Abstract
Background
The treatment of choice for hepatocellular carcinoma (HCC) is surgical resection but only a small percentage of patients are operative candidates. Percutaneous radiofrequency interstitial thermal ablation (RFA) has proved to be effective in the treatment of unresectable HCC. However, there is a sub-group of patients who may benefit from a laparoscopic rather than a percutaneous approach. Laparoscopic RFA offers the combined advantages of improved tumor staging based on the intracorporeal ultrasound examination and safer access to liver lesions that are difficult or impossible to treat with a percutaneous approach. The aim of our review was to evaluate the advantages and limitations of the laparoscopic approach, according to the criteria of evidence-based medicine.
Conclusions
Laparoscopic RFA of HCC proved to be a safe and effective technique, at least in terms of the short- and mid-term results. This technique may be indicated in selected cases of HCC when percutaneous RFA is very difficult or contraindicated.
Keywords: Hepatocellular carcinoma, Liver cirrhosis, Laparoscopic ultrasound, Radiofrequency interstitial thermal ablation
Sommario
Background
Il trattamento ottimale dell'epatocarcinoma è la resezione epatica. Tuttavia, solo una piccola percentuale di pazienti può essere sottoposta a intervento. La termoablazione mediante radiofrequenza per via percutanea si è dimostrata efficace nel trattamento degli epatocarcinomi non resecabili. Tuttavia, esiste un sottogruppo di pazienti che potrebbe essere adatto a un approccio per via laparoscopica. Il razionale di questa tecnica è quello di associare i vantaggi di una miglior stadiazione assicurata dalla valutazione ecografica intraoperatoria con un sicuro approccio alle lesioni epatiche difficili o impossibili da trattare per via percutanea. Lo scopo di questa revisione critica del tema è quello di valutare vantaggi e limiti dell'approccio laparoscopico secondo i criteri di una medicina basata sull'evidenza.
Conclusioni
La radiofrequenza per via laparoscopica del'epatocarcinoma si è dimostrata una metodica sicura ed efficace almeno a medio-termine. Questa tecnica può essere indicata in casi selezionati quando l'approccio percutaneo alla lesione da trattare è molto difficile o controindicato.
Introduction
Radiofrequency interstitial thermal ablation was initially used as an attempt to achieve local control in cases of unresectable liver tumors. More recently, however, its use has been considered with curative intent [1]. An increasing number of patients with cirrhosis and hepatocellular carcinoma (HCC) are being treated with radiofrequency ablation (RFA), but these patients are still less in number than those with metastatic lesions [2]. Radiofrequency ablation is most commonly performed as a percutaneous procedure [3,4], but there is a sub-group of patients who may benefit from laparoscopic RFA [5–8]. The latter approach combines the advantages of improved staging accuracy based on the intracorporeal ultrasound (US) examination [9] with increased safety in the elimination of liver lesions that are difficult or impossible to treat percutaneously [10]. The aim of our review was to evaluate the advantages and limitations of RFA from a surgeon's point of view, according to the levels of evidence (Table 1) [11].
Table 1.
Levels of evidence
| Level | Therapy/prevention, aetiology/harm | Diagnosis |
|---|---|---|
| 1a | SR (with homogeneitya) of RCTs | SR (with homogeneitya) of level 1 diagnostic studies; clinical decision rules with 1b studies from different clinical centres |
| 1b | Individual RCT (with narrow confidence interval) | Validating cohort study with good reference standards; or clinical decision rules tested within one clinical centre |
| 1c | All or none case-series | Absolute SpPins and SnNoutsb |
| 2a | SR (with homogeneitya) of cohort studies | SR (with homogeneitya) of level > 2 diagnostic studies |
| 2b | Individual cohort study (including low quality RCT; e.g., <80% follow-up) | Exploratory cohort study with good reference standards; clinical decision rules after derivation, or validated only on split-sample or databases |
| 2c | “Outcomes” research; ecological studies | |
| 3a | SR (with homogeneitya) of case-control studies | SR (with homogeneitya) of 3b and better studies |
| 3b | Individual case-control study | Non-consecutive study; or without consistently applied reference standards |
| 4 | Case-series (and poor quality cohort and case-control studies) | Case–control study, poor or non-independent reference standard |
| 5 | Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” | Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” |
Source: Ref. [11].
SR = systematic review and RCT = randomized controlled trials.
By homogeneity we mean a systematic review that is free of worrisome variations (heterogeneity) in the directions and degrees of results between individual studies.
An “Absolute SpPin” is a diagnostic finding whose specificity is so high that a positive result rules-in the diagnosis. An “Absolute SnNout” is a diagnostic finding whose sensitivity is so high that a negative result rules-out the diagnosis.
Laparoscopic staging
Intraoperative US (IOUS) is the most accurate diagnostic technique for detecting focal liver lesions [12]. However, in patients with cirrhosis and HCC, some of the nodules detected with this approach are non-neoplastic [13]. Its accuracy can be enhanced by the use of second-generation US contrast agents, which improves the power of differentiating new nodules detected by intraoperative US in cirrhotic livers [14].
The use of laparoscopic ultrasound (LUS) overcomes the two major limitations of laparoscopy, i.e., inspection limited to the surface of the organs and the impossibility of evaluation based on palpation of structures. LUS provides the advantages of an IOUS examination, in terms of similar accuracy, with a mini-invasive access [15]. However, at the moment, LUS technology does not permit the use of a US contrast agent. The problem of differentiating new malignant nodules from other kinds of lesions remains open: from a technical point of view, it can be very difficult to insert a fine needle into deep-seated hepatic tumors using this type of probe [10] (Fig. 1). Some authors have evaluated the potential benefits of LUS in staging liver tumors. Only some of these reports dealt with patients with liver cirrhosis and hepatocellular carcinoma (Table 2) [16–21].
Fig. 1.
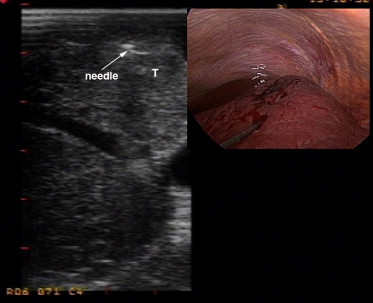
Ultrasound and intraoperative images with radiofrequency needle electrode. The tip of the electrode has been positioned inside the target liver nodule.
Table 2.
Published series of laparoscopic ultrasound (LUS) staging of hepatocellular carcinoma (HCC)
| References | No. of patients | HCC (% of total patients) | Further information |
|---|---|---|---|
| John et al. [16] | 43 | 15 | Better staging in 42% |
| Tandan et al. [15] | 31 | 10 | Avoidance of useless LPT in 10% |
| Ido et al. [17] | 186 | 100 | Better staging in 12% |
| Lo et al. [18] | 198 | 100 | Avoidance of useless LPT in 16% |
| D'Angelica et al. [19] | 401 | 8 | Avoidance of useless LPT in 20% |
| De Castro et al. [20] | 76 | 43 | Avoidance of useless LPT in 24% |
| Montorsi et al. [21] | 132 | 100 | Avoidance of useless LPT in 14% |
LPT = laparotomy.
There is a reasonable amount of evidence supporting the value of laparoscopic staging and its potential impact on the management of patients with HCC and liver cirrhosis (level 2b evidence), as shown by the results of our study published in 2002 [21]. Our updated personal experience now includes 207 patients who have been evaluated with LUS. In 53 (25%) of these cases, LUS revealed new malignant liver lesions that had not been detected with preoperative US or computed tomography (iodized-oil and/or spiral). In 19 cases, the new tumors were located in the same liver segment, near the primary lesion, and in 34 cases, in different liver segments (Fig. 2). Prior to the LUS examination, 130 of the 207 patients were believed to have a solitary HCC; in 24 of these cases (18%) new malignant nodules were identified. New malignant lesions were detected in 29 (38%) of the 77 patients with a preoperative diagnosis of two or more nodules. The likelihood of identifying new malignancies intraoperatively is thus higher when more than one lesion has been detected preoperatively (p = 0.0022).
Fig. 2.
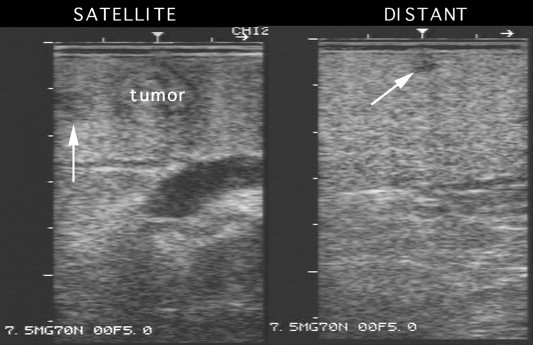
Laparoscopic ultrasonographic (LUS) images of new, sub-centimeter lesions (satellite and at a distance from the primary tumor) missed by preoperative computed tomography and ultrasonography.
Most of the new nodules were very small with a mean diameter of 10.8 ± 4.5 mm (median: 10 mm). In our experience, the results of LUS modified the original treatment plan in 49 of the 53 patients with new malignant nodules: 11 patients underwent only laparoscopic exploration, 32 required an additional session of interstitial therapy, and in 4 other cases an additional non-anatomic resection was performed. In the remaining four cases, the new malignant lesion was close to the primary nodule and was therefore included in the planned segmental hepatic resection.
Comparison with liver surgery
It is currently unclear whether accurate understanding of the stage of disease can benefit the patient, with respect to prognosis and treatment planning (level 2b evidence). An answer to this question could be obtained by analyzing the results of prospective studies, comparing patients treated with RFA and those who underwent surgical resection with IOUS evaluation (Table 3). There are five studies of this type in the literature [22–26], but only one was a randomized controlled trial (RCT) [26]. Some biases are present: none of the studies examined the impact on outcome of the more accurate staging based on IOUS, which is the most important advantage of using a therapeutic modality associated with IOUS examination. Our study is the only one that evaluated this aspect, comparing the IOUS findings obtained during liver resection with the results of LUS during RFA [23]. It is reasonable to assume that IOUS-based staging will influence the incidence of HCC recurrences. In fact, four studies [22–25] documented lower rates of intrahepatic recurrence after surgery than after RFA (in one, the difference was limited to local recurrences [24]), while the RCT [26] showed no differences. The lower recurrence rates observed in the majority of these studies might be determined at least in part by improved IOUS-based staging. Two of the studies [22,25] also found better survival rates after surgery, while no differences were observed in the other three. The similar overall survival rates associated with RFA in these studies may be the result of repeat ablation after local recurrence. The most successful predictor of outcome seems to be the underlying state of the liver. However, in patients with small nodules, RFA may be a potential alternative to liver resection [27]. For larger tumors that are still resectable, surgery is unquestionably the best option, although laparoscopic RFA could eliminate some of the problems associated with percutaneous RFA, by allowing intraoperative staging and/or more aggressive treatment of the nodule. However, use of RFA as an alternative to surgery in patients with resectable HCCs cannot be justified. The role of RFA in HCC patients who are surgical candidates is still controversial, and it is not sufficiently well-established to be considered as the equivalent of surgery (level 2b evidence). More work needs to be done to define the role of RFA as an alternative to resection, but compared with percutaneous RFA, the use of laparoscopic RFA with LUS examination is more likely to provide the advantages of surgical resection with IOUS staging.
Table 3.
Published series of HCCs treated with radiofrequency ablation (RFA) vs. surgical resection
| Author | Study type | Patients and treatment | Size HCC | Recurrence rates | Survival rates |
|---|---|---|---|---|---|
| Vivarelli et al. [22] | Retrospective | 79 RES | <5 cm | 50% RES* | 65% RES* |
| Comparative | 79 P-RFA | 66% P-RFA* | 33% P-RFA* | ||
| 3 Years | 3 Years | ||||
| Montorsi et al. [23] | Retrospective | 40 RES | <5 cm | 30% RES* | 73% RES |
| Comparative | 58 L-RFA | 53% L-RFA* | 61% L-RFA | ||
| 3 Years | |||||
| Hong et al. [24] | Retrospective | 93 RES | <4 cm | 45% RES° | 84% RES |
| Comparative | 55 P-RFA | 58% P-RFA° | 73% P-RFA | ||
| 3 Years | |||||
| Wakai et al. [25] | Retrospective | 85 RES | <4 cm | 2% RES* | 75% RES* |
| Comparative | 64 P-RFA | 27% P-RFA* | 55% P-RFA* | ||
| 5 Years | |||||
| Chen et al. [26] | RCT | 90 RES | <5 cm | 31% RES | 73% RES |
| 90 P-RFA | 40% P-RFA | 69% P-RFA | |||
| 3 Years | 3 Years | ||||
RCT = randomized controlled trial; L-RFA = laparoscopic RFA; P-RFA = percutaneous RFA; and RES = resection.
*p < 0.05; and °p < 0.05 only for local recurrences.
Today, the majority of RFA procedures are still performed percutaneously. In the absence of data from randomized trials, there is no consensus among experts about which approach is the best [27]. Laparoscopic access can be used successfully to treat patients with tumor characteristics that have been associated with unsuccessful treatment with percutaneous RFA, i.e., superficial lesions located near other organs, deep-seated tumors, multiple lesions, or large HCCs [28,29]. Laparoscopy is less invasive than laparotomy, but it also offers decreased access to the liver and limits the positioning of US and radiofrequency ablation probes.
Effects of the laparoscopic approach
Several authors [5–7,29–37] have evaluated the potential benefits of laparoscopic RFA for treatment of liver tumors, but only a few of these reports involved series with more than 10 patients with liver cirrhosis and hepatocellular carcinoma (Table 4) [29–31,36,37]. Because laparoscopic RFA is a relatively new treatment modality for HCC, the data in the literature consist largely of preliminary observations with short periods of follow-up. Complete necrosis was achieved in 86% [7] to 100% [6,29,31,33,34] of the lesions treated, although in some situations a second procedure was required to ablate residual tumor tissue. Intrahepatic recurrence of HCC is common with all types of treatment (excluding liver transplantation), and incidence rates following laparoscopic RFA range from 0% [7,32] to 54% [36,37] after mean follow-ups of 6.8–24.2 months.
Table 4.
Published series of hepatocellular carcinomas (HCCS) treated with laparoscopic radiofrequency ablation
| Authors | No. of patients | Child–Pugh classes | Ablative device | Total necrosis, n (%) | Morbidity, n (%) | HCC recurrence, n (%) | Mean follow-up (months) |
|---|---|---|---|---|---|---|---|
| Ido et al. [29] | 15 | 8A; 4B; 3C | Microwave | 15 (100) | 0 | 2 (13) | 16.8 |
| Goletti et al. [7] | 7 | – | Cooled | 6 (86) | 0 | 1 (14) | 17 |
| Seki et al. [30] | 24 | 21A; 3B | Microwave | 22 (92) | 3 (12)a | 13 (54) | 18 |
| Podnos et al. [31] | 12 | 12B | Multi-array | 12 (100) | 1 (8)a | 1 (8) | 7 |
| Cuschieri et al. [6] | 2 | – | Multi-array | 2 (100%) | 0 | 0 | 11.1 |
| Chung et al. [5] | 4 | – | Multi-array | – | 0 | 2 (50) | 8.2 |
| Siperstein et al. [32] | 4 | – | Multi-array | – | – | 0 | 13.9 |
| Machi et al. [33] | 3 | – | Multi-array | 3 (100) | 1 (33)b | – | 6.8 |
| Okano et al. [34] | 6 | 3A; 3C | Microwave | 6 (100) | 0 | 3 (50) | 20.2 |
| Topal et al. [35] | 8 | 3A; 4B; 1C | Cooled | – | 1 (12)e | 3 (37) | – |
| Santambrogio et al. [36,37] | 104 | 61A; 43B | Cooled | 88 (87) | 9 (9)b,c,d | 55 (54) | 24.2 |
Pneumothorax.
Ascites.
Intraperitoneal bleeding.
Jaundice.
Local abscess.
One of the largest series reported to date is the one we analyzed in 2003–2005 [36,37]. The present report analyzes the efficacy of laparoscopic RFA based on updated data: 153 patients with HCC and liver cirrhosis have now been treated with laparoscopic RFA. The laparoscopic approach proved to be useful for the treatment of superficial tumors (30 patients). It allowed direct visualization and displacement of surrounding structures (gallbladder, colon, stomach), which reduced the risks of damage during ablation of the liver tumor. The group of patients with HCCs located near these visceral structures was not significantly different from the group with tumors in other areas of the liver, in terms of total necrosis rates (93% vs. 86%; p = 0.284), HCC recurrences (50% vs. 57%; p = 0.468), or survival curves (p = 0.863). Furthermore, no major complications occurred in this group of patients, and this finding is in contrast with the complication rate reported for percutaneous thermoablation of tumors located close to other visceral structures [29]. In 73 patients, the main nodule was located deep in the liver, and RFA was deemed very difficult without laparoscopy and LUS guidance. In 38 cases, the lesion proved impossible to localize with percutaneous ultrasound. These 73 patients presented rates of total necrosis (87% vs. 88%; p = 0.96) and HCC recurrence (52% vs. 66%; p = 0.128), and survival curves (p = 0.863) that were similar to those whose tumors could be well visualized with percutaneous US. These findings suggest that laparoscopic RFA can be used to treat liver lesions that are poor candidates for percutaneous RFA.
On the other hand, when our data were analyzed based on the number of HCC nodules being treated (single vs. multiple), laparoscopic and percutaneous RFA produced similar results [27,28]. In both cases, complete necrosis rates are higher in patients with a single lesion than in those with multiple lesions (92% vs. 80%; p = 0.02). However, these two groups were not significantly different in terms of survival (p = 0.424) or HCC recurrence (55% vs. 57%; p = 0.773) rates. Similar findings have been reported regarding the impact on outcomes of HCC diameter [27,28,30]. In 98 patients with lesions smaller than 3 cm, total necrosis was achieved in 92 cases (94%), and this rate is significantly better than that recorded in the other 47 patients with lesions larger than 3 cm (74%) (p = 0.009). However, nodule size did not have a statistically significant impact on survival (p = 0.572) or HCC recurrences (55% vs. 57%; p = 0.790). The number and dimensions of the HCC nodules seem to influence total necrosis rates achieved with both laparoscopic and percutaneous RFA [2,3,38,39]. However, they do not seem to have any effect on the recurrence rate associated with laparoscopic RFA. This difference is difficult to explain based on the current literature data [27].
Laparoscopic RFA seems to be associated with good rates of total necrosis even in patients with HCCs that are relatively difficult to treat percutaneously due to inaccessible positions, multiplicity, or large tumor volumes (level 3b evidence). Additional studies are needed to determine whether laparoscopic RFA is also superior to the percutaneous approach in unselected patients.
The choice of the approach to be used for RFA of an HCC is usually dictated by the training and experience of the operator. Increased training and experience are accompanied by improvement in patient selection, choice of the appropriate approach, electrode-placement and ablation techniques [10], and perioperative management. Laparoscopic RFA is technically more demanding than the percutaneous approach because it requires experience in both laparoscopic ultrasound and laparoscopic, ultrasound-guided needle placement. In any case, the indications for laparoscopic RFA are likely to expand with the increasing experience and skill of the laparoscopic surgeon (level-4 evidence).
Thus far, we have excluded large tumors (>5 cm) from our studies [36,37]. Other authors have reported good results in these cases [4], although longer treatment and multiple electrode insertions may be necessary to achieve complete necrosis of the lesion. Interruption of hepatic blood flow has been shown to increase the volume of tissue that can be ablated with a single needle insertion in both experimental and clinical settings [40]. However, this approach has not been extensively studied in cases involving an HCC located in proximity to major hepatic vessels in a cirrhotic liver. Scott et al. [41] showed that ablations performed in a pig model with hepatic inflow occlusion were superior in size, shape, and symmetry to those performed under conditions of normal perfusion. However, no data are available on the safety of this approach. During RFA, blood flow protects vascular and biliary structures, and the absence of blood flow could thus result in thermal damage to blood vessels or bile ducts [42]. Two cases of vascular injury have been reported following open RFA performed during the Pringle maneuver [43].
Considering the risk of portal thrombosis associated with the Pringle maneuver, we do not favor its use. Moreover, it is important to recall that the pneumoperitoneum induced during laparoscopy already reduces hepatic blood flow by 30–40%, and this factor alone may increase the area of liver tissue necrosis that can be produced with RFA [42]. In the last 3 years, we have modified the technique in this way. Tumor vascularity was evaluated with color Doppler imaging (Fig. 3). Under ultrasonographic guidance, the RF electrode was then directed towards the tumor with direct puncture of the nearby blood vessel. The ablation cycle lasted 2–4 min. A second color Doppler study was performed to confirm that the vascular area had been completely ablated, and the nodule itself was then treated with conventional insertion of the RF electrode [10]. With this technique, we have treated 27 patients. At the 1-month CT evaluation, total necrosis was confirmed in all 27 patients. In contrast, in the group of 118 patients treated with standard laparoscopic RFA, total necrosis was observed in only 85% of cases (p = 0.03). Further analysis is necessary to determine whether this new technique also has a positive impact on recurrences and survival.
Fig. 3.
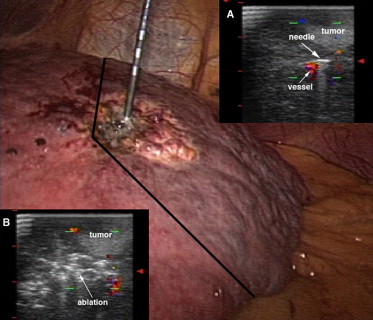
Tumor vascularity was evaluated with color Doppler imaging. Under ultrasonographic guidance, the tip of the needle was directed towards the tumor with direct puncture of the nearby blood vessel (inset A) and the ablation cycle lasted 2–4 min (inset B). Under laparoscopic vision, it is possible to see the color change of the liver surface produced by RFA.
Complications
The laparoscopic procedure proved to be feasible and safe with a low rate of serious complications [21,23,29,31]. In our series, only two patients required surgical intervention for hemorrhage at a trocar site. Bleeding from hepatic puncture sites or trocar insertion sites is a potential problem, but it can be readily recognized and treated laparoscopically. The laparoscopic approach allowed good hemostasis in nearly all cases in which active post-treatment bleeding occurred at the puncture site or trocar insertion sites. Treatment-related side effects (pain and fever) were mild, and the clinical course of the procedure was quite similar to that of diagnostic laparoscopy. Percutaneous access seems to be associated with a similar rate of complications, but they are more severe than those seen with laparoscopic approach: in a report published in 2002, 14 deaths were described in a group of patients treated with percutaneous RFA, while none occurred when laparoscopic access was used [42].
In one of our cases (1%), laparoscopic RFA was associated with parietal seeding. This problem can sometimes be eliminated by preliminary insertion of a 14-gauge intravenous needle or a 2-mm trocar through the abdominal wall. The 18-gauge needle electrode is then inserted through the larger-gauge sheath. In the case cited above, this type of protection could not be used because the lesion to be treated was large and a cluster needle had to be used. A review of the literature shows that the risk of electrode-track seeding varies widely, with rates ranging from 0.2% to 12.5% in patients treated with percutaneous RFA. The reasons for these differences are not clear [42,44,45].
Laparoscopic RFA seems to be a safer procedure. It is possible to prevent some complications, such as bleeding or visceral perforation, for certain patients at a risk of visceral lesions (level 2a evidence). Laparoscopy may often be the approach of choice to reduce the risk of electrode-track seeding (level 2c evidence).
Conclusions
In conclusion, laparoscopic RFA represents a safe, effective treatment for HCCs that are not amenable to surgical resection, especially when percutaneous RFA is deemed very difficult or impossible. The main advantages of the laparoscopic approach include better protection of vital structures and more accurate staging. Its use can therefore be proposed in appropriately well-selected patients (level 2c evidence).
References
- 1.Sutherland L.M., Williams J.A.R., Padbury R.T.A., Gotley D.C., Stokes B., Maddern G.J. Radiofrequency ablation of liver tumors: a systematic review. Arch Surg. 2006;141:181–190. doi: 10.1001/archsurg.141.2.181. [DOI] [PubMed] [Google Scholar]
- 2.Ng K.K., Lam C.M., Poon R.T., Ai V., Tso W.K., Fan S.T. Thermal ablative therapy for malignant liver tumors: a critical appraisal. J Gastroenterol Hepatol. 2003;18:616–629. doi: 10.1046/j.1440-1746.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 3.Lencioni R., Cioni D., Bartolozzi C. Percutaneous radiofrequency thermal ablation of liver malignancies: techniques, indications, imaging findings, and clinical results. Abdom Imaging. 2001;26:345–360. doi: 10.1007/s002610000194. [DOI] [PubMed] [Google Scholar]
- 4.Livraghi T., Goldberg S.N., Lazzaroni S. Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 5.Chung M.H., Wood T.F., Tsioulias G.J., Rose D.M., Bilchik A.J. Laparoscopic radiofrequency ablation of unresectable hepatic malignancies. A phase 2 trial. Surg Endosc. 2001;15:1020–1026. doi: 10.1007/s00464-001-0026-2. [DOI] [PubMed] [Google Scholar]
- 6.Cuschieri A., Bracken J., Boni L. Initial experience with laparoscopic ultrasound-guided radiofrequency thermal ablation of hepatic tumours. Endoscopy. 1999;31:318–321. doi: 10.1055/s-1999-16. [DOI] [PubMed] [Google Scholar]
- 7.Goletti O., Lencioni R., Armillotta N. Laparoscopic radiofrequency thermal ablation of hepatocarcinoma: preliminary experience. Surg Laparosc Endosc Percutan Tech. 2000;10:284–290. [PubMed] [Google Scholar]
- 8.Montorsi M., Santambrogio R., Bianchi P. Radiofrequency interstitial thermal ablation (LRF) of hepatocellular carcinoma (HCC) in liver cirrhosis: role of the laparoscopic approach. Surg Endosc. 2001;15:141–145. doi: 10.1007/s004640000242. [DOI] [PubMed] [Google Scholar]
- 9.Lo C.M., Lai E.C.S., Liu C.L., Fan S.T., Wong J. Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg. 1998;227:527–532. doi: 10.1097/00000658-199804000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santambrogio R., Bianchi P., Pasta A., Palmisano A., Montorsi M. Ultrasound-guided interventional procedures of the liver during laparoscopy. Technical considerations. Surg Endosc. 2002;16:349–354. doi: 10.1007/s004640090082. [DOI] [PubMed] [Google Scholar]
- 11.CEBM Centre for evidence-based medicine—levels of evidence. May 2001. <http://www.cebm.net> Available from:
- 12.Kokudo N., Bandai Y., Imanishi H. Management of new hepatic nodules detected by intraoperative ultrasonography during hepatic resection for hepatocellular carcinoma. Surgery. 1996;119:634–640. doi: 10.1016/s0039-6060(96)80187-5. [DOI] [PubMed] [Google Scholar]
- 13.Takigawa Y., Sugawara Y., Yamamoto J. New lesions detected by intraoperative ultrasound during liver resection for hepatocellular carcinoma. Ultrasound Med Biol. 2001;27:151–156. doi: 10.1016/s0301-5629(00)00346-x. [DOI] [PubMed] [Google Scholar]
- 14.Torzilli G., Olivari N., Moroni E. Contrast-enhanced intraoperative ultrasonography in surgery for hepatocellular carcinoma in cirrhosis. Liver Transpl. 2004;10(2 Suppl. 1):S34–S38. doi: 10.1002/lt.20050. [DOI] [PubMed] [Google Scholar]
- 15.Tandan V.R., Asch M., Margolis M., Page A., Gallinger S. Laparoscopic vs open intraoperative ultrasound examination of the liver: a controlled study. J Gastrointest Surg. 1997;1:146–151. doi: 10.1016/s1091-255x(97)80102-3. [DOI] [PubMed] [Google Scholar]
- 16.John T.G., Greig J.D., Crosbie J.L., Miles W.F.A., Garden O.J. Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound. Ann Surg. 1994;6:711–719. doi: 10.1097/00000658-199412000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ido K., Nakazawa Y., Isoda N. The role of laparoscopic US and laparoscopic US-guided aspiration biopsy in the diagnosis of multicentric hepatocellular carcinoma. Gastrointest Endosc. 1999;50:523–526. doi: 10.1016/s0016-5107(99)70076-3. [DOI] [PubMed] [Google Scholar]
- 18.Lo C.M., Fan S.T., Liu C.L. Determining resectability for hepatocellular carcinoma: the role of laparoscopy and laparoscopic ultrasonography. J Hepatobiliary Pancreat Surg. 2000;7:260–264. doi: 10.1007/s005340070046. [DOI] [PubMed] [Google Scholar]
- 19.D'Angelica M., Fong Y., Weber S. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol. 2003;10:183–189. doi: 10.1245/aso.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 20.De Castro S.M., Tilleman E.H., Busch O.R. Diagnostic laparoscopy for primary and secondary liver malignancies: impact of improved imaging and changed criteria for resection. Ann Surg Oncol. 2004;11:522–529. doi: 10.1245/ASO.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Montorsi M., Santambrogio R., Bianchi P., Dapri G., Spinelli A., Podda M. Perspectives and drawbacks of minimally invasive surgery for hepatocellular carcinoma. Hepatogastroenterology. 2002;49:56–61. [PubMed] [Google Scholar]
- 22.Vivarelli M., Guglielmi A., Ruzzenente A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montorsi M., Santambrogio R., Bianchi P. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg. 2005;9:62–68. doi: 10.1016/j.gassur.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Hong S.N., Lee S.Y., Choi M.S. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005;39:247–252. doi: 10.1097/01.mcg.0000152746.72149.31. [DOI] [PubMed] [Google Scholar]
- 25.Wakai T., Shirai Y., Suda T. Long-term outcomes of hepatectomy vs percutaneous ablation for treatment of hepatocellular carcinoma ≤4 cm. World J Gastroenterol. 2006;28:546–552. doi: 10.3748/wjg.v12.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M.S., Li J.Q., Zheng Y. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulier S., Ni Y., Jamart J., Ruers T., Marchal G., Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillams A.R. Radiofrequency ablation in the management of liver tumors. Eur J Surg Oncol. 2003;29:9–16. doi: 10.1053/ejso.2002.1346. [DOI] [PubMed] [Google Scholar]
- 29.Ido K., Isoda N., Kawamoto C. Laparoscopic microwave coagulation therapy for solitary hepatocellular carcinoma performed under laparoscopic ultrasonography. Gastrointest Endosc. 1997;45:415–420. doi: 10.1016/s0016-5107(97)70155-x. [DOI] [PubMed] [Google Scholar]
- 30.Seki S., Sakaguchi H., Kadoya H. Laparoscopic microwave coagulation therapy for hepatocellular carcinoma. Endoscopy. 2000;32:591–597. doi: 10.1055/s-2000-9014. [DOI] [PubMed] [Google Scholar]
- 31.Podnos Y.D., Henry G., Ortiz J.A. Laparoscopic ultrasound with radiofrequency ablation in cirrhotic patients with hepatocellular carcinoma: technique and technical considerations. Am Surg. 2001;67:1181–1184. [PubMed] [Google Scholar]
- 32.Siperstein A., Garland A., Engle K. Local recurrence after laparoscopic radiofrequency thermal ablation of hepatic tumors. Ann Surg Oncol. 2000;7:106–113. doi: 10.1007/s10434-000-0106-x. [DOI] [PubMed] [Google Scholar]
- 33.Machi J., Oishi A.J., Mossing A.J., Furumoto N.L., Oishi R.H. Hand-assisted laparoscopic ultrasound-guided radiofrequency thermal ablation of liver tumors: a technical report. Surg Laparosc Endosc Percutan Tech. 2002;12:160–164. doi: 10.1097/00129689-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Okano H., Shiraki K., Inoue H. Laparoscopic microwave coagulation therapy for small hepatocellular carcinoma on the liver surface. Oncol Rep. 2002;9:1001–1004. [PubMed] [Google Scholar]
- 35.Topal B., Aerts R., Penninckx F. Laparoscopic radiofrequency ablation of unresectable liver malignancies: feasibility and clinical outcome. Surg Laparosc Endosc Percutan Tech. 2003;13:11–15. doi: 10.1097/00129689-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Santambrogio R., Podda M., Zuin M. Safety and efficacy of laparoscopic radiofrequency of hepatocellular carcinoma in patients with liver cirrhosis. Surg Endosc. 2003;17:1826–1832. doi: 10.1007/s00464-002-8960-1. [DOI] [PubMed] [Google Scholar]
- 37.Santambrogio R., Opocher E., Costa M., Cappellani A., Montorsi M. Survival and intra-hepatic recurrences after laparoscopic radiofrequency of hepatocellular carcinoma in patients with liver cirrhosis. J Surg Oncol. 2005;89:218–226. doi: 10.1002/jso.20204. [DOI] [PubMed] [Google Scholar]
- 38.Bleicher R.J., Allegra D.P., Nora D.T., Wood T.F., Foshag L.J., Bilchik A.J. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10:52–58. doi: 10.1245/aso.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Komorizono Y., Oketani M., Sako K. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
- 40.Rossi S., Garbagnati F., Lencioni R. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 41.Scott D.J., Fleming J.B., Watumull L.M., Lindberg G., Tesfay S.T., Jones D.B. The effect of hepatic inflow occlusion on laparoscopic radiofrequency ablation using simulated tumors. Surg Endosc. 2002;16:1286–1291. doi: 10.1007/s004640080167. [DOI] [PubMed] [Google Scholar]
- 42.Mulier S., Mulier P., Ni Y. Complications of radiofrequency coagulation of liver tumors. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 43.Shen P., Fleming S., Westcott C., Challa V. Laparoscopic radiofrequency ablation of the liver in proximity to major vasculature: effect of the Pringle maneuver. J Surg Oncol. 2003;83:36–41. doi: 10.1002/jso.10235. [DOI] [PubMed] [Google Scholar]
- 44.De Sio I., Castellano L., De Girolamo V. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001;34:609–610. doi: 10.1002/hep.510340327. [DOI] [PubMed] [Google Scholar]
- 45.Llovet J.M., Vilana R., Bru C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]


