Abstract
Introduction
Niemann–Pick disease (NPD) types A and B are lipid storage disorders. NPD type A is a fatal disorder of infancy. Type B is a non-neuronopathic form observed in children and adults. It is associated with enlargement of the liver, spleen, or both, and nodular splenomegaly may be detected with ultrasound.
Methods
A 21-year-old female was admitted to the Emergency Room with fever, pharyngitis, and left upper quadrant abdominal pain. Labwork revealed anemia, thrombocytopenia, increased levels of AST, ALT, GGT, AF, LDH, triglycerides, and total cholesterol and low levels of HDL-cholesterol. PCR blood assays for CMV and EBV were both negative. Chest X-ray was unremarkable. Transabdominal B-mode ultrasound (US) revealed splenomegaly (long axis: >22 cm), an irregular subcapsular hypoechoic lesion in the superior pole that was consistent with splenic infarction, and multiple round highly echogenic nodes measuring 1–5 cm in diameter. Contrast-enhanced ultrasonography (CEUS) was performed using SonoVue® (Bracco).
Results
The presence of a splenic infarction was confirmed. The nodular lesions showed arterial-phase enhancement with late parenchymal phase wash-out. 18F-FDG-PET revealed splenic nodular uptake. Primary splenic lymphoma was suspected, and the patient underwent open splenectomy. The diagnosis was type B NPD with splenic hemangiomas.
Discussion
CEUS confirmed the diagnosis and extent of splenic infarction, but the nodular atypical enhancement pattern together with nodular 18F-FDG-PET uptake was misleading, suggesting as it did lymphoproliferative involvement of the spleen.
Keywords: CEUS, Niemann–Pick disease, Nodular splenomegaly
Sommario
Introduzione
La malattia di Niemann-Pick (NPD) tipo A e B è una patologia da accumulo di lipidi. Il tipo A è un disordine fatale dell'infanzia. Il tipo B è una forma non-neuronopatica ossevata sia nei bambini che negli adulti con possibile riscontro di epatomegalia e/o splenomegalia (nodulare) durante un esame ecografico. Il tipo C dipende da un difetto nel trasporto del colesterolo.
Metodi
Una donna di 21 anni si è presentata al Pronto Soccorso con febbre, faringodinia e dolore al quadrante addominale superiore sinistro. Gli esami ematochimici hanno evidenziato anemia, piastrinopenia, aumento delle AST, ALT, GGT, FA, LDH trigliceridi, colesterolo totale, e ridotto HDL. La PCR per CMV ed EBV era negativa. La radiografia del torace era negativa. L'ecografia transaddominale ha rilevato splenomegalia (>22 cm long axis) con una lesione ipoecogena irregolare subcapsulare al polo superiore compatibile con infarto splenico e la presenza di multiple lesioni nodulari iperecogene con diametro da 1 cm fino a 5.
Risultati
È stata quindi eseguita una ecografia con mezzo di contrasto con SonoVue® (Bracco) che ha confermato la presenza di un infarto splenico. Le lesioni nodulari mostravano un enhancement in fase arteriosa con wash out in fase parenchimatosa tardiva. La 18F-FDG-PET ha mostrato un uptake nodulare splenico. Nel sospetto di un processo linfoproliferativo è stata eseguita una splenectomia. La diagnosi è stata di NPD tipo B con infarto splenico e le lesioni nodulari sono risulate essere emangiomi.
Discussione
Concludendo, la CEUS ha confermato la diagnosi e l'estenzione dell'infarto splenico, ma l'enhancement nodulare atipico supportato dalle immagini 18F-FDG-PET è stato fuorviante, suggerendo l'ipotesi di lesioni linfomatose.
Introduction
Niemann–Pick disease (NPD) types A and B are lipid storage disorders caused by deficiency of the lysosomal enzyme, acid sphingomyelinase (ASM), and intracellular accumulation of its substrate, sphingomyelin. ASM-deficient NPD is inherited as a recessive trait [1]. Type A is a severe neurodegenerative disorder. Hepatosplenomegaly, moderate lymphadenopathy, and psychomotor retardation are evident by 6 months of life and are followed by rapid neurodegeneration leading to premature death by 3 years of age. Type B NPD is a non-neuronopathic disorder. Most diagnoses are made in infancy or childhood when enlargement of the liver, spleen, or both is detected during a routine physical examination. The clinical course of type B NPD is quite heterogeneous. In mildly affected patients, the splenomegaly may not be noted until adulthood. Disease manifestations may be minimal and the life-span normal.
Niemann–Pick disease type C is a fatal neurovisceral lipid storage disease of autosomal inheritance. Its clinical spectrum ranges from rapidly fatal disease to a chronic, adult-onset neurodegenerative disease.
Cases of nodular splenomegaly in NPD have been described [2–4] including one in which the nodules resembled hemangiomas.
In this study, nodular splenomegaly in type B NPD was investigated by means of B-mode sonography, contrast-enhanced ultrasonography (CEUS), and positron emission tomography with 2-[fluorine-18]fluoro-2-deoxy-d-glucose (18F-FDG-PET).
Materials and methods
A 21-year-old female was admitted to the Emergency Room because of fever, pharyngitis, and left upper quadrant abdominal pain. A complete blood count and blood chemistry studies revealed anemia (hemoglobin 9.3 g/dl), thrombocytopenia (62.000/μL), and increased levels of aspartate transaminase (104 U/L), alanine transaminase (90 U/L), gamma-glutamyltransferase (71 U/L), alkaline phosphatase (525 U/L), lactate dehydrogenase (1053 U/L), triglycerides (197 mg – normal 40–150), and total cholesterol (234 mg/dl) with a low level of high density lipoprotein -cholesterol (34 mg/dl – normal 45–65). Polymerase chain reaction (PCR) blood assays for cytomegalovirus and Epstein Barr virus were both negative. Hepatitis A and C and human immunodeficiency virus (HAV, HCV, HIV, respectively) infections, toxoplasmosis, Widal-Wright, and Bartonella were negative. The patient had been vaccinated against Hepatitis B (HBV) infection and antiHBs titers were 19.4 IU/ml. The chest X-ray was unremarkable.
Transabdominal B-mode ultrasonography was performed with a portable MyLab25 scanner (Esaote) and a convex transducer (3.5–5.0 MHz). It revealed marked splenomegaly (long axis: >22 cm), an irregular hypoechoic lesion in the supcapsular region of the superior pole, which was highly suggestive of splenic infarction, and multiple round well-defined highly echogenic nodes measuring from 1 to 5 cm (Fig. 1a,b). One of the hyperechoic nodules was close the suspected area of infarction (Fig. 2a,b). No abdominal lymphadenopathies were detected. The superficial portions of lateral cervical, submandibular, supra- and subclavicular, axillary, and inguinal lymph nodes were scanned with a linear transducer (7.5–10 MHz), but no pathologic ultrasound features were detected [5]. The liver had a normal US appearance with no detectable focal lesions.
Fig. 1.
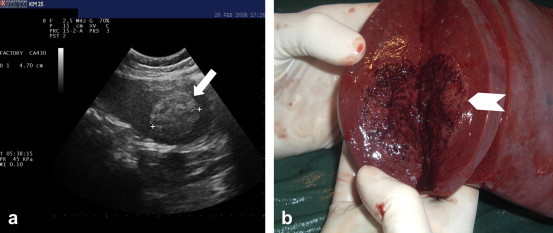
(a) B-mode US: well-defined hyperechogenic nodule in the spleen measuring 4.7 cm in diameter (white arrow). (b) Macroscopically, the nodule appeared brownish and spongy (white arrowhead).
Fig. 2.
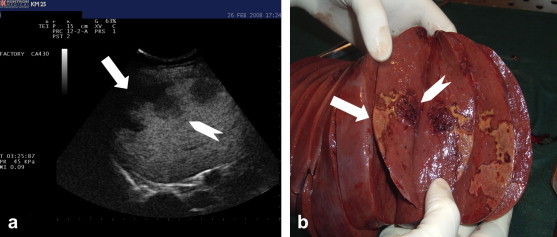
(a) Late phase CEUS (3 min) showing splenic infarction(white arrow) and a close nodule (white arrowhead). (b) Macroscopic appearance of the splenic infarction (white arrow) and the spleen node (white arrowhead).
18F-FDG-PET was performed with a GE Discovery scanner. Images were acquired 45 min after i.v. injection of 10 mCi 18F-FDG.
CEUS was performed with a portable MyLab25 sonographer (Esaote) with harmonic contrast-tuned imaging technology and a convex phased-array 3.5-MHz transducer. After written consent was obtained, an ultrasound contrast agent (SonoVue®, Bracco) consisting of phospholipid-stabilized sulfur hexafluoride microbubbles was injected as an i.v. bolus (2.4 ml), and the line was immediately flushed with 5 mL saline. The left upper quadrant of the abdomen was continuously scanned for 5 min until the enhancement effect began to decrease. A very low mechanical index (0.06–0.08) was used to minimize disruption of the microbubbles, and the sound beam was focused on deeper layers of the region of interest.
Results
CEUS confirmed the presence of a splenic infarct in the superior pole of the spleen, which was manifested by the absence of arterial and late-phase enhancement of the irregular hypoechoic subcapsular lesion (Fig. 2a). The nodular lesions displayed enhancement during the arterial phase with wash-out during the late parenchymal phase, resulting in lesions that appeared mildly hypoechoic with respect to the surrounding splenic parenchyma. This was later confirmed by a second 2.4-mL bolus of contrast medium (Fig. 3a–c). In Fig. 3a, the splenic parenchyma appeared hyperechoic as a result of the first injection of contrast medium. The nodule, which was initially hypoechoic because of the previous-wash out, began to show enhancement 18 s after injection of the second bolus. Complete enhancement occurred at 39 s (Fig. 3b) and wash-out at 2 min (Fig. 3c).
Fig. 3.
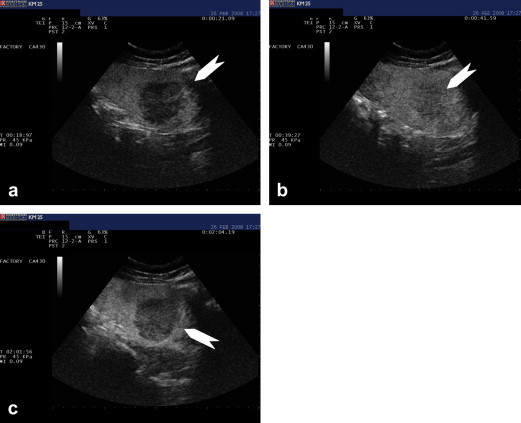
CEUS performed after injection of a second SonoVue® bolus (2.4 ml). (a) Contrast medium enhancement at 18 s. (b) Contrast medium enhancement at 39 s. (c) Contrast medium enhancement at 2.02 min.
18F-FDG-PET revealed multiple areas of uptake in correspondence with the nodular lesions (standard uptake by the largest node: 5.5) (Fig. 4).
Fig. 4.
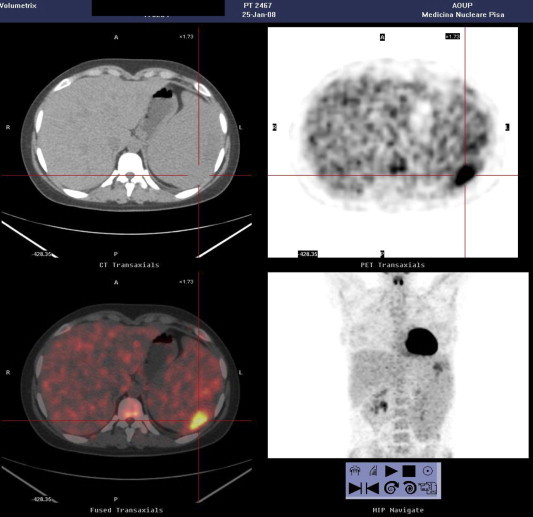
18F-FDG-PET showing uptake in correspondence with the splenic nodules (standard uptake by the largest nodule: 5.5).
A primary splenic lymphoproliferative disorder was suspected, and the patient underwent open splenectomy. The postoperative phase was complicated by blood loss requiring multiple units of packed red cells. (Splenectomy involving organs measuring >15 cm is reportedly associated with greater blood loss, longer operating times, and longer hospital stay [6].) The patient also developed low-grade fever, which resolved with broad spectrum antibiotics. Twenty days after surgery, the patient was discharged, fully recovered.
Macroscopically (Fig. 5) the spleen weighed 1200 g and measured 25 × 14 × 5 cm. The presence of the superior pole infarct was confirmed. The nodular lesions appeared brownish and spongy (Fig. 1b). One was located adjacent to the infarction, and the 2 lesions had been distinguished from one another in part by the different patterns they produced on CEUS pattern (Fig. 2a). Immunohistochemistry findings were consistent with a final diagnosis of Neumann-Pick Disease: diffusely positive CD68 and oil Red-O staining, weak periodic acid-Schiff stain positivity, negative Perls staining (Fig. 6a,b). CD34 immunohistochemical staining revealed dilated vessels within the nodules (Fig. 7a,b) (compared with vessels in the surrounding splenic parenchyma), which were consistent with the diagnosis of hemangiomas. A bone marrow biopsy performed just before splenectomy revealed marrow involvement by NPD (Fig. 6b). The diagnosis of NPD was later confirmed by genetic analysis.
Fig. 5.
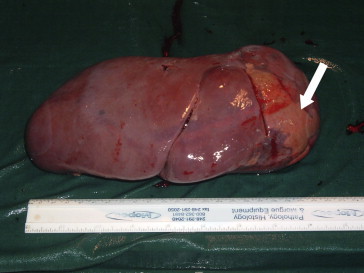
The spleen measured 25 × 14 × 5 cm and weighed 1200 g. It contained an obvious subcapsular infarct in the superior pole (white arrow).
Fig. 6.
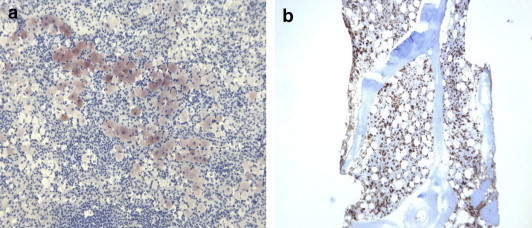
Microscopical appearance of the splenic parenchyma. (a) Oil Red O stain for lipid droplets in macrophages (5×). (b) Bone marrow biopsy showing foam cells (PG-M1 ×5).
Fig. 7.
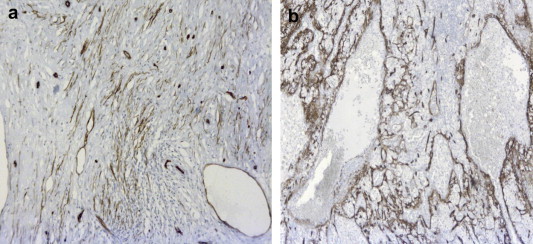
(a) CD34 staining in the splenic parenchyma. (b) CD34 staining in the splenic nodes show vessels that are ectasic (compared with those of the normal parenchyma). These findings are consistent with hemangioma (5×).
Discussion
NPD types A and B are lipid storage disorders caused by deficiency of the lysosomal enzyme, acid sphingomyelinase (ASM), and the subsequent intracellular accumulation of its substrate, sphingomyelin. Type A is a fatal disorder of infancy, and neurodegeneration proceeds rapidly and leads to death within 3 years. Type B is a non-neuronopathic form observed in children and adults. Patients frequently survive into adulthood. (Onset may occur anywhere from childhood to adulthood with wide phenotypic variability.) [1,7].
Enlarged livers and spleens are the most consistent clinical findings, although respiratory complications may also be present [8] Patients usually have elevated levels of triglycerides and LDL-cholesterol, low HDL-cholesterol levels, and sometimes a history of coronary artery disease. Other possible clinical findings include fatigue, joint pain, bleeding, headaches, gastrointestinal pain, and bone fractures [9].
It is more frequent in individuals of Middle Eastern and North African descent with an estimated incidence ranging from ∼0.5 to 1 per 100,000 births. However, the true incidence is likely to be higher because the estimates cited above are based solely on cases referred to biochemistry laboratories for enzyme studies. NPD type C is a fatal neurovisceral lipid storage disease of autosomal inheritance. The histological hallmark of NPD is the pathological “foam cell” or “Niemann–Pick cell.” This histiocyte is found in clinically involved tissues and organs, particularly those of the monocyte-macrophage system. In the NPD patient, tissues without at least some foam cells are rare. In the terminal stages of the disease, the organs of the monocyte-macrophage system, such as the lymph nodes and spleen, are often completely infiltrated with these cells [10].
There are very few reports on B-mode US findings associated with splenic involvement in NPD. In case reports regarding NPD type B or C presenting with splenic nodules, the nodules were described as well-defined and highly echogenic. In one case, the features of the nodes were similar to those of hemangiomas.
The hyperechogenicity of the splenic nodules has previously been attributed to their high lipid content. This has been reported in previous cases of NPD type C presenting with nodular splenomegaly and mental retardation [11].
In our patient, B-mode sonography revealed splenomegaly with hyperechoic nodes and a probable splenic infarct. The average spleen measures about 110–120 mm (length) by 70 mm (width) by 40 mm (thickness) and weighs 150–200 g. Splenomegaly may accompany certain infections, and it may also be caused by tumors, blood sequestration, blood supply disorders, or portal hypertension [12,13]. Compared with other parenchymal organs, the spleen is rarely the site of primary focal lesions [14], whose prevalence in the general population is estimated at 0.1% [15].
Primary malignant lesions of the spleen are rare and include non-Hodgkin's lymphoma, Hodgkin's disease, and hemangiosarcoma. There are no definitive data on the CEUS patterns associated with these entities. The rare benign splenic tumors are usually incidental findings. They include cysts, hemangiomas, hamartomas, and lymphangiomas. On B-mode US, splenic hemangiomas and splenic hamartomas may appear hypoechoic, isoechoic, or hyperechoic [16].
Vascular neoplasms are the most common primary non-hematolymphoid tumors. They can present dramatically with spontaneous splenic rupture and massive hemoperitoneum. Hemangioma is the most common benign primary vascular neoplasm of the spleen. On sonograms, hemangiomas may appear as well-defined, intrasplenic or pedunculated, echogenic masses. Some are solid; others are complex cysts. Echogenic calcifications with acoustic shadowing may be present [17].
Malignant lesions in the spleen are mostly hypoechogenic (97% of cases). They occur most frequently in patients with lymphoma (80% of focal splenic lesions). In Hodgkin's disease, the lesions may be of various sizes or may occur within diffuse infiltration. In high-grade non-Hodgkin lymphomas (NHLs), the lesions often exceed 30 mm, while in low-grade NHL there are areas of diffuse infiltration and small focal lesions (<10 mm). Metastatic lesions of the spleen are estimated to occur in 0.5–9% of patients with neoplastic diseases, mainly those with malignancy of the lungs, breast, pancreas, or prostate, or melanoma [18].
In one study, the authors evaluated the focal echogenic lesions of the spleen reflecting lymphomatous involvement. Focal splenic lesions were found in 178 patients with malignant lymphoma. Focal echo-rich splenic infiltrates were predominantly seen in patients with low-grade NHL (9 out of 11). In all 9 cases, the infiltrate measured less than 3 cm in diameter, and the spleen itself measured over 8 × 20 cm [19].
On ultrasound, most splenic lymphomas are characterized by low reflectivity and indistinct margins. Highly reflective, complex lesions and “target sign” lesions have also been described, but are uncommon [13].
CEUS makes it possible to study the microvasculature of focal splenic lesions. Splenic artery opacification starts about 12 s after the injection of the SonoVue® bolus. Subsequently, there is inhomogeneous enhancement of the splenic parenchyma that resembles the well-known zebra-striped pattern seen on dynamic computed tomography (CT) or magnetic resonance imaging (MRI) [14]. During the first minute after Sonovue injection, small arteries are seen radiating from the splenic hilum; venous opacification is always limited. Approximately 50 s after injection, the splenic parenchyma becomes uniformly enhanced and remains so for up to 5–7 min. SonoVue® produces spleen-specific enhancement that lasts longer (5 min) than the blood-pool and liver enhancement, probably because there is some degree of parenchymal uptake. The site of accumulation for this second-generation microbubble agent is unknown, but it may be within the reticuloendothelial system or sinusoids [20]. Compared with the left kidney, which shows intense but transient enhancement, the spleen appears hypoechoic during the early phase of opacification and hyperechoic during the late phase [16].
This unique splenic specificity is therefore useful for the detection of splenic tissue and the characterization of focal splenic lesions.
Small capillary hemangiomas usually show an enhancement pattern similar to that of adjacent splenic tissue and therefore remain constantly isoechoic. A hyperechoic lesion on baseline images that becomes undetectable on enhanced scans is diagnosed as a hemangioma. Larger, cavernous hemangiomas show a greater degree of enhancement, with rapid or slow opacification and centripetal or diffuse fill-in patterns. Contrast enhancement is very dense and prolonged in large hemangiomas, and there may be posterior shadowing. Lymphoma nodules and blood-borne metastases can be isoechoic (undetectable) or hypoechoic, but after injection of contrast medium they appear as clear, hypoechoic defects. The lesion-to-parenchyma contrast gradient increases progressively from the arterial phase to the late phase of parenchymal enhancement [21].
Our patient was symptomatic with anemia and thrombocytopenia and no signs of neuronopathy. (She was a student with no impairment of cognitive function.) Her abdominal symptoms were mostly caused by the infarct in the superior pole of the spleen.
The multiple, well-defined hyperechogenic spleen nodules found on B-mode sonography may initially raise the suspicion of hemangiomas, hamartomas, or more rarely splenic lymphoma.
As noted above, the CEUS patterns observed in primary splenic lymphomas has never been definitively described. The late-phase washout of the spleen nodes in our patient – although mild – suggested further diagnostic insights.
For all the above mentioned reasons, 18F-FDG-PET was performed, and it showed multiple areas of uptake in correspondence with the spleen nodules.
Our patient had a CEUS pattern that did not resemble the CEUS enhancement pattern typically associated with hemangioma [22].
The late-phase wash-out of the splenic nodes in our NPD patient (hemangiomas) could be related to the spleen-specific enhancement produced by SonoVue®, which lasts longer than the blood-pool and liver enhancement [20].
The splenic nodules presented a CEUS pattern with late parenchymal phase wash-out and 18F-FDG-PET uptake, both of which were highly suggestive of splenic lymphoma.
Given the complexity of the differential diagnosis, the decision was made to perform splenectomy. The aim of this procedure was not only diagnostic: it might also have been curative if the patient had been found to have primary splenic lymphomas without other organ involvement. Two splenic lymphoma variants have been described that tend to be limited, at least initially, to the spleen: the form characterized by circulating villous lymphocytes and marginal zone lymphoma [23].
The presence of ectasic vessels within the splenic nodes of our patient could be a reflection of the crucial role played by macrophages in regulating angiogenesis during wound healing and during tumor progression. These cells produce a number of proangiogenic factors, including vascular endothelial growth factor, tumor necrosis factor alpha, granulocyte macrophage colony-stimulating factor, interleukin (IL)-1, and IL-6, as well as other factors that can regulate angiogenesis, including matrix metalloproteinases and nitric oxide [24].
Tumor-associated macrophages have been shown regulate the angiogenic switch, an essential step for the progression of mammary tumors to malignancy [25].
The nodular 18F-FDG uptake in our case was misleading. However, intense 18F-FDG uptake is observed not only in malignant tissues, but also in inflammatory lesions (e.g., sarcoidosis, tuberculosis, fungal infections, abdominal abscesses) [26].
There have been reports of false-positive 18F-FDG-PET scans caused by lipoid pneumonia (a benign condition frequently associated with inadvertent aspiration or inhalation of oily substances) mimicking a solid pulmonary nodule. The histopathological specimen contained cholesterol clefts, foam cells, pseudogranuloma formation, and a lymphocytic infiltrate [27–29].
A variety of congenital enzyme deficiencies cause splenomegaly related to the accumulation of histiocyte-macrophages in the red pulp of the spleen. The foamy cytoplasm of these cells is a result of the accumulation of various types of phospholipids, particularly sphingomyelin in NPD. The presence of these cells with possible active metabolic activity might explain the 18F-FDG uptake observed in the spleen nodules and the angiogenesis with ectasic vessels found in our patient.
The patient was later definitively diagnosed with type B NPD based on genetic analysis.
To the best of our knowledge, this is the first report describing the CEUS enhancement pattern in splenic nodular NPD.
In conclusion, CEUS was useful in confirming the diagnosis and extent of the splenic infarction that was responsible for our patient's abdominal pain, but together with the PET features, it was misleading in terms of the nature of the splenic nodes. The CEUS pattern of enhancement was in fact highly suggestive of malignant lesions, whereas the nodules proved instead to be hemangiomas with an atypical CEUS enhancement pattern.
Conflict of interest statement
The authors have no conflict of interest.
References
- 1.Schuchman E.H. The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann–Pick disease. J Inherit Metab Dis. 2007;30:654–663. doi: 10.1007/s10545-007-0632-9. [DOI] [PubMed] [Google Scholar]
- 2.Schubert F. Echogenic splenic tumours in type B Niemann–Pick disease. Australas Radiol. 1994;38:127–129. doi: 10.1111/j.1440-1673.1994.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 3.Fröhlich E., Harzer K., Heller T., Rühl U. Ultrasound echogenic splenic tumors:nodular manifestation of type C Niemann–Pick disease. Ultraschall Med. 1990;11:119–122. doi: 10.1055/s-2007-1011543. [DOI] [PubMed] [Google Scholar]
- 4.Omarini L.P., Frank-Burkhardt S.E., Seemayer T.A., Mentha G., Terrier F. Niemann–Pick disease type C: nodular splenomegaly. Abdom Imaging. 1995;20(2):157–160. doi: 10.1007/BF00201528. [DOI] [PubMed] [Google Scholar]
- 5.Leboulleux S., Girard E., Rose M., Travagli J.P., Sabbah N., Caillou B. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007 Sep;92(9):3590–3594. doi: 10.1210/jc.2007-0444. [DOI] [PubMed] [Google Scholar]
- 6.Pugliese R., Maggioni D., Scandroglio I., Sansonna F., Grilloa G., Di Lernia S. Splenectomy in haematologic diseases. Clinical indications and surgical technique. Chir Ital. 2005;57:283–291. [PubMed] [Google Scholar]
- 7.Dardis A., Zampieri S., Filocamo M., Burlina A., Bembi B., Pittis M.G. Functional in vitro characterization of 14 SMPD1 mutations identified in Italian patients affected by Niemann–Pick Type B disease. Hum Mutat. 2005;26:164. doi: 10.1002/humu.9353. [DOI] [PubMed] [Google Scholar]
- 8.Mendelson D.S., Wasserstein M.P., Desnick R.J., Glass R., Simpson W., Skloot G. Type B Niemann–Pick disease:findings at chest radiography, thin-section CT, and pulmonary function testing. Radiology. 2006;238:339–345. doi: 10.1148/radiol.2381041696. [DOI] [PubMed] [Google Scholar]
- 9.McGovern M.M., Pohl-Worgall T., Deckelbaum R.J., Simpson W., Mendelson D., Desnick R.J. Lipid abnormalities in children with types A and B Niemann–Pick disease. J Pediatr. 2004;145:77–81. doi: 10.1016/j.jpeds.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 10.Mills S.E., Carter D., Greenson J.K., Oberman H.A., Reuter V.E., Stoler M.H. 4th ed. Lippincott Williams & Wilkins (LWW); 2004. Sternberg's diagnostic surgical pathology. p. 792–4. [chapter 18] [Google Scholar]
- 11.Vanier M.T., Rodriguez-Lafrasse C., Rousson R., Mandon G., Boué J., Choiset A. Prenatal diagnosis of Niemann–Pick type C disease: current strategy from an experience of 37 pregnancies at risk. Am J Hum Genet. 1992;51:111–122. [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews M.W. Ultrasound of the spleen. World J Surg. 2000;24:183–187. doi: 10.1007/s002689910031. [DOI] [PubMed] [Google Scholar]
- 13.Peddu P., Shah M., Sidhu P.S. Splenic abnormalities:a comparative review of ultrasound, microbubble-enhanced ultrasound and computed tomography. Clin Radiol. 2004;59:777–792. doi: 10.1016/j.crad.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Goerg C., Schwerk W.B., Goerg K. Splenic lesions: sonographic patterns, follow up, differential diagnosis. Eur J Radiol. 1991;13:59–66. doi: 10.1016/0720-048x(91)90058-4. [DOI] [PubMed] [Google Scholar]
- 15.Wernecke K., Peters P.E., Kruger K.G. Ultrasonographic patterns of focal hepatic and splenic lesions in Hodgkin's and non-Hodgkin's lymphoma. Br J Radiol. 1987;60:655–660. doi: 10.1259/0007-1285-60-715-655. [DOI] [PubMed] [Google Scholar]
- 16.Gorg C. The forgotten organ: contrast enhanced sonography of the spleen. Eur J Radiol. 2007;64:189–201. doi: 10.1016/j.ejrad.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Abbott R.M., Levy A.D., Aguilera N.S., Gorospe L., Thompson W.M. Primary vascular neoplasms of the spleen: radiologic–pathologic correlation. Radiographics. 2004;24:1137–1163. doi: 10.1148/rg.244045006. [DOI] [PubMed] [Google Scholar]
- 18.Robertson F., Leander P., Ekberg O. Radiology of the spleen. Eur Radiol. 2001;11:80–95. doi: 10.1007/s003300000528. [DOI] [PubMed] [Google Scholar]
- 19.Riera-Knorrenschild J., Görg C., Dennhardt N., Restrepo I., Neubauer A. Focal echogenic involvement in malignant lymphoma:a diagnostic problem. Ultraschall Med. 2000;21:3–7. doi: 10.1055/s-2000-8642. [DOI] [PubMed] [Google Scholar]
- 20.Lim A.K., Patel N., Eckersley R.J., Taylor-Robinson S.D., Cosgrove D.O., Blomley M.J. Evidence for spleen-specific uptake of a microbubble contrast agent:a quantitative study in healthy volunteers. Radiology. 2004;231:785–788. doi: 10.1148/radiol.2313030544. [DOI] [PubMed] [Google Scholar]
- 21.Catalano O., Sandomenico F., Vallone P., D'Errico A.G., Siani A. Contrast-enhanced sonography of the spleen. Semin Ultrasound CT MR. 2006;27:426–433. doi: 10.1053/j.sult.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Claudon M., Cosgrove D., Albrecht T., Bolondi L., Bosio M., Calliada F. EFSUMB study group. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)-update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 23.Catovsky D., Matutes E. Splenic lymphoma with circulating villous lymphocites/splenic marginal-zone lymphoma. Semin Hematol. 1999;36:148–154. [PubMed] [Google Scholar]
- 24.Lin E.Y., Li J.F., Gnatovskiy L., Deng Y., Zhu L., Grzesik D.A. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 25.Dirkx A.E., Oude Egbrink M.G., Wagstaff J., Griffioen A.W. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 26.Jerusalem G., Beguin Y., Fassotte M.F., Belhocine T., Hustinx R., Rigo P. Early detection of relapse by whole-body positron emission tomography in the follow-up of patients with Hodgkin's disease. Ann Oncol. 2003;14:123–130. doi: 10.1093/annonc/mdg011. [DOI] [PubMed] [Google Scholar]
- 27.Mokhlesi B., Angulo-Zereceda D., Yaghmai V. False-positive FDG-PET scan secondary to lipoid pneumonia mimicking a solid pulmonary nodule. Ann Nucl Med. 2007;21:411–414. doi: 10.1007/s12149-007-0042-y. [DOI] [PubMed] [Google Scholar]
- 28.Tahon F., Berthezène Y., Hominal S., Blineau N., Guérin J.C., Cinotti L. Exogenous lipoid pneumonia with unusual CT pattern and FDG positron emission tomography scan findings. Eur Radiol. 2002;12(Suppl. 3):S171–S173. doi: 10.1007/s00330-002-1659-9. [DOI] [PubMed] [Google Scholar]
- 29.Talwar A., Mayerhoff R., London D., Shah R., Stanek A., Epstein M. False-positive PET scan in a patient with lipoid pneumonia simulating lung cancer. Clin Nucl Med. 2004;29:426–428. doi: 10.1097/01.rlu.0000129123.61966.e0. [DOI] [PubMed] [Google Scholar]


