Abstract
The hepatic vasculature is highly complex. The hepatic artery (a branch of the celiac tripod) and the portal vein (formed by the confluence of the splenic and superior mesenteric veins) provide a dual blood supply while venous drainage is guaranteed by the hepatic veins. There are also numerous anatomic variants that can involve one or more of the system’s three components.
Hepatic artery variants are the least common. Ten types have been identified, including several that are fairly frequent and others that are quite rare, and the variation generally involves the extrahepatic portion of the vessel. Portal vein variants are found in around 20% of the population. They can involve the main portal trunk or segmental branches. Variants of the hepatic veins are the most common. They involve the number and course (supernumerary veins) or the number, course, and openings (accessory veins).
Knowledge of portal vein and hepatic vein variants, which are extremely common, is of prime importance for precise localization of lesions. Hepatic artery variants are equally important for surgical treatment of hepatic disease, especially liver transplantation, where it is essential for preoperative workup and postoperative follow-up of the recipient as well as for assessment of potential donors.
Keywords: Sonography, Liver, Sonography – liver, Hepatic artery, Portal vein, Hepatic veins
Sommario
La vascolarizzazione del fegato è complessa: arteria epatica, ramo del tripode celiaco, e vena porta, costituita dalla confluenza delle vene splenica e mesenterica superiore, forniscono una doppia vascolarizzazione; mentre il drenaggio è assicurato dalle vene sovrepatiche, inoltre vi è un numero significativo di varianti che possono interessare separatamente o contemporaneamente i tre i sistemi.
Le varianti dell’arteria epatica, meno frequenti rispetto alle altre, sono state classificate in dieci tipi di cui alcuni più comuni, altri del tutto eccezionali, riguardano principalmente il tratto extra-epatico del vaso. Quelle della vena porta, che si riscontrano in circa il 20% della popolazione, possono interessare sia i rami principali che i segmentari. Quelle delle vene sovrapatiche, le più frequenti, possono riguardare il numero ed il decorso (vene soprannumerarie) o il numero ed il decorso, ma anche lo sbocco (vene accessorie).
La conoscenza delle varianti portali e delle vene sovrepatiche, estremamente frequenti, è di primaria importanza per la possibilità di localizzare con precisione eventuali lesioni; non meno importante è la conoscenza delle varianti dell’arteria epatica, in relazione alle terapie chirurgiche, in particolare per quello che riguarda i trapianti, sia nella fase pre-operatoria, sia nella valutazione del potenziale donatore, sia nel follow-up.
Introduction
The hepatic artery and portal vein provide the liver with a dual blood supply, and venous drainage is ensured by the hepatic veins [1–3].
The common hepatic artery gives rise to the gastroduodenal and right gastric arteries and then continues as the proper hepatic artery, which passes anteriorly over the portal vein and runs to the left of the main bile duct. At the level of the hilum, it divides into right and left branches that run alongside the right and left branches of the portal vein.
The portal vein is derived from the union of the splenic and superior mesenteric veins, which takes place behind the head of the pancreas. At the hepatic level, the vein divides into two main branches, the left and right portal veins (Fig. 5A). The right portal vein gives rise to a branch that supplies the caudate lobe and then to anterior and posterior branches, which in turn subdivide to form two superior and two inferior branches for the four segments. The left branch runs horizontally, crossing over the round ligament (umbilical segment), where it gives rise to two branches that supply the II and III segments followed by two terminal branches that supply the IV segment [4].
Fig. 5.
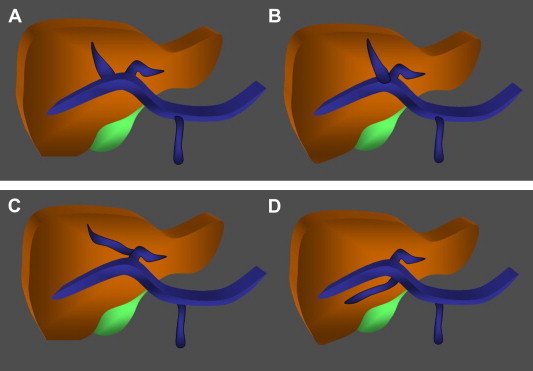
Portal vein variants. A. The portal vein is formed by the confluence of the superior mesenteric and splenic veins. At the hepatic level it divides to form the two main branches (left and right); B. Anatomic variant characterized by trifurcation of the main portal vein. The right portal vein is absent. It is replaced by a right anterior branch and a right posterior branch, which arise together with the left branch. C. Anatomic variant characterized by the presence of a right anterior segmental branch that originates from the left portal vein. D. Anatomic variant characterized by a right posterior segmental branch that originates from the main portal vein.
The hepatic veins (generally three in number) drain the liver and empty into the inferior vena cava near the diaphragm. The right hepatic vein drains segments V and VII, the middle branch drains segments IV, V, and VII, and the left branch drains segments II and III.
In 60% of all patients, the middle and left hepatic veins merge to form a common trunk that empties into the inferior vena cava [5] (Fig. 9A).
Fig. 9.
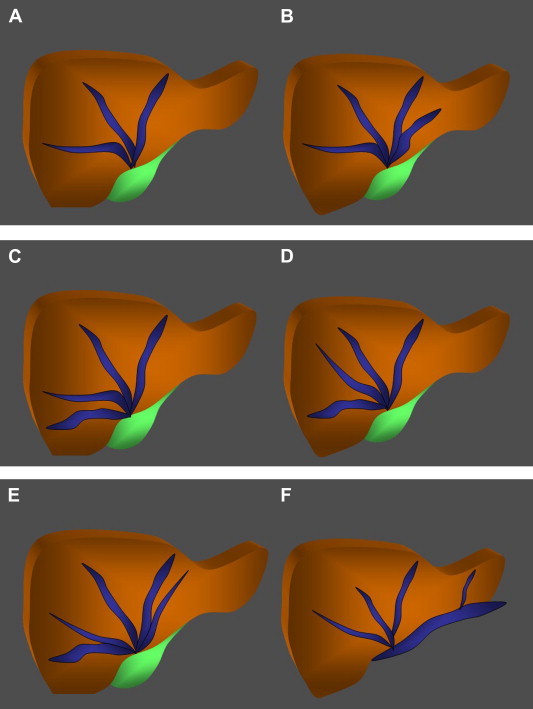
Hepatic vein variants. A. The hepatic veins drain the liver. There are generally three of these veins, which open into the inferior vena cava near the diaphragm. In 60% of individuals, the middle and left hepatic veins form a common trunk that drains into the vena cava. B. Anatomic variant characterized by the presence of two left hepatic veins. C. Anatomic variant characterized by the presence of two right hepatic veins. D. Anatomic variant characterized by the presence of two middle hepatic veins. E. Anatomic variant characterized by the presence of two right hepatic veins and two left hepatic veins. F. Anatomic variant characterized by the presence of a left accessory hepatic vein.
There are also a considerable number of anatomic variations that can involve one or more of the three systems (Figs. 1, 2, 5 and 9).
Fig. 1.
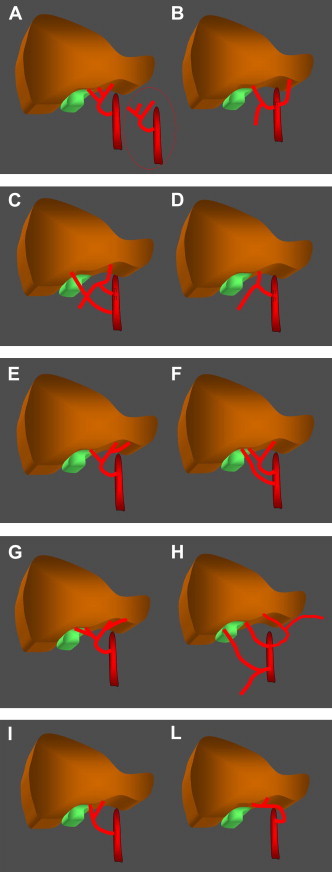
Classification of hepatic artery variants according to Michels. A. Type I is characterized by the presence of a middle hepatic artery that arises from the left or right branch of the proper hepatic artery. B. In type II, the common hepatic artery gives rise to the gastroduodenal and right hepatic arteries, and the left branch is replaced by the left gastric artery. C. In type III, the common hepatic artery gives rise exclusively to the gastroduodenal and left hepatic arteries. The right hepatic artery is replaced by the superior mesenteric artery. D. Type IV – the common hepatic artery divides to form the gastroduodenal and middle hepatic arteries. E. Type V is characterized by the presence of a left accessory hepatic artery that arises from the left hepatic artery. F. Type VI is characterized by the presence of a right accessory hepatic artery that arises from the superior mesenteric artery. G. In type VII there are right and left accessory hepatic arteries. H. Type VIII is a combination of substituted arteries (right or left) plus and inverted accessory artery. I. Type IX – the hepatic artery is replaced by the superior mesenteric artery. L. Type X – the hepatic artery is replaced by the left gastric artery.
Fig. 2.
Sonographic appearance of the type III variant. The common hepatic artery gives rise only to the gastroduodenal and left hepatic arteries while the right hepatic artery is replaced by a branch (arrows) of the superior mesenteric artery (hollow arrow).
Hepatic artery
Michels identified 10 anatomic variants of the hepatic artery (Fig. 1) [6,7] and reported their relative frequencies based on the dissection of 200 cadavers. Other variants exist although they are much less common.
According to Michels, in the type I variant (Fig. 1A) (frequency 55%) the right and left hepatic arteries arise from the proper hepatic artery whereas the middle hepatic artery originates from either the right or left branch; in 10% of all subjects, it is a separate branch of the proper hepatic artery.
In type II (Fig. 1B) (10%) the common hepatic artery divides to form the gastroduodenal artery and the right hepatic artery, and the left branch is replaced by the left gastric artery. If instead the common hepatic artery gives rise only to the gastroduodenal and left hepatic arteries, the right hepatic artery is substituted by the superior mesenteric artery (type III) (Fig. 1C and Fig. 2) (11%). In types II and III, the middle hepatic artery originates from the non-substituted branch of the hepatic artery.
In type IV (Fig. 1D) (1%), the common hepatic artery divides to form the gastroduodenal and middle hepatic arteries; the right and left hepatic arteries are both substituted.
In type V (Fig. 1E and Fig. 3) (8%), there is an accessory left hepatic artery that originates from the left gastric artery.
Fig. 3.
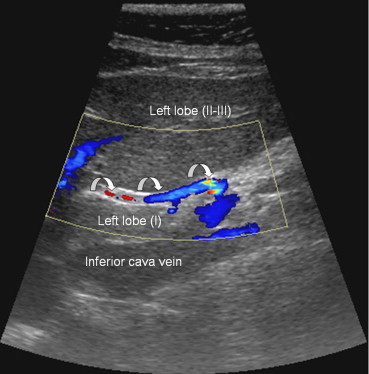
Sonographic appearance of the type V variant. The left hepatic artery (curved arrow) originates from the left gastric artery and runs alongside the venous ligament.
Type VI (Fig. 1F) (7%) is characterized by the presence of an accessory right hepatic artery that arises from the superior mesenteric artery whereas there is an accessory right hepatic artery as well as an accessory left hepatic artery in type VII (Fig. 1G) (1%).
In type VIII (Fig. 1H) (2%), replaced arteries on the right or left are combined with an inverse accessory artery.
If the hepatic artery is missing altogether, it can be replaced by the superior mesenteric artery (Fig. 4 and Fig. 1I) (type IX) (4.5%) or by the left gastric artery (type X) (Fig. 1L) (0.5%) [8,9].
Fig. 4.
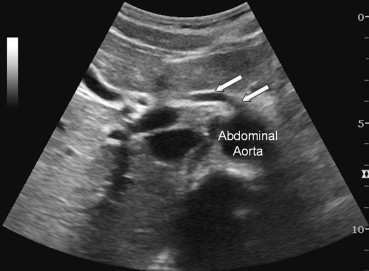
Sonographic appearance of the type IX variant. The entire hepatic artery is replaced by the superior mesenteric artery (hollow arrows).
Portal vein
The portal vein is formed by the confluence of the superior mesenteric vein and splenic vein, which occurs behind the head of the pancreas. At the hepatic level, it divides to form the right and left portal veins, which give rise to segmental branches that penetrate the various hepatic segments accompanied by branches of the hepatic artery. Variants of this pattern (Fig. 5) are encountered in around 20% of the population [10].
The most common consists in trifurcation of the portal vein (Fig. 5B) (7.8–10.8%), which gives rise to a left portal vein and two branches on the right (the right anterior and right posterior portal veins).
The second most common variant (4.7–5.8%) is characterized by the presence of a right posterior segmental branch that arises, from the main portal vein (Fig. 5D).
In 2.9–4.3% of all individuals, there is a right anterior segmental branch that arises from the left branch (Fig. 5C).
Other variants can be observed as a result of malpositioning of the gall bladder. They include anomalous course of the horizontal segment and umbilical portion [of the portal vein] [11,12].
Variability has also been described at the segmental level (Fig. 6–8). The single branches that normally supply the II and III segments can each be duplicated, and segment IV, which is often the largest segment of the left lobe, can receive as many as seven portal branches (Fig. 6B). The branches supplying the right lobe can also vary in number and course [13].
Fig. 6.
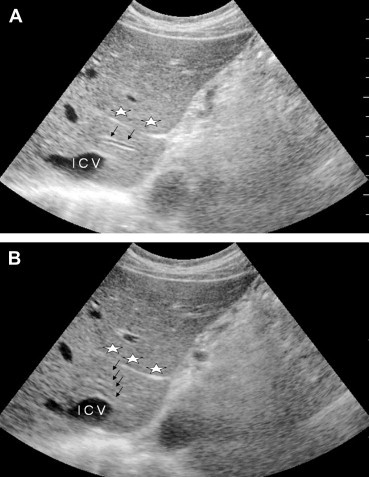
Sonographic appearance of the portal vein variants. The first hepatic segment may contain one (A) or more (black arrows) (B) portal branches, lying between the venous ligament (stars) and the inferior vena cava (IVC).
Fig. 7.
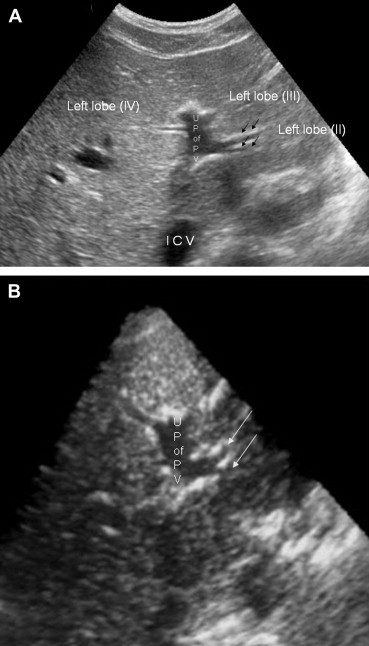
Sonographic appearance of the portal vein variants. The portal branch that supplies segment II, which arises from the umbilical portion of the left portal vein (U P of P V), divides prematurely to form secondary branches (black arrows) (A), as seen in the 3-dimensional reconstruction (white arrows) (B).
Fig. 8.
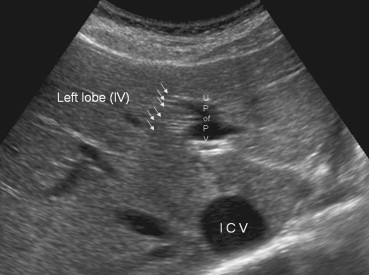
Sonographic appearance of the portal vein variants. Hepatic segment IV, which is often the largest segment of the left lobe, receives a variable number of portal branches (up to seven) (white arrows).
The hepatic veins
The most frequent anatomic variants are those involving the hepatic veins. The most common is characterized by the presence of [one or more] accessory vessels (Fig. 9F, Fig. 10A–C) that drain separately into the vena cava. This usually involves a right inferior hepatic vein (frequency: 52.2%); less frequent variants include the presence of two accessory hepatic veins (12%) or accessory veins that drain the caudate lobe (Fig. 10A) (12%) [14].
Fig. 10.
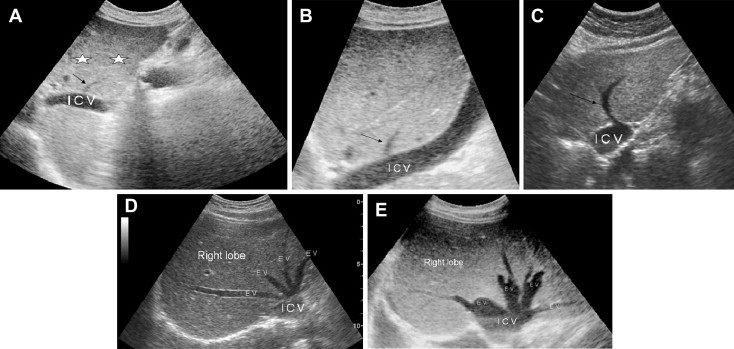
Sonographic appearance of the hepatic vein variants. A. Accessory hepatic vein of segment I (black arrow), venous ligament (stars). B. Accessory hepatic vein of segment II (black arrow). C. Left accessory hepatic vein (black arrow). D. Anatomic variant characterized by the presence of two left hepatic veins. E. Anatomic variant characterized by the presence of two right hepatic veins and two left hepatic veins.
Supernumerary hepatic veins are also common (Fig. 9 B–E, and Fig. 10D, E) (30%). There may be two left hepatic veins (Fig. 9B) (19%), two right hepatic veins (Fig. 9C) (8%), and/or two middle hepatic veins (Fig. 9D) (3%) [15,16].
A rare variant that is important from a surgical point of view is characterized by the precocious division of the vein that drains the right superoanterior segment (VIII) in the middle hepatic vein [17].
Congenital malformations
Congenital malformations [involving the hepatic vasculature] are rare. They may be associated with symptoms or discovered by chance during sonographic examinations requested for various reasons.
Those affecting [18] the portal system include the formation of a prepancreatic portal vein (frequently associated with situs inversuso or other congenital malformations); duplication of the portal vein; and agenesis of the portal vein itself [19] or of one of its main branches.
Agenesis is believed to be related to developmental failure or to thrombosis of the segmental branch [20,21].
Another type of malformation (Fig. 11) involves arterovenous shunting caused by Rendu-Osler disease [22], an autosomal dominant disorder characterized by vascular dysplasia. Its prevalence in the general population is 1–2/100,000 [21]. The various types of angiodysplasia have been demonstrated with angiography, including arterial aneurysms, arterovenous fistulas, phleboectasia, and angiomas, all of which can also be visualized sonographically (Fig. 11).
Fig. 11.
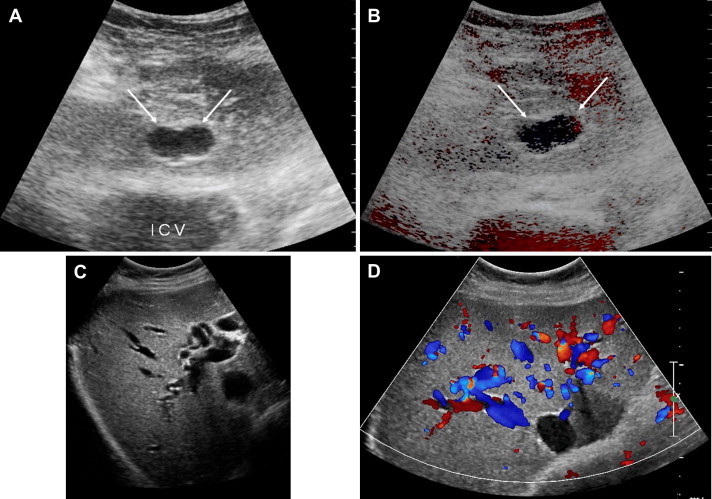
Appearance on ultrasound (A, C) and color-Doppler (B, D) of the angiodysplastic changes associated with Rendu-Osler disease, including arterial aneurysms, arterovenous fistulas, and phlebectasia.
Conclusions
The identification and localization of hepatic lesions represent an important part of routine clinical practice, and they require a thorough knowledge of the anatomical aspects of the liver [1,2]. The concept of the liver as an organ that can be divided into segments is now universally accepted.
The subdivision is based on hepatic vascularization, and the various segments are defined by the branches of the portal vein that they contain and by the hepatic veins that separate them [1,2,5].
Knowledge of the anatomic variants of the portal and hepatic veins, which are extremely common, is of prime importance for precise localization of lesions and it can also be useful during insertion of a TIPS (transjugular intrahepatic portosystemic shunt) [23]. Familiarity with hepatic vein variants is also important for surgical treatment of hepatic disease, especially liver transplantation, where it is essential for preoperative workup and postoperative follow-up of the recipient as well as for assessment of potential donors [7,24,25].
The vascular anatomy of the liver is undeniably complex, and the situation is further complicated by the fact that, the inferior vena cava, the openings of the hepatic veins, and the main branches of the portal veins can always be visualized sonographically, but the intrasegmental vessels (portal branches, arterial branches, accessory hepatic veins) can rarely be identified [1]. Although superior results can undoubtedly be obtained with multislice CT or MRI, ultrasound is still the method of choice for use in a number of situations, and a thorough knowledge of the sonographic appearance of the normal hepatic vascular tree and its anatomic variants is absolutely indispensable.
Conflict of interest statement
The authors have no conflict of interest.
References
- 1.Draghi F., Rapaccini G.L., Fachinetti C., de Matthaeis N., Battaglia S., Abbattista T. Ultrasound examination of the liver: normal vascular anatomy. J. Ultrasound. 2007;10:5–11. doi: 10.1016/j.jus.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lafortune M., Madore F., Patriquin H., Breton G. Segmental anatomy of the liver: a sonographic approach to the Couinaud nomenclature. Radiology. 1991 Nov;181(2):443–448. doi: 10.1148/radiology.181.2.1924786. [DOI] [PubMed] [Google Scholar]
- 3.Winter T.C., 3rd, Nghiem H.V., Freeny P.C., Hommeyer S.C., Mack L.A. Hepatic arterial anatomy: demonstration of normal supply and vascular variants with three-dimensional CT angiography. Radiographics. 1995;15:771–780. doi: 10.1148/radiographics.15.4.7569128. [DOI] [PubMed] [Google Scholar]
- 4.Gallego C., Velasco M., Marcuello P., Tejedor D., De Campo L., Friera A. Congenital and acquired anomalies of the portal venous system. Radiographics. 2002 Jan–Feb;22(1):141–159. doi: 10.1148/radiographics.22.1.g02ja08141. [DOI] [PubMed] [Google Scholar]
- 5.Lafortune M., Lepanto L. Liver anatomy: echography and Doppler. J Radiol. 2002 Feb;83(2 Pt. 2):235–246. [PubMed] [Google Scholar]
- 6.Michels N.A. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 1966 Sep;112(3):337–347. doi: 10.1016/0002-9610(66)90201-7. [DOI] [PubMed] [Google Scholar]
- 7.Coskun M., Kayahan E.M., Ozbek O., Cakir B., Dalgic A., Haberal M. Imaging of hepatic arterial anatomy for depicting vascular variations in living related liver transplant donor candidates with multidetector computed tomography: comparison with conventional angiography. Transplant Proc. 2005 Mar;37(2):1070–1073. doi: 10.1016/j.transproceed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Covey A.M., Brody L.A., Maluccio M.A., Getrajdman G.I., Brown K.T. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002 Aug;224(2):542–547. doi: 10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- 9.Quiroga S., Sebastià C., Pallisa E., Castellà E., Perez-Lafuente M., Alvarez-Castells A. Improved diagnosis of hepatic perfusion disorders: value of hepatic arterial phase imaging during helical CT. Radiographics. 2001 Jan–Feb;21(1):65–81. doi: 10.1148/radiographics.21.1.g01ja0165. [DOI] [PubMed] [Google Scholar]
- 10.Madoff D.C., Hicks M.E., Vauthey J.N., Charnsangavej C., Morello F.A., Jr., Ahrar K. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002;22:1063–1076. doi: 10.1148/radiographics.22.5.g02se161063. [DOI] [PubMed] [Google Scholar]
- 11.Covey A.M., Brody L.A., Getrajdman G.I., Sofocleous C.T., Brown K.T. Incidence, patterns, and clinical relevance of variant portal vein anatomy. AJR Am J Roentgenol. 2004 Oct;183(4):1055–1064. doi: 10.2214/ajr.183.4.1831055. [DOI] [PubMed] [Google Scholar]
- 12.Chen J.S., Yeh B.M., Wang Z.J., Roberts J.P., Breiman R.S., Qayyum A. Concordance of second-order portal venous and biliary tract anatomies on MDCT angiography and MDCT cholangiography. AJR Am J Roentgenol. 2005 Jan;184(1):70–74. doi: 10.2214/ajr.184.1.01840070. [DOI] [PubMed] [Google Scholar]
- 13.Dirisamer A., Friedrich K., Schima W. Anatomy and variants of hepatic segments, vessels, and bile ducts. Radiologe. 2005 Jan;45(1):8–14. doi: 10.1007/s00117-004-1150-5. [DOI] [PubMed] [Google Scholar]
- 14.Sahani D., Mehta A., Blake M., Prasad S., Harris G., Saini S. Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics. 2004;24:1367–1380. doi: 10.1148/rg.245035224. [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Torres A., Fernández-Cuadrado J., Pinella I., Parrón M., de Vicente E., López-Santamaría M. Multidetector CT in the evaluation of potential living donors for liver transplantation. Radiographics. 2005;25:1017–1030. doi: 10.1148/rg.254045032. [DOI] [PubMed] [Google Scholar]
- 16.Lee V.S., Morgan G.R., Lin J.C., Nazzaro C.A., Chang J.S., Teperman L.W. Liver transplant donor candidates: associations between vascular and biliary anatomic variants. Liver Transpl. 2004 Aug;10(8):1049–1054. doi: 10.1002/lt.20181. [DOI] [PubMed] [Google Scholar]
- 17.Bach T.T., Tung T.T., Lang T.D. Vascular complications in hepatic resection. Chirurgie. 1994–1995;120(13):179–185. [PubMed] [Google Scholar]
- 18.Gallego C., Miralles M., Marín C., Muyor P., González G., García-Hidalgo E. Congenital hepatic shunts. Radiographics. 2004;24:755–772. doi: 10.1148/rg.243035046. [DOI] [PubMed] [Google Scholar]
- 19.Altavilla G., Cusatelli P. Ultrastructural analysis of the liver with portal vein agenesis: a case report. Ultrastruct Pathol. 1998 Nov–Dec;22(6):477–483. doi: 10.3109/01913129809032284. [DOI] [PubMed] [Google Scholar]
- 20.Chevallier P., Oddo F., Baldini E., Peten E.P., Diaine B., Padovani B. Agenesis of the horizontal segment of the left portal vein demonstrated by magnetic resonance imaging including phase-contrast magnetic resonance venography. Eur Radiol. 2000;10(2):365–367. doi: 10.1007/s003300050057. [DOI] [PubMed] [Google Scholar]
- 21.Draghi F., Precerutti M., Danesino G.M. Vascular abnormalities in the fingers of patients affected with hereditary hemorrhagic telangiectasia (HHT) as assessed by color Doppler sonography. Am J Med Genet A. 2005 May 15;135(1):106–109. doi: 10.1002/ajmg.a.30649. [DOI] [PubMed] [Google Scholar]
- 22.Ricci P., Cantisani V., Lombardi V. Is color-Doppler us a reliable method in the follow-up of transjugular intrahepatic portosystemic shunt? J. Ultrasound. 2007 March 10:22–27. doi: 10.1016/j.jus.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi M., Pennini G., Lai Q., Ginanni Corradini S., Drudi F.M., Pugliese F. Liver transplantation. J. Ultrasound. 2007 March;10:28–45. doi: 10.1016/j.jus.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drudi F.M., Pagliara E., Cantisani V., Arduini F., D’Ambrosio U., Alfano G. Post-transplant hepatic complications: imaging findings. J. Ultrasound. 2007 March 10:53–58. doi: 10.1016/j.jus.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Draghi F., Spinazzola A., Abbati D., Precerutti M., Fachinetti C. Multi-organ donor: pre-explant sonographic hepatic evaluation. J. Ultrasound. 2007 March;10:5–11. [Google Scholar]


