Abstract
Vesiculodeferentography was used in the past to evaluate suspected cases of obstruction of the seminal ducts. Over the years, numerous attempts have been made to improve the technique used to perform this examination and to render it less invasive. Its use is currently indicated in selected cases, where it is combined with functional studies like seminal tract washout and followed by immediate interventions to correct the alterations revealed. Vesiculodeferentography includes collection of the contents of the seminal vesicles, which can later be used in vitro assisted fertilization procedures.
Keywords: Vesiculodeferentography, Transrectal sonography, Infertility
Sommario
La vesciculodeferentografia, esame utilizzato in passato nei casi di sospette ostruzioni delle vie seminali, è stato negli anni oggetto di numerosi studi allo scopo di migliorarne la tecnica di esecuzione e renderla meno invasiva. Attualmente è indicata solo in casi selezionati, in associazione a un'indagine funzionale quale il Seminal Tract Washout e prevedendo un contemporaneo – o in via subordinata, successivo – atto terapeutico.
Ovviamente, peraltro, la procedura – ipso facto – prevede il prelievo di materiale dalla seminale e ciò può consentire una successiva procedura di fecondazione assistita in vitro.
Introduction
Throughout history societies have viewed infertility as a problem. The first references to infertility are found in Egyptian papyri dating back to 2200 BC, and there are numerous Bible verses on the importance of procreation (“…be fruitful and multiply, and fill the earth…” – Genesis, 1:28) and on the concept of God's curse on those that are infertile (“Write this man down as childless…” – Jeremiah, 22:30) [1]. Hippocrates (460–370 BC) wrote the first treatise on semen, while Roman physicians were the first to recognize lead poisoning, sexual promiscuity, and hot baths as causes of decline in fertility. (Some of these conditions are still considered significant in this sense.) In Italy 48,000 of the 240,000 couples that marry each year discover that they have conception difficulties within 2 years of their marriage. Over 20,000 of these couples consult a physician for problems of infertility, and about half of these undergo assisted fertilization treatments [2]. The prevalence of childless couples reported in northern Europe and North America during the 20th century ranged from 8.4% to 21.5% [3]. Epidemiological studies in the general population show conception rates of 80–85% after 12 months of sexual activity without contraception [4]. In the remaining couples, who do not achieve pregnancy within 2 years, outcomes differ. Half of these have no apparent andrologic or gynecologic anomalies, and they achieve pregnancy within the next 2 years [5]. However, only 22–35% of couples in which the male has moderate to severe oligoasthenoteratospermia achieve spontaneous pregnancy within the next 12 years [6]. Stickler et al. [3] have reported that a couple's fertility is correlated with coital frequency and with the woman's age, which is more important than that of the man. However, in older men the ejaculate is both qualitatively and quantitatively inferior. The sperm count is lower, the sperm that are present are less mobile, and there is a higher frequency of chromosomal anomalies, which sometimes lead to spontaneous abortions [7]. Numerous studies have attempted to analyze factors that influence fertility. Those that have been verified include:
-
1.
cigarette smoking [8];
-
2.
physical exercise, diet, and changes in body weight [9], less evident in males [10];
-
3.
psychological and emotional factors [11];
-
4.
medical and surgical pathologies, varicocele, cryptorchidism, and acute orchialgia;
-
5.
drugs;
-
6.
pelvic infections and sexually transmitted diseases.
Infertility affects approximately 15–20% of all couples. Its main causes include [12]:
-
•
male pathology in 30–40%;
• female pathology in 30–40%;
-
•
problems involving both partners in 20%;
-
•
idiopathic causes in the remaining 10–20%.
Table 1 shows the percentages of incidence of the different pathologies responsible for male infertility, based on series reported in the literature.
Table 1.
Causes of male infertility
| Associated pathology | Dubin and Amelar [38] (% on 1294 pz) | Van Zyl et al. [39] (% on 596 pz) | WHO(% on 7057 pz) | Behre et al. [40] (% on 7802 pz) |
|---|---|---|---|---|
| Infection | – | 26 | 6.6 | 9.0 |
| Varicocele | 39 | 24 | 12.3 | 16.6 |
| Chromosomal anomalies | 3 | 12 | 2.1 | – |
| Cryptorchidism | 4 | 3 | – | 8.5 |
| Endocrinopathies | 9 | 2 | 0.6 | 8.9 |
| Obstructions | 7 | 3 | – | 1.5 |
| Idiopathic | 38 | 30 | 48.5 | 31.7 |
| Sexual | – | – | 1.7 | 5.7 |
| Testicular neoplasia | – | – | – | 2.3 |
| Systemic diseases | – | – | – | 5.0 |
The incidence of infertility in couples (especially that secondary to male factors) is obviously destined to increase due to the effect of social factors – which raise the mean age at which pregnancy is first attempted – and changes in the environment [7].
A relatively modest percentage of male infertility cases, 30–67% those with azoospermia [38], is caused by obstruction-stenosis of the seminal ducts. This type of pathology is of particular interest because it is potentially curable with the use of surgery.
Ejaculatory duct obstruction is clinically manifested by the appearance of symptoms, such as painful ejaculation, penile pain, hemospermia, and infertility [13,14].
The causes of these disorders can be congenital or acquired. The former type includes atresia or stenosis of the ejaculatory duct, ectopic ureter, utricular cysts, and Mullerian or Wolffian duct cysts, which can compress the ejaculatory ducts [15]. Among the latter causes are urethral catheterization and endoscopy procedures, infections or inflammatory states, and obstructions due to calculi [16].
However, in many cases, these obstructions are considered idiopathic.
The diagnosis of obstructive infertility does not require diagnostic imaging. However, its subsequent treatment is impossible unless the site and characteristics of the obstruction are known. We focused our attention on the use of vesiculodeferentography (VDG) in this setting.
Materials and methods
Our experience with non-surgical VDG, which began in the 1980s, prompted us to conduct a literature search based on PubMed and specific texts found in various university libraries and that of our Institution. For the PubMed search we used the key words vesiculodeferentography, ejaculatory duct obstruction, male infertility, and seminal duct imaging and considered studies conducted since 1979. We also reviewed all available books on urology and urologic radiology published over the last 5 years. Our objective was to assess the opinions of other authors on the role and function of VDG in the diagnosis and treatment of male infertility.
VDG is a diagnostic radiological method that evaluates the morphology and the patency of the distal seminal ducts. It was developed in 1913 by Belfield, who had been describing injectional intradeferential drug therapy since 1903. Use of the method spread thanks to the work of Boreau, who published a book in French in 1935 on radiographic imaging of the seminal ducts. An English edition of this work was published in 1974 [17,18].
VDG is carried out after surgical exposure of the vas deferens. Its increasing use led to the “endoscopic” tendencies advocated by Merricks in 1949. However, despite technological advances in urologic equipment, endoscopic catheterization of the ejaculatory ducts was not widely used for various reasons: first of all it was difficult to perform, due to the location of the orifices of the ducts, in the posterolateral portion of the seminal colliculus (Veru Montanum) and the fragility of the ducts themselves. It also carried the risk of ascendant infections [19,20].
For teaching purposes, VDG can be classified as anterograde or retrograde and by the manner in which it is performed (direct catheterization of the ejaculatory ducts or direct puncture, the latter involving transrectal or transperineal access).
Traditional surgery calls for exposure of the vasa deferentia, under local anesthesia, with the aid of an Allis forceps. The tunica is stripped from each vas deferens for a length of approximately 1 cm, and the denuded segments are held under tension between two vascular loops [21,22].
The lumen is then cannulated with a short (9.5 mm) 25-G Butterfly needle. In expert hands, this procedure does not cause local damage [23], and consequently it can be repeated multiple times on the same patient (at least in theory). Some authors, to eliminate the risk of secondary stenosis, prefer to cannulate the vas deferens via deferentectomy [24,25].
Non-ionic contrast medium is then injected into the seminal ducts. As the contrast medium advances, real-time radiographic images are recorded (as open pelvis anteroposterior views according to Chevassu) on a television chain. Opacification begins in the vas deferens and subsequently extends to the ampullae, the seminal vesicles, and the ejaculatory ducts. At the end of the examination reflux of the contrast medium is observed in the bladder.
Complications include stenosis of the vas deferens (caused by cannulation or by inflammatory reactions to the contrast medium), ischemia (caused by traction, lesions, or spasm of the vasa deferentia), hematomas, and spermatic granuloma.
VDG is a fairly invasive technique that requires anesthesia. The procedure itself can also lead to obstruction of the seminal duct, as well as damage caused by the ionizing radiation.
In 1979, in the attempt to obviate the damage caused by surgical preparation of the vasa deferentia and the difficulties involved in the endoscopic approach, Meyer proposed blind needle puncture of the seminal vesicles through the rectal ampullae [26]. This approach naturally proved to be quite traumatic, and it was also difficult to maintain sterility.
Exactly 20 years ago, we published a short article in Italian entitled “Vesciculodeferentografia transperineale sotto guida ecografica: prime esperienze” [Transperineal vesiculodeferentography under ultrasound guidance: Initial experiences”] [27], which described transperineal access to the seminal vesicles through the so-called “triangle of safety”. The procedure was performed under ultrasound (US) guidance achieved by the placement of a biplanar endorectal probe. In other words, our procedure was an adaptation of the well-known standardized approach widely used for biopsies of the prostate (Fig. 1).
Fig. 1.
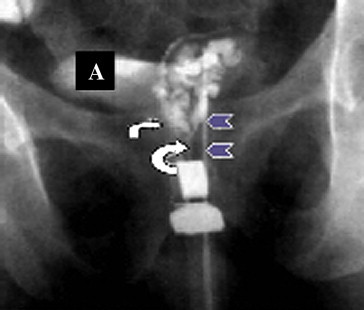
A normal seminal vesiculogram. Contrast medium is injected through a needle (arrow heads) to opacify the left seminal vesicle, the ampullae of the vas deferens (small arrow), and the ejaculatory duct (large arrow). The contrast medium expands into the bladder (A), reflecting the patency of the ejaculatory duct.
More specifically, after the induction of local anesthesia, an 18-G needle was inserted directly into the seminal vesicle under radiologic guidance, and the contents were collected by aspiration for biochemical analyses, sperm counts, and studies of sperm morphology (Fig. 2).
Fig. 2.
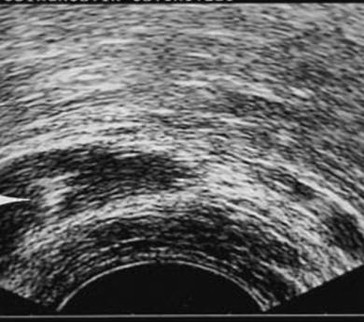
Direct puncture of the seminal vesicles under transrectal ultrasound guidance. This transverse scan shows the tip of the needle (which is hyperechoic) within the right seminal vesicle.
Contrast medium was then injected directly into the vesicle. In a series of approximately 70 patients, complete examination of the seminal duct (possibly on both sides), from the epididymis to the opening of the duct in the bladder wall, was achieved in 80% of the cases.
In 50% of the cases characterized by obstruction, we observed leakage of the contrast medium into the soft tissues contiguous to the seminal vesicles or around the needle. The leakage was caused by the elevated pressure created by the contrast agent within the obstructed seminal ducts, which could not be further dilated. We are personally convinced that the pressure exerted by the contrast medium reopened numerous suboccluded seminal ducts. The fact that approximately 20% of our patients experienced significant improvement after the diagnostic procedure alone seems to confirm this hypothesis.
Our manuscript was rejected by the most important international journals of that era on the grounds that the procedure was impossible to perform or associated with technical difficulties. The truth is that the entire procedure could be performed easily in less than 20 min provided that the seminal vesicles were fully distended (as a result of an appropriate period of abstinence from sexual activity) – an aspect that is verified sonographically prior to insertion of the needle. After the procedure, patients received antibiotic coverage for 5–7 days, and as a result, there were no inflammatory complications.
Although they were probably unaware of our previous publication, Abe et al. in 1989 [28] and Asch and Toi in 1991 [29] described what proved to be an adapted version of our method. It involved transrectal access to the seminal vesicles with an end-fire US probe. This type of access is obviously easier to learn and to perform, and it is also less painful for the patient. However, there is an obvious risk of potentially dangerous inflammatory complications.
In 1997, Jones et al. published an elegant article on their experience with this technique [30]. Using 17-G needles that were 30-cm-long, these authors achieved good visualization of the seminal ducts and collected abundant specimens of the vesicle contents in a large number of cases. They used this technique on 12 patients, and anesthesia was required only in those cases in which the procedure was followed by invasive recanalization of the ejaculatory duct. They stressed the advantages of the transrectal approach, which included lower complication rates, improved visualization of the more distal portions of the reproductive tract, and the fact that therapeutic interventions (aspiration of prostatic or periprostatic cysts, elimination of ejaculatory duct obstructions) could be carried out during the examination itself.
Similar procedures were described by Killi et al. in 1999 [31] and by Apaydin et al. in 2004 [32]. The technique developed by the latter group (in 10 patients) involved the use of an ultrasound contrast agent (Levovist®, Schering) mixed with methylene blue. This enabled the authors to assess the passage of the contrast medium through the ampullae all the way to the bladder but, owing to the imaging technique used, they were not able to assess the vasa deferentia. In the same study, the authors evaluated the efficacy of this technique combined with transurethral resection of the ejaculatory ducts in eliminating distal stenoses. Compared with previously used methods, the new approach proved to be more effective and associated with fewer complications. As noted in earlier studies, it was also easy to perform. More recently, in 2006, Lawler et al. proposed the use of transrectal vesiculodeferentography followed by endoluminal recanalization of obstructed seminal ducts using a balloon catheter. The results of this study were also interesting from both the diagnostic and therapeutic points of view [33]. VDG allowed clear visualization only of more obvious anomalies. The study also showed that radiological changes in the seminal ducts do not necessarily reflect loss of functional patency; even more important, functional obstructions may be associated with normal findings [34].
As these results demonstrate, VDG needs to be combined with functional studies like seminal tract washout (STW), which should be performed at the same time and in the same center [18,35]. STW consists in anterograde lavage of the seminal ducts starting from the proximal-most segment of the vas deferens. This study and counts of the sperm recovered from the bladder (Sp-STW) can reveal whether or not there are sperm downstream from the epididymis. (An Sp-STW count of 0 combined with testicular biopsy evidence of more or less normal gametogenesis is indicative of complete obstruction of the proximal duct at the epididymal or intratesticular level.) An Sp-STW that is better than the semen analysis indicates altered ejaculatory emptying and spermatic stasis at the level of the distal seminal ducts. To perform an STW the vas deferens is first catheterized (as for VDG), and 25–30 ml of 0.9% saline plus 0.5 ml of 10% methylene blue are injected (on each side). Resistance to the passage of the lavage fluid should be noted. (There is generally a progressive decrease in resistance as the dense material filling the ducts is removed.) The liquid recovered from the bladder (via catheterization or collection of the urine passed with the first two post-procedure voidings) is centrifuged and subjected to a sperm count (Sp-STW). In selected subjects with hypoposia, oligoazoospermia, reduced levels of biochemical vesicular markers, and sonographic evidence of seminal vesicle distention, Colpi et al. [34] found high sperm counts in the liquid collected from the bladder after STW performed during VDG. The absence of obstruction at the level of the ejaculatory ducts in these patients was consistent with functional disturbances of ejaculatory emptying at the level of the distal seminal ducts [36]. These investigators later used STW during VDG in patients with anatomic subtotal obstructions (congenital or acquired) of the distal seminal ducts, and a modified version of this diagnostic procedure has also been used therapeutically, that is, for the recovery of sperm for use in assisted fertilization procedures from men with neurologic forms of an ejaculation or post-inflammatory subtotal obstruction of the ejaculatory ducts [37]. In theory, STW could also be done during a VDG performed via direct transrectal or transperineal puncture of the seminal vesicles although there are currently no reports on this approach in the literature.
Conclusions
The use of magnetic resonance imaging for studies of the seminal ducts is limited by problems of low spatial resolution. Multislice CT has also been used for this purpose but this approach is associated with unacceptable radiation exposure of the gonads (and patient). In light of these considerations, VDG appears to have a precise albeit limited role in the study of obstructive male infertility in determining the site of the obstruction prior to surgical or (better yet) mini-invasive forms of treatment. It can also play a relatively important role in oncology, where it is occasionally used to demonstrate infiltration of the seminal vesicle by prostate cancers (or other tumors). In this setting it is superior to MRI in terms of safety and spatial resolution.
In any case, VDG is too invasive for widespread use as an exclusively diagnostic procedure, but it can be justified when it is associated with a therapeutic intervention, e.g., micro- or endosurgical repair of obstructions revealed by the diagnostic procedure, recovery of sperm for assisted fertilization procedures. It is also important to recall that in clinically selected cases, standard US itself can be of value in the detection of obstructive pathology. This is especially true of studies performed with sophisticated, last-generation equipment and comparative pre- and post-ejaculatory studies done with 3D technology (which is far more sensitive to volumetric changes).
Conflict of interest
The authors have no conflict of interest.
References
- 1.Shamma F.N., DeCherney A.H. Infertilità: una prospettiva storica. In: Keye W.R., Chang R.J., Rebar R.W., Soules M.R., editors. Infertilità – valutazione e trattamento. Edizione Italiana Verduci; Roma: 1997. pp. 3–7. [Google Scholar]
- 2.Menchini Fabris G.F., Turchi P. Infertilità maschile. Epidemiologia ed eziopatogenesi. In: Molinatti F., Fontna D., editors. Andrologia. Fisiopatologia e clinica. Verduci; Roma: 1997. p. 175. [Google Scholar]
- 3.Stickler R.C. Fattori che influenzano la fertilità. In: Keye W.R., Chang R.J., Rebar R.W., Soules M.R., editors. Infertilità – valutazione e trattamento. Edizione Italiana Verduci; Roma: 1997. pp. 8–19. [Google Scholar]
- 4.Vessey M.P., Wright N.H., Mc Pherson K. Fertility after stopping different methods of contraception. Br Med J. 1978 Feb 4;1(6108):265–267. doi: 10.1136/bmj.1.6108.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller D.W., Strickler R.C., Warren J.C. Appleton-Century-Croft; 1984. Clinical infertility. [Google Scholar]
- 6.Schoysman R. Valutazione del fattore maschile: che cosa è determinante? G SIFES. 1994;1:9–16. [Google Scholar]
- 7.Scaravelli G. Istituto Superiore Sanità, Registro Nazionale Procreazione Medicalmente Assistita; 2007. Infertilità maschile e femminile. [Google Scholar]
- 8.Howe G., Westoff C., Vessey M. Effects of age, cigarette smoking and other factors on fertility: findings in a large prospective study. Br Med J. 1985;290:1697–1700. doi: 10.1136/bmj.290.6483.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebar R.W. Exercise and reproduction. Semin Reprod Endocrinol. 1983;3:1–88. [Google Scholar]
- 10.Schinfeld J.S. Effects of athletics on male reproduction and sexuality. Med Aspects Hum Sex. 1989;23:67–74. [Google Scholar]
- 11.Paulson J.D., Haarmann B.S., Salerno R.L. An investigation of the relationship between emotional maladjustment and infertility. Fertil Steril. 1988;49:258–262. doi: 10.1016/s0015-0282(16)59712-x. [DOI] [PubMed] [Google Scholar]
- 12.Skakkebaek N.E., Giwerman A., de Kretser D. Pathogenesis and management of male infertility – review article. Lancet. 1994;343:1473. doi: 10.1016/s0140-6736(94)92586-0. [DOI] [PubMed] [Google Scholar]
- 13.Pryor J.P., Hendry W.F. Ejaculatory duct obstruction in subfertile males: analysis of 87 patients. Fertil Steril. 1991;56:725–730. doi: 10.1016/s0015-0282(16)54606-8. [DOI] [PubMed] [Google Scholar]
- 14.Turek P.J. Seminal vesicle and ejaculatory duct surgery. In: Glenn Graham., editor. Urologic surgery. 5th ed. Lippincott-Raven; Jr. Philadelphia: 1998. pp. 477–486. sect. VII. [Google Scholar]
- 15.Littrup P.J., Lee F., Mcleary R.D. Transrectal US of the seminal vesicles and ejaculatory ducts: clinical correlation. Radiology. 1988;168:625. doi: 10.1148/radiology.168.3.3043543. [DOI] [PubMed] [Google Scholar]
- 16.Fish H., Lambert S.M., Goluboff E.T. Management of ejaculatory duct obstruction: etiology, diagnosis and treatment. World J Urol. 2006;24(6):604–610. doi: 10.1007/s00345-006-0129-4. Review. [DOI] [PubMed] [Google Scholar]
- 17.Colpi G.M., Franco G., Greco E. Linee guida su: la azoospermia. Parte prima: la diagnosi. G Ital Androl. 1998;5:2–13. [Google Scholar]
- 18.Colpi G.M., Negri L., Mariani M.E. Semen anomalies due to voiding defects of ampullo-vesicular tract. Andrologia. 1990;22:206–218. doi: 10.1111/j.1439-0272.1990.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 19.Colpi G.M., Negri L., Nappi R.E. Is transrectal ultrasonography a reliable diagnostic approach in ejaculatory duct sub-obstruction? Hum Rep. 1997;12:2186–2191. doi: 10.1093/humrep/12.10.2186. [DOI] [PubMed] [Google Scholar]
- 20.Aggour A., Mostafa H., Maged W. Endoscopic management of ejaculatory duct obstruction. Int Urol Nephrol. 1998;30(4):481–485. doi: 10.1007/BF02550229. [DOI] [PubMed] [Google Scholar]
- 21.Colpi G.M., Negri L., Scroppo F.I. Epididymal ultrasonographic findings in case of obstructive pathology. Acta Chir Hung. 1994;34:299–302. [PubMed] [Google Scholar]
- 22.Piediferro G. Corso di aggiornamento: Il trattamento dell'Infertilità maschile. Ospedale San Paolo; Milano: 19/05/2000. La diagnostica invasiva: indicazioni attuali. [Google Scholar]
- 23.Payne S.R., Pryor J.P., Parks C.M. Vasography, its indication and complications. Br J Urol. 1985;57:215–217. doi: 10.1111/j.1464-410x.1985.tb06427.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein M. Verduci; Roma: 1996. Chirurgia dell'infertilità maschile. [Google Scholar]
- 25.Keye W.R., Jr., Chang R.J., Rebar R.W. Verduci; Roma: 1997. Infertilità: valutazione e trattamento. [Google Scholar]
- 26.Meyer J.J., Hartig P.R., Koos G.W. Transrectal seminal vesiculography. J Urol. 1979;121:129–130. doi: 10.1016/s0022-5347(17)56693-8. [DOI] [PubMed] [Google Scholar]
- 27.Solivetti F.M. Vesciculodeferentografia transperineale sotto guida ecografica: prime esperienze. Acta Urol Ital. 1988;II(3):197. [Google Scholar]
- 28.Abe M., Watanahc H., Kojima M. Puncture of the seminal vesicles guided by a transrectal real-tie linear scanner. J Clin Ultrasound. 1989;17:173–178. doi: 10.1002/jcu.1870170303. [DOI] [PubMed] [Google Scholar]
- 29.Asch M.R., Toi A. Seminal vesicles: imaging and intervention using transrectal ultrasound. J Ultrasound Med. 1991;10:19–23. doi: 10.7863/jum.1991.10.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Jones T.R., Zagoria R.J., Jarow J.P. Transrectal US-guided seminal vesiculography. Radiology. 1997;205:276–278. doi: 10.1148/radiology.205.1.9314999. [DOI] [PubMed] [Google Scholar]
- 31.Killi R.M., Pourbagher A., Semerci B. Transrectal ultrasonography-guided echo-enhanced seminal vesiculography. BJU Int. 1999;84:521–523. doi: 10.1046/j.1464-410x.1999.00255.x. [DOI] [PubMed] [Google Scholar]
- 32.Apaydin E., Killi R.M., Turna B. Transrectal ultrasonography-guided echo-enhanced seminal vesiculography in combination with transurethral resection of the ejaculatory ducts. BJU Int. 2004;93:1110–1112. doi: 10.1111/j.1464-410X.2003.04790.x. [DOI] [PubMed] [Google Scholar]
- 33.Lawler L.P., Cosin O., Jarow J.P. Transrectal US-guided seminal vesiculography and ejaculatory duct recanalitazion and balloon dilatation for treatment of chronic pelvic pain. J Vasc Interv Radiol. 2006;17(1):169–173. doi: 10.1097/01.rvi.0000186956.00155.26. [DOI] [PubMed] [Google Scholar]
- 34.Colpi G.M., Casella F., Zanollo A. Functional voiding disturbances of the ampullo-vesicular seminal tract: a cause of male infertility. Acta Eur Fertil. 1987;18:165–179. [PubMed] [Google Scholar]
- 35.Colpi G.M., Negri L., Scroppo F.I. Seminal tract washout: a new diagnostic tool in complicated cases of male infertility. J Androl. 1994;15:17s–22s. [PubMed] [Google Scholar]
- 36.Ichijo S., Sigg C., Nagasawa M. Vasoseminal vesiculography before and after ejaculation. Urol Int. 1981;36:35. doi: 10.1159/000280391. [DOI] [PubMed] [Google Scholar]
- 37.Colpi G.M., Negri L., Stamm J. Full-term pregnancy obtained with sperm recovered by seminal tract washout from an anejaculating spinal cord injured man. J Urol. 1992;148:1266–1267. doi: 10.1016/s0022-5347(17)36886-6. [DOI] [PubMed] [Google Scholar]
- 38.Dubin L., Amelar R.D. Etiologic factors in 1294 consecutive cases of male infertility. Fertil Steril. 1971;22:469–474. doi: 10.1016/s0015-0282(16)38400-x. [DOI] [PubMed] [Google Scholar]
- 39.Van Zyl J.A., Menkveld R., van Kotze T.J., Retief A.E., van Niekerk W.A. Oligozoospermia: a seven-year survey of the incidence, chromosomal aberrations, treatment and pregnancy rate. Int J Fertil. 1975;20:129–132. [PubMed] [Google Scholar]
- 40.Behre H.M., Kliesch S., Schudel F., Nieschlag E. Clinical relevance of scrotal and transrectal ultrasonography in andrological patients. Int J Androl. 1995;18(Suppl. 2):27–31. [PubMed] [Google Scholar]


