Abstract
Introduction
Ultrasound elastography (US-E) is a helpful tool for the diagnosis of thyroid cancer. In acromegaly, multinodular goiter is a common occurrence while the prevalence of thyroid cancer is still matter of debate. Our aims were to evaluate thyroid nodules in acromegaly and to assess the accuracy of US-E in providing information on their nature (benign vs. malignant) using cytological analysis as a reference.
Materials and methods
US-E was performed in 25 patients with acromegaly (active in 10 cases, medically controlled in 8, and cured by pituitary surgery in 7), each of whom had at least one solid thyroid nodule. A total of 90 nodules were classified according to the elastography scores (ES): ES1 and ES2 for soft nodules, ES3 and ES4 for an elastic lesions. FNAC was performed in 78.6% of the ES 4 lesions and 54.1% of the ES 3 nodules.
Results
Fourteen of the 90 nodules (15.5%) displayed an ES of 1, 25 (27.7%) an ES of 2, 37 (41.3%) an ES of 3, and 14 (15.5%) an ES of 4. The prevalence of hard nodules in patients with active acromegaly (68.9%) was greater than that observed in patients with cured (44.4%) or controlled (52.5%) acromegaly. The prevalence of hard nodules in the total series (56.7%) was higher than that reported in nonacromegalic goitrous subjects. All thyroid nodules subjected to FNAC were negative for malignant cells and follicular lesions.
Discussion
Acromegaly (particularly active forms) is associated with a high prevalence of stiff thyroid nodules that exceeds that observed in nonacromegalic patients with goiters (33.7%). However, these nodules were never malignant at cytology, and their firmness is probably due to fibrosis. US-E therefore appears to be of limited value for the diagnosis of thyroid cancer in patients with acromegaly.
Keywords: Thyroid nodule, Elastography, Fibrosis, Acromegaly
Sommario
Introduzione
L’elastosonografia (US-E) è un utile strumento per la diagnosi del carcinoma tiroideo. Se nell’acromegalia lo struma multinodulare ha un’elevata prevalenza, ancora non vi è accordo sulla possibilità di un’aumentata prevalenza delle neoplasie maligne tiroidee. Obiettivi dello studio sono stati: a) valutare i noduli tiroidei nell’acromegalia con l’US-E; b) stabilire l’accuratezza di questa tecnica nel fornire informazioni sulla loro natura, usando l’esame citologico come parametro di riferimento.
Materiali e metodi
L’US-E è stata eseguita in 25 pazienti acromegalici (7 guariti dalla chirurgia ipofisaria, 8 controllati dalla terapia medica, 10 con patologia attiva) che presentavano almeno un nodulo solido. Un totale di 90 noduli sono stati classificati secondo un punteggio elastosonografico (ES) in quattro classi di durezza: ES1 e ES2 per i noduli più elastici, ES3 e ES4 per quelli più duri. L’agoaspirato (FNAC) è stato effettuato nel 78.6% dei noduli ES 4 e nel 54,1% dei noduli ES 3.
Risultati
Dei 90 noduli, 14 (15,5%) mostravano un ES 1, 25 (27,7%) un ES 2, 37 (41,3%) un ES 3 e 14 (15,5%) un ES 4. La prevalenza di noduli duri nei pazienti con acromegalia attiva (68,9%) era maggiore di quella osservata nei pazienti guariti (44,4%) e nei pazienti controllati (52,5%). Nell’intera popolazione di acromegalici la prevalenza di noduli duri (56,7%) era maggiore di quella riportata dalla letteratura nei pazienti non acromegalici con struma multinodulare (33,7%). Tutti i noduli sottoposti a FNAC sono risultati negativi per cellule tumorali maligne o per sospetta malignità.
Discussione
Questo studio ha dimostrato un’elevata prevalenza di noduli duri nell’acromegalia, soprattutto nei pazienti con patologia attiva. Tale prevalenza è maggiore di quella riportata in letteratura nei pazienti non acromegalici con patologia nodulare tiroidea. Questi noduli, tuttavia, non sono risultati maligni all’esame citologico e potrebbero essere di natura fibrosa. In conclusione, l’US-E sembra essere uno strumento di valore limitato nella diagnosi del tumore della tiroide nell’acromegalia.
Introduction
Acromegaly is a slowly progressive disease caused by growth hormone (GH) hypersecretion. It is characterized by several complications. Thyroid disease, usually multinodular, is a common finding in patients with acromegaly [1–5]. The functional, scintigraphic, and ultrasonographic features of goiters in these patients have been fully documented [3], but thus far there are no data on the ultrasound elastographic characteristics of these lesions. Ultrasound elastography (US-E) is a new dynamic technique for estimating tissue stiffness [6,7] that has been likened to “electronic palpation”. The basic principle of US-E is that tissue compression produces a strain that is inversely proportional to the firmness of the tissue. It involves measurement of the degree of distortion of the US beam caused by the application of an external force [6].
Compared with normal tissues, malignant lesions are often characterized by greater stiffness [8], and this feature has been exploited to allow US-E differentiation of cancers and benign lesions in various organs, including the prostate, pancreas, breast, and lymph nodes [9–11]. Recently, US-E has also been used to evaluate the elasticity of thyroid nodules, and it has been proposed as an effective, non-invasive tool for diagnosing thyroid cancer [12–18]. Thus far, no data have been published on the use of US-E for assessing thyroid nodules in patients with acromegaly, who are suspected to have an increased incidence of thyroid cancer [19–21].
The aims of this study were to evaluate the elastic properties of acromegaly-associated thyroid nodules with US-E and to investigate the diagnostic accuracy of this technique in detecting thyroid cancer in this population.
Materials and methods
The study population consisted of 35 patients with acromegaly who were consecutively referred to the Division of Endocrinology of our Institution. Diagnosis of acromegaly and definition of active disease were based on clinical signs, high baseline GH levels, GH values greater than 1 μg/l after standard oral glucose loading, and serum IGF-I values that were above the normal range for age and sex [22]. Conventional preliminary B-mode US and color power Doppler were performed in all subjects (US). Patients with no evidence of solid thyroid nodules (i.e., 5 patients with normal thyroid glands, 3 with Hashimoto’s thyroiditis, 1 who had undergone thyroidectomy for papillary carcinoma, 1 with cystic lesions) were excluded from the study. Each of the other 25 patients (15 women and 10 men, mean age 57.2 ± 12.14 years) had at least one solid thyroid nodule. They were enrolled in the study, which was approved by the Ethics Committee of our Institute, and all underwent US-E after providing written informed consent.
Ten patients presented active acromegaly according to the above-mentioned criteria: 2 of these had newly diagnosed disease, 3 had undergone unsuccessful pituitary surgery (followed in 1 case by unsuccessful radiotherapy), and the remaining 5 had disease that was poorly controlled by medical treatment (One of the latter patients had also been treated unsuccessfully with radiotherapy). In seven patients, the acromegaly had been cured with pituitary surgery alone. In the remaining 8 patients, the disease was controlled by medical treatment (somatostatin analogues alone [n = 5] or associated with dopamine agonists [n = 2] or pegvisomant [n = 1]) (5 + 2 + 1). Five of these 8 patients had previously had pituitary surgery without postoperative radiotherapy, 1 had received pituitary irradiation alone, and in the remaining 2 cases, treatment had been exclusively medical. All patients were euthyroid with normal serum calcitonin levels at the time of enrolment.
All thyroid US and US-E examinations were performed by the same endocrinologist, who was skilled in thyroid US (M.A.). A Hitachi Logos E, EUB 7500 (Esaote SpA, Genoa, Italy) was used with a 6–13 MHz linear probe. Each thyroid nodule was carefully examined to identify the following: nature (solid, liquid, or mixed); echogenicity (hyperechoic, isoechoic or hypoechoic compared with the normal parenchyma); margins (regular or irregular); size; homogeneity (homogeneous or inhomogeneous); calcifications (coarse or microcalcifications, these latter presenting as hyperechoic spots less than 2 mm in diameter without acoustic shadowing); flow pattern type (I. No blood flow; II. Predominantly perinodular with little or no intranodular blood flow; III. Predominantly intranodular with little or no perinodular flow) [23].
The volume of each thyroid lobe was calculated with the formula for an ellipse (π/6 × length × width × thickness) and the results summed to obtain total thyroid volume [24]. After standard US, US-E was performed on all solid nodules by the same operator. Two US images were acquired (before and after tissue compression with the probe), and tissue displacement was tracked by assessing the propagation of the imaging beam. Dedicated software was used to obtain accurate measurements of the tissue distortion (Combined autocorrelation method [CAM], Hitachi Medical, Tokyo, Japan). The US-elastogram was superimposed on the B-mode image. Color coding was used to indicate the magnitude of strain: red (soft tissue), green (intermediate stiffness), and blue (hard, inelastic tissue). The color pattern of the thyroid lesion was compared with that of the surrounding tissue to define its elastic properties. Lesion elasticity was rated according to a 4-point elasticity score (ES) based on the overall pattern [15,16,18,25]. An ES of 1 was assigned to nodules that were uniformly elastic (homogeneously green). An ES of 2 was assigned to nodules that were predominantly elastic (green) with some areas of peripheral and/or central stiffness (blue). An ES of 3 reflected stiffness (blue) in a large portion of the examined area with some peripheral and/or central areas of elasticity (green), and nodules than were uniformly inelastic nodules (homogenously blue) were rated ES 4. To minimize inter- and intraobserver variability involving free-hand compression of the lesions, we used a device that provided real-time measurement of the force applied expressed on a scale of 1–5. Pressure was constantly maintained at an intermediate level (level 3 or 4) throughout the examination.
To obtain reliable elasticity images, we positioned the region of interest over the nodule so that it also included a sufficient amount of normal thyroid parenchyma. When mixed (solid-cystic) nodules were examined, the ES was assigned only to the solid component of the lesion. In nodules with diameters exceeding 30 mm, the cranial portion of the lesion was examined before the caudal region to avoid artifacts. Multiple frames were acquired, and numerous elastographic images were generated. The best-fit B-mode sonogram–elastogram image pairs were used to assign the ES. Images were reviewed by a second skilled US examiner (M.M.). Interobserver agreement on the scoring of US parameters was 90.0%. In particular, concordant scores were assigned to 81 of the 90 nodules by the two examiners. In 9 cases the final score was agreed upon after joint re-examination of the recorded images.
Cytological examination of material obtained by fine-needle aspiration (FNAC) was used as reference standard to establish the benign/malignant nature of the lesion. Because of its high sensitivity and specificity, this technique is widely considered the best single test for this type of differentiation.
FNAC was performed under US guidance with a 22-gauge needle attached to a 20-mL syringe. The adequacy of the aspirates was defined according to the guidelines of the Papanicolau Society [26]. Criteria for selecting the nodule to be aspirated were: a) size greater than 6 mm; b) simultaneous presence of at least two US features associated with malignancy, i.e., hypoechogenicity, irregular margins, intralesional vascularization, and spot microcalcifications [23,27–29]; c) ES scores indicative of stiffness (ES 3 or 4). In patients with more than 3 stiff nodules, only the most suspicious lesions were biopsied.
On the whole, FNAC was performed in 31 (60.8%) of the hard lesions, 20 (54.1%) of those with an ES of 3 and 11 (78.6%) of those with an ES of 4. Aspirates were not obtained from 20 other hard nodules. Ten of these lesions belonged to patients with multinodular disease and at least 2 other suspicious nodules had been recently aspirated. The nonsampled nodules clearly displayed US characteristics associated with benignity (isoechogenicity, regular margins, type I or II flow pattern, no microcalcifications). In the other 10, FNAC was not performed because patient consent was lacking (n = 6) or because the nodules were very small.
All stiff nodules not undergoing FNAC were monitored (is not biopsy!) with US and US-E for at least 12 months.
Statistical analysis
Continuous variables were expressed as means ± SEM. Statistical analysis of quantitative and qualitative data was performed with ANOVA and the chi-square test, respectively. A p-value of less than 0.05 was considered significant.
Results
In the 25 patients with acromegaly, the mean thyroid volume was 20.2 ± 9.41 ml (range 7.9–42.9). A total of 102 thyroid nodules were found (1–10 per patient). The mean size of the nodules was 10.7 ± 6.21 mm (range 2–39).
The characteristics of the nodules on conventional US are shown in Table 1.
Table 1.
Ultrasonographic features of thyroid nodules in patients with acromegaly (percentage in brackets).
| Nature | solid (85.3) | mixed (3.9) | cystic (10.8) | |
| Localization | right (52.9) | left (36.3) | isthmus (10.8) | |
| Echogenicity | hypo (63.7) | iso (23.6) | hyper (1.8) | ane (10.9) |
| Homogeneity | hom (62.7) | inh (37.3) | ||
| Margins | regular (78.4) | irregular (21.6) | ||
| Calcifications | absent (93.2) | micro (2.9) | coarse (3.9) | |
| Vascularization | type I (56.8) | type II (32.4) | type III (10.8) |
Abbreviations: right, right lobe; left, left lobe; hypo, hypoechoic; iso, isoechoic; hyper, hyperechoic; ane, anechoic; hom, homogeneous; inh, inhomogeneous; micro, microcalcifications.
Eleven cystic nodules and 1 completely calcific lesion were excluded, leaving 90 nodules for US-E examination: 18 in patients whose disease had been cured, 40 in patients whose disease was pharmacologically controlled, and 32 in patients with active disease. Thirty-nine of the nodules were soft and 51 were hard. Fourteen (15.5%) had an ES of 1, 25 lesions (27.7%) had an ES of 2, 37 (41.3%) had an ES of 3, and the remaining 14 (15.5%) had an ES of 4. Conventional ultrasonographic and elastographic features of the thyroid nodules in each score class are shown in Figs. 1–4. The majority of the stiff nodules had none of the conventional ultrasonographic features suggestive of malignancy (hypoechogenicity, inhomogeneity, irregular margins, intralesional vascularization and microcalcifications) with the exception of hypoechogenicity.
Fig. 1.
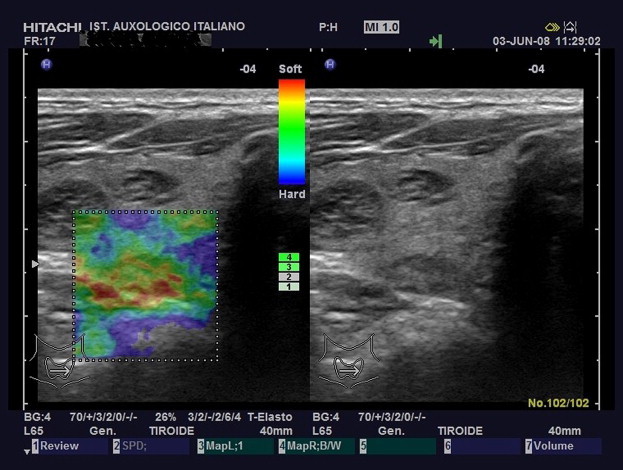
Elasticity Score 1. The whole nodule was homogenously green at US-E evaluation.
Fig. 2.
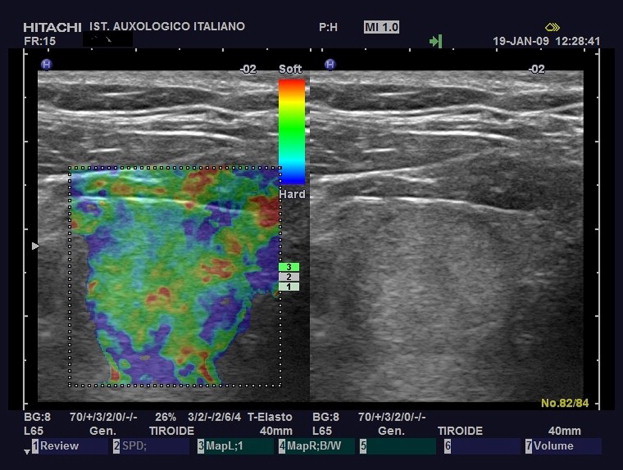
Elasticity Score 2. The nodule displays elasticity (green) in a large portion of the examined area with some peripheral and/or central areas of stiffness (blue).
Fig. 3.
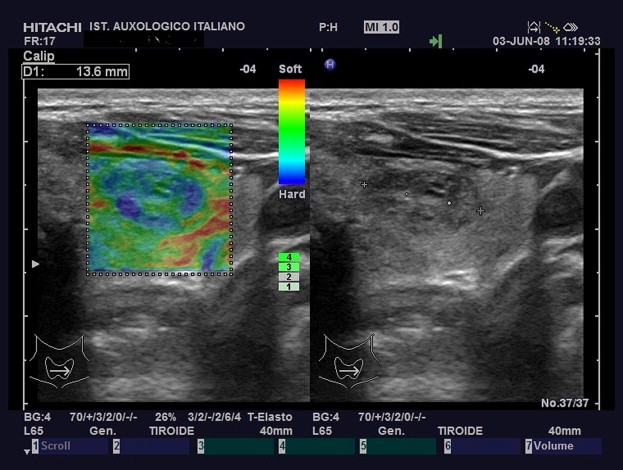
Elasticity Score 3.The nodule displays stiffness (blue) in a large portion of the examined area with some peripheral and/or central areas of elasticity (green).
Fig. 4.

Elasticity Score 4.The entire nodule is uniformly blue indicating stiffness.
Fig. 5 shows the distribution of ESs as a function of the activity status of the acromegaly. Cured and controlled disease statuses were associated with similar prevalences of hard (ES 3–4) and soft nodules (ES 1–2), but in patients with active disease hard lesions predominated. In addition, the prevalence of hard nodules in the latter patients (68.9%) was higher than that observed in patients with cured (44.4%) or controlled (52.5%) disease although this difference was not statistically significant (p = 0.09) (Fig. 5).
Fig. 5.

Distribution of elastographic scores according to the activity status of the acromegaly: a) cured; b) controlled; c) active.
In one patient who had previously undergone radioiodine therapy for hyperthyroidism, 7 nodules were found, 5 of which were hard (ES 3 for 3 nodules, ES 4 for the other 2).
During the 12-month follow-up, no changes in US or US-E characteristics were observed in the stiff nodules that were not subjected to FNAC.
All 31 of the thyroid nodules undergone FNAC (is not a biopsy) (20 ES 3 lesions and 11 ES 4 nodules) were negative for malignancy or suspect malignancy (follicular lesions), and none of the smears were inadequate or nondiagnostic.
Discussion
US-E, a dynamic technique for estimating tissue stiffness, has proved to be useful in differentiating cancers from benign lesions in the prostate, breast, pancreas, and lymph nodes [9–11]. It has recently been used to evaluate the elasticity of thyroid nodules, and some investigators feel that it is the best available non-invasive tool for the diagnosis of thyroid cancer [12–18]. Exploiting the fact that malignant thyroid nodules are generally harder than their benign counterparts, US-E has identified patients at high risk for malignancy with high sensitivity (82–97%) and specificity (81–100%) [12,13,15] although it appears to be less accurate in the identification of follicular carcinomas [15–17].
To the best of our knowledge, this is the first attempt to define the ultrasonographic and elastographic features of thyroid nodules in patients with acromegaly. Conventional US revealed thyroid nodules in 25 of the 35 patients we examined, which is consistent with the prevalence figures reported in the literature [5]. The major finding of our study is the high prevalence of hard nodules detected with US-E in these patients. The prevalence of ES3 and ES4 nodules in our study population (56.8%) is definitely higher than that reported in patients without acromegaly (33.7%, including some that were in fact malignant) [15]. The prevalence of hard nodules was also higher in patients with active acromegaly, whereas soft and hard lesions appeared to be equally distributed in those whose acromegaly had been cured or controlled. It is also interesting to note that none of the hard nodules found in our patients was malignant at the cytological examination.
Excess secretion of GH and IGF-I causes thyroid overgrowth, which is usually considered one of the typical forms of visceromegaly that characterize acromegaly [1–4].
Benign thyroid overgrowth is a common phenomenon in this pathology, but whether or not acromegaly is associated with an increase in the prevalence of malignant thyroid nodules is still a matter of debate [5,19,20].
Thus far, studies using US-E to investigate thyroid nodules have been performed in nonacromegalic patients who were already scheduled for surgery because their nodules (single or multiple) were either large or displayed signs of possible malignancy at FNAC [12,13]. In other series, this technique has been used only for nodules larger than 10 mm [15].
We performed US-E on all of the nodules found in our patients with acromegaly. At variance with findings in nonacromegalic subjects, where closer correlation between malignancy and nodule stiffness is observed [12,13,15,16,18], FNAC revealed no signs of malignancy in the nodules found in our patients, despite the high frequency of stiff lesions.
Therefore, it seems that in patients with acromegaly, the hardness of thyroid nodules at US-E does not seem to predict the malignant nature of the lesions. A benign process—nodular fibrosis perhaps—may be responsible for the frequent lack of elasticity displayed by many thyroid nodules in patients with acromegaly. Indeed, histologically proven fibrosis in thyroid nodules appears to be associated with a high stiffness index at US-E [17]. In addition, several hard nodules were detected in one of our patients who had previously undergone radioiodine treatment, which is known to induce fibrosis [30], and US-E is known to be a valuable tool for estimating the degree of fibrosis in cirrhotic livers [31].
GH and IGF-I are both known to increase collagen synthesis and deposition [32], and their excess secretion can induce fibrosis. This phenomenon might also contribute to acromegalic cardiomyopathy [5]. Fibrosis might also develop in the thyroid gland, and this could explain the higher prevalence of hard nodules in acromegalic (vs. nonacromegalic) goiters. Along this line, the highest number of stiff lesions—twice the number of soft nodules—was found in the patients with active acromegaly, i.e., those with elevated serum levels of GH and IGF-I.
The lack of thyroid cancer in our study population, despite the high prevalence of hard nodules, is consistent with the fact that the vast majority of the stiff nodules of our series displayed none of the conventional ultrasonographic features associated with malignancy, with the exception of hypoechogenicity, and recent reports indicate that the latter is a typical feature of nodular fibrosis [33].
In conclusion, our US-E study demonstrated a high prevalence of hard thyroid nodules in patients with acromegaly, particularly those with active disease. Interestingly, none of the lesions turned out to be malignant at cytological examination. The hypothesis that nodular fibrosis might account for this elastographic pattern is certainly conceivable, but histopathological confirmation is essential. At this point, US-E appears to be of limited value for detecting thyroid malignancies in patients with acromegaly.
Conflict of interest statement
The authors have no conflict of interest.
References
- 1.Miyakawa M., Saji M., Tsushima T., Wakai K., Shizume K. Thyroid volume and serum thyroglobulin levels in patients with acromegaly: correlation with plasma insulin-like growth factor 1 levels. J Clin Endocrinol Metab. 1988;67:973–978. doi: 10.1210/jcem-67-5-973. [DOI] [PubMed] [Google Scholar]
- 2.Cheung N.W., Boyages S.C. The thyroid gland in acromegaly: an ultrasonographic study. Clin Endocrinol. 1997;46:545–549. doi: 10.1046/j.1365-2265.1997.1680985.x. [DOI] [PubMed] [Google Scholar]
- 3.Kasagi K., Shimatsu A., Miyamoto S., Misaki T., Sakahara H., Konishi J. Goiter associated with acromegaly: sonographic and scintigraphic findings of the thyroid gland. Thyroid. 1999;9:791–796. doi: 10.1089/thy.1999.9.791. [DOI] [PubMed] [Google Scholar]
- 4.Gasperi M., Martino E., Manetti L., Arosio M., Porretti S., Faglia G. Prevalence of thyroid diseases in patients with acromegaly: results of an Italian multi-center study. J Endocrinol Invest. 2002;25:240–245. doi: 10.1007/BF03343997. [DOI] [PubMed] [Google Scholar]
- 5.Colao A., Ferone D., Marzullo P., Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25:102–152. doi: 10.1210/er.2002-0022. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi P., Insana M.F., Hall T.J. Ultrasonic and elasticity imaging to model disease-induced changes in soft tissue structure. Med Image Anal. 1998;2:325–338. doi: 10.1016/s1361-8415(98)80014-5. [DOI] [PubMed] [Google Scholar]
- 7.Ophir J., Alam S.K., Garra B., Kallel L., Konofagou E., Krouskop T. Elastography: ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H. 1999;213:203–233. doi: 10.1243/0954411991534933. [DOI] [PubMed] [Google Scholar]
- 8.Siperstein A.E., Clark O.H. Thyroid diseases: tumors, carcinoma of follicular epithelium, surgical therapy. In: Braverman L.E., Utiger R.D., editors. Werner and Ingbar’s the thyroid: a Fundamental and clinical Text. 8th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 898–899. [Google Scholar]
- 9.Cochlin D.L., Ganatra R.H., Griffiths D.F. Elastography in the detection of prostatic cancer. Clin Radiol. 2002;57:1014–1020. doi: 10.1053/crad.2002.0989. [DOI] [PubMed] [Google Scholar]
- 10.Garra B.S., Cespedes E.I., Ophir J., Spratt S.R., Zuurbier R.A., Magnant C.M. Elastography of breast lesions: initial clinical results. Radiology. 1997;202:79–86. doi: 10.1148/radiology.202.1.8988195. [DOI] [PubMed] [Google Scholar]
- 11.Giovannini M., Hookey L.C., Bories E., Pesenti C., Monges G., Delpero J.R. Endoscopic ultrasound elastography: the first step towards virtual biopsy? preliminary results in 49 patients. Endoscopy. 2006;38:344–348. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 12.Lyshchik A., Higashi T., Asato R., Tanaka S., Ito J., Mai J.J. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202–211. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]
- 13.Rago T., Santini F., Scutari M., Pinchera A., Vitti P. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92:2917–2922. doi: 10.1210/jc.2007-0641. [DOI] [PubMed] [Google Scholar]
- 14.Bae U., Dighe M., Dubinsky T., Minoshima S., Shamdasani V., Kim Y. Ultrasound thyroid elastography using carotid artery pulsation: preliminary study. J Ultrasound Med. 2007;26:797–805. doi: 10.7863/jum.2007.26.6.797. [DOI] [PubMed] [Google Scholar]
- 15.Asteria C., Giovanardi A., Pizzocaro A., Cozzaglio L., Morabito A., Somalvico F. US-Elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. 2008;18:523–531. doi: 10.1089/thy.2007.0323. [DOI] [PubMed] [Google Scholar]
- 16.Rubaltelli L., Corradin S., Dorigo A., Stabilito M., Tregnaghi A., Borsato S. Differential diagnosis of benign and malignant thyroid nodules at elastosonography. Ultraschall Med. 2008;21:175–179. doi: 10.1055/s-2008-1027442. [DOI] [PubMed] [Google Scholar]
- 17.Dighe M., Bae U., Richardson M.L., Dubinsky T.J., Minoshima S., Kim Y. Differential diagnosis of thyroid nodules with US elastography using carotid artery pulsation. Radiology. 2008;248:662–669. doi: 10.1148/radiol.2482071758. [DOI] [PubMed] [Google Scholar]
- 18.Tranquart F., Bleuzen A., Pierre-Renoult P., Chabrolle C., Sam Giao M., Lecomte P. Elastosonography of thyroid lesion. J Radiol. 2008;89:35–39. doi: 10.1016/s0221-0363(08)70367-6. [DOI] [PubMed] [Google Scholar]
- 19.Tita P., Ambrosio M.R., Scollo C., Carta A., Gangemi P., Bondanelli M. High prevalence of differentiated thyroid carcinoma in acromegaly. Clin Endocrinol. 2005;63:61–67. doi: 10.1111/j.1365-2265.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 20.Siegel G., Tomel Y. Is there an association between acromegaly and thyroid carcinoma? A critical review of the literature. Endocr Res. 2005;31:51–58. doi: 10.1080/07435800500229177. [DOI] [PubMed] [Google Scholar]
- 21.Baris D., Gridley G., Ron E., Weiderpass E., Mellemkjaer L., Ekbom A. Acromegaly and cancer risk: a cohort study in Sweden and Denmark. Cancer Causes Control. 2002;13:395–400. doi: 10.1023/a:1015713732717. [DOI] [PubMed] [Google Scholar]
- 22.Giustina A., Barkan A., Casanueva F.F., Cavagnini F., Frohman L., Ho K. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000;85:526–529. doi: 10.1210/jcem.85.2.6363. [DOI] [PubMed] [Google Scholar]
- 23.Rago T., Vitti P., Chiovato L., Mazzeo S., De Liperi A., Miccoli P. Role of conventional ultrasonography and color flow-doppler sonography in predicting malignancy in “cold” thyroid nodules. Eur J Endocrinol. 1998;138:41–46. doi: 10.1530/eje.0.1380041. [DOI] [PubMed] [Google Scholar]
- 24.Brunn J., Block U., Ruf G., Bos I., Kunze W.P., Scriba P.C. Volumetric analysis of thyroid lobes by real-time ultrasoundDtsch Med Wochenschr. 1981;106:1338–1340. doi: 10.1055/s-2008-1070506. (author’s transl) [DOI] [PubMed] [Google Scholar]
- 25.Itoh A., Ueno E., Tohno E., Kamma H., Takahashi H., Shiina T. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 26.The papanicolau society of cytopathology task force on standards of practice. Guidelines of the papanicolau society of cytopathology for the examination of the fine-needle aspiration specimens from thyroid nodules. Mod Pathol. 1996;9:710–715. [PubMed] [Google Scholar]
- 27.Frates M.C., Benson C.B., Charboneau J.W., Cibas E.S., Clark O.H., Coleman B.G. Management of thyroid nodules detected at US: society of radiologist in ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 28.Rago T., Vitti P. Role of thyroid ultrasound in the diagnostic evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:913–928. doi: 10.1016/j.beem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Watters D.A., Ahuja A.T., Evans R.M., Chick W., King W.W., Metreweli C. Role of ultrasound in the management of thyroid nodules. Am J Surg. 1992;164:654–657. doi: 10.1016/s0002-9610(05)80728-7. [DOI] [PubMed] [Google Scholar]
- 30.Shih W.J., Mitchell B., Schott J.C. Scarred atrophic thyroid after I-131 therapy for graves’ disease documented at autopsy. J Natl Med Assoc. 2002;94:915–919. [PMC free article] [PubMed] [Google Scholar]
- 31.Castera L. Assessing liver fibrosis. Expert Rev Gastroenterol Hepatol. 2008;2:541–552. doi: 10.1586/17474124.2.4.541. [DOI] [PubMed] [Google Scholar]
- 32.Fruchtman S., Simmons J.G., Michaylira C.Z., Miller M.E., Greenhalgh C.J., Ney D.M. Suppressor of cytokine signaling-2 modulates the fibrogenic actions of GH and IGF-1 in intestinal mesenchymal cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:342–350. doi: 10.1152/ajpgi.00413.2004. [DOI] [PubMed] [Google Scholar]
- 33.Chen S.J., Yu S.N., Tzeng J.E., Chen Y.T., Chang K.Y., Cheng K.S. Characterization of the major histopathological components of thyroid nodules using sonographic textural features for clinical diagnosis and management. Ultrasound Med Biol. 2009;35:201–208. doi: 10.1016/j.ultrasmedbio.2008.08.017. [DOI] [PubMed] [Google Scholar]


