholar]
- 23.Gould J, Reeve HL, Vaughan PF, Peers C. Nicotinic acetylcholine receptors in human neuroblastoma (SH-SY5Y) cells. Neurosci Lett. 1992;145:201–204. doi: 10.1016/0304-3940(92)90022-y. [DOI] [PubMed] [Google Scholar]
- 24.Miller SW, Trimmer PA, Parker WD, Jr, Davis RE. Creation and characterization of mitochondrial DNA-depleted cell lines with “neuronal-like” properties. J Neurochem. 1996;67:1897–1907. doi: 10.1046/j.1471-4159.1996.67051897.x. [DOI] [PubMed] [Google Scholar]
- 25.Inesi G, Hua S, Xu C, Ma H, Seth M, Prasad AM, Sumbilla C. Studies of Ca2+ATPase (SERCA) inhibition. J Bioenerg Biomembr. 2005;37:365–368. doi: 10.1007/s10863-005-9472-1. [DOI] [PubMed] [Google Scholar]
- 26.Michelangeli F, East JM. A diversity of SERCA Ca2+ pump inhibitors. Biochem Soc Trans. 2011;39:789–797. doi: 10.1042/BST0390789. [DOI] [PubMed] [Google Scholar]
- 27.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–17071. [PubMed] [Google Scholar]
- 28.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 30.Green DR, Wang R. Calcium and energy: Making the cake and eating it too? Cell. 2010;142:200–202. doi: 10.1016/j.cell.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010;67:3407–3423. doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 34.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 35.Spat A, Szanda G, Csordas G, Hajnoczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 2008;44:51–63. doi: 10.1016/j.ceca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabadkai G, Duchen MR. Mitochondria: The hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 37.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 39.Modrego PJ. The effect of drugs for Alzheimer disease assessed by means of neuroradiological techniques. Curr Med Chem. 2006;13:3417–3424. doi: 10.2174/092986706779010289. [DOI] [PubMed] [Google Scholar]
- 40.Krishnan KR, Charles HC, Doraiswamy PM, Mintzer J, Weisler R, Yu X, Perdomo C, Ieni JR, Rogers S. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer's disease. Am J Psychiatry. 2003;160:2003–2011. doi: 10.1176/appi.ajp.160.11.2003. [DOI] [PubMed] [Google Scholar]
- 41.Henigsberg N, Kalember P, Hrabac P, Rados M, Bajs M, Kovavic Z, Loncar M, Madzar T. 1-H MRS changes in dorsolateral prefrontal cortex after donepezil treatment in patients with mild to moderate Alzheimer's disease. Coll Antropol. 2011;35(Suppl 1):159–162. [PubMed] [Google Scholar]
- 42.Jessen F, Traeber F, Freymann K, Maier W, Schild HH, Block W. Treatment monitoring and response prediction with proton MR spectroscopy in AD. Neurology. 2006;67:528–530. doi: 10.1212/01.wnl.0000228218.68451.31. [DOI] [PubMed] [Google Scholar]
- 43.Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: Implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- 44.Clark JB. N-acetyl aspartate: A marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- 45.Patel TB, Clark JB. Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J. 1979;184:539–546. doi: 10.1042/bj1840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeuffer J, Tkac I, Choi IY, Merkle H, Ugurbil K, Garwood M, Gruetter R. Localized in vivo 1H NMR detection of neurotransmitter labeling in rat brain during infusion of [1-13C] D-glucose. Magn Reson Med. 1999;41:1077–1083. doi: 10.1002/(sici)1522-2594(199906)41:6<1077::aid-mrm1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 47.Tune L, Tiseo PJ, Ieni J, Perdomo C, Pratt RD, Votaw JR, Jewart RD, Hoffman JM. Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer disease: Results of a 24-week, double-blind, placebo-controlled study. Am J Geriatr Psychiatry. 2003;11:169–177. [PubMed] [Google Scholar]
- 48.Asai M, Fujikawa A, Noda A, Miyoshi S, Matsuoka N, Nishimura S. Donepezil- and scopolamine-induced rCMRglu changes assessed by PET in conscious rhesus mon keys. Ann Nucl Med. 2009;23:877–882. doi: 10.1007/s12149-009-0316-7. [DOI] [PubMed] [Google Scholar]
- 49.Keller C, Kadir A, Forsberg A, Porras O, Nordberg A. Long-term effects of galantamine treatment on brain functional activities as measured by PET in Alzheimer's disease patients. J Alzheimers Dis. 2011;24:109–123. doi: 10.3233/JAD-2010-101290. [DOI] [PubMed] [Google Scholar]
- 50.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guizzetti M, Moore NH, Giordano G, Costa LG. Modulation of neuritogenesis by astrocyte muscarinic receptors. J Biol Chem. 2008;283:31884–31897. doi: 10.1074/jbc.M801316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shega JW, Ellner L, Lau DT, Maxwell TL. Cholinesterase inhibitor and N-methyl-D-aspartic acid receptor antagonist use in older adults with end-stage dementia: A survey of hospice medical directors. J Palliat Med. 2009;12:779–783. doi: 10.1089/jpm.2009.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rainer M, Mucke HA, Kruger-Rainer C, Kraxberger E, Haushofer M, Jellinger KA. Cognitive relapse after discontinuation of drug therapy in Alzheimer's disease: Cholinesterase inhibitors versus nootropics. J Neural Transm. 2001;108:1327–1333. doi: 10.1007/s007020100009. [DOI] [PubMed] [Google Scholar]
- 54.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 55.Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246:2425–2429. [PubMed] [Google Scholar]
- 56.Beattie DS, Basford RE, Koritz SB. The turnover of the protein components of mitochondria from rat liver, kidney, and brain. J Biol Chem. 1967;242:4584–4586. [PubMed] [Google Scholar]
- 57.Khan AA, Wilson JE. Studies of turnover in mammalian subcellular particles: Brain nuclei, mitochondria and microsomes. J Neurochem. 1965;12:81–86. doi: 10.1111/j.1471-4159.1965.tb11942.x. [DOI] [PubMed] [Google Scholar]
- 58.Gross NJ, Getz GS, Rabinowitz M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem. 1969;244:1552–1562. [PubMed] [Google Scholar]
- 59.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 60.Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, Richardson S. Efficacy of donepezil in mild cognitive impairment: A randomized placebo-controlled trial. Neurology. 2004;63:651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 61.Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK, Truyen L, Mayorga AJ, Wang D, Brashear HR, Nye JS. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70:2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 62.Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, Xu Y, Murthy AK. Donepezil treatment of patients with MCI: A 48-week randomized, placebo-controlled trial. Neurology. 2009;72:1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 63.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 64.Shanks M, Kivipelto M, Bullock R, Lane R. Cholinesterase inhibition: Is there evidence for disease-modifying effects? Curr Med Res Opin. 2009;25:2439–2446. doi: 10.1185/03007990903209332. [DOI] [PubMed] [Google Scholar]
- 65.Doody RS, Geldmacher DS, Gordon B, Perdomo CA, Pratt RD. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427–433. doi: 10.1001/archneur.58.3.427. [DOI] [PubMed] [Google Scholar]
- 66.Farlow MR. Do cholinesterase inhibitors slow progression of Alzheimer's disease? Int J Clin Pract Suppl. 2002:37–44. [PubMed] [Google Scholar]
- 67.Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54:2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 68.Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swerdlow RH. Mitochondrial medicine and the neurodegenerative mitochondriopathies. Pharmaceuticals. 2009;2:150–167. doi: 10.3390/ph2030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swerdlow RH. Role and treatment of mitochondrial DNA-related mitochondrial dysfunction in sporadic neurode-generative diseases. Curr Pharm Des. 2011;17:3356–3373. doi: 10.2174/138161211798072535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caccamo A, Fisher A, LaFerla FM. M1 agonists as a potential disease-modifying therapy for Alzheimer's disease. Curr Alzheimer Res. 2009;6:112–117. doi: 10.2174/156720509787602915. [DOI] [PubMed] [Google Scholar]
- 72.Davis AA, Fritz JJ, Wess J, Lah JJ, Levey AI. Deletion of M1 muscarinic acetylcholine receptors increases amyloid pathology in vitro and in vivo. J Neurosci. 2010;30:4190–4196. doi: 10.1523/JNEUROSCI.6393-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]