Abstract
Purpose
To evaluate the efficiency of an original slow freezing protocol on the quality and function of human ovarian cortex.
Methods
Human ovarian tissues were cryopreserved using a freezing medium supplemented with propanediol and raffinose as cryoprotectants and antioxidants (L-glutamine, taurine). Samples were then frozen using a faster cooling rate than the usual one. Viability and morphology of follicles, DNA fragmentation in follicles and stroma as well as histology of the vascular endothelium were analyzed before and after freezing/thawing. Moreover, a functional analysis was performed based on the evaluation of follicular growth and development in thawed ovarian tissues that were cultured in vitro.
Results
Our freezing/thawing protocol allows preservation of a high proportion of viable follicles and the preservation of the different follicle developmental stages (p > 0.05 versus fresh control). 70.5 ± 5.2 % of follicles retained an intact morphology after cryopreservation (p = 0.04). Stroma cells but not follicles exhibited a slight increase of DNA fragmentation after thawing (p < 0.05). Microvessel endothelium within thawed tissues appeared to be preserved. Granulosa cells showed signs of proliferation in follicles cultured for 12 days. Secretion of 17β-oestradiol significantly increased during in vitro culture.
Conclusions
This protocol leads to good preservation of ovarian integrity and functionality post-thawing and thus appears as a suitable technique of ovarian tissue cryopreservation in clinical settings. Further research could be extended to optimize conditions of in vitro culture.
Keywords: Human ovarian tissue, Slow freezing, DNA fragmentation, In vitro culture
Introduction
Constant progress in the diagnosis and treatments has greatly improved the life expectancy of adolescents and young women at risk of premature ovarian failure (POF) due to oncological and non-oncological disorders [13, 37]. Ovarian tissue cryopreservation is the only way to preserve fertility of patients who cannot delay their treatment or who are not sexually mature enough to undergo ovarian stimulation for oocyte or embryo cryopreservation [31, 32].
Controlled-rate freezing is currently the favored freezing method worldwide for therapeutic ovarian tissue cryopreservation. Despite encouraging results [9], adverse effects on structure and functionality of human ovarian tissue have been reported after cryopreservation [41]. Previous studies reported the beneficial effects of 1,2-propanediol (PrOH) in ovarian tissue cryopreservation [11, 14] and raffinose for cold storage of various human tissues before graft [29, 51]. Moreover, antioxidant supplements such as taurine and L-glutamine in freezing media have been found to reduce cryoinjuries [4, 40]. In an earlier study, follicular growth was observed after subcutaneous xenografting of human ovarian tissue cryopreserved according an original protocol using a serum-free freezing medium supplemented with PrOH and raffinose as well as antioxidants, and a faster freezing program than the usual one [15, 39]. Nevertheless, further studies of the quality and functionality of thawed ovarian tissue are needed to support the feasibility of this freezing protocol in clinical settings.
Cortical ovarian tissue contains many different cell types such as stroma cells, follicles each formed by one oocyte and granulosa cells, and blood vessels. Adequate preservation of stroma and vascular system is of fundamental importance due to their critical role in follicular development and restoration of gonadal function after grafting [47]. Therefore, the efficiency of a cryopreservation protocol has to be evaluated not only by the analysis of the follicles but also of other cells contained in cortical ovarian tissue. In vitro culture of thawed ovarian tissue provides a complementary approach to xenograft for appreciating the functionality of ovarian tissue after thawing [42]. Hence, with the aim to further evaluate the efficiency of our original slow freezing procedure, we analyzed the quality of the different cortical ovarian elements (follicles, stroma cells and vascular system) before and after freezing. In addition, a functional analysis was performed based on the evaluation of follicular growth and development in thawed ovarian tissues that were cultured in vitro.
Materials and methods
Unless otherwise indicated, all products were purchased from Sigma-Aldrich (France).
Ovarian tissue
This study was approved by the regional research ethics committee. Ovarian cortical samples from 13 patients were collected during endoscopic surgery for benign cysts, after written informed consent. The mean age of the women was 28.6 ± 1.5 years [± Standard Error of the Mean (SEM)]. For each patient, a piece of ovarian cortex overlying the cyst was excised with scissors and without electrocoagulation. The specimens were immediately immersed in the basal “medium A” at 4 °C and transported to the laboratory on ice, as previously described [38]. “Medium A” was composed of: NaCl (94.7 mmol/l), KCl (4.8 mmol/l), MgSO4 (0.8 mmol/l), NaH2PO4 (1.0 mmol/l), NaHCO3 (25.0 mmol/l), CaCl2 (1.8 mmol/l), sodium lactate (21.3 mmol/l), sodium pyruvate (0.3 mmol/l), D-glucose (5.5 mmol/l), L-glutamine (25.0 mmol/l), taurine (0.5 mmol/l), and 0.5 % of human serum albumin (Vitrolife Sweden AB, Sweden). The cortex was cut into slices of about 1 cm2 in area and 1 mm in thickness. One fragment was fixed overnight at 4 °C in Alcohol-Formalin-Acetic acid (AFA) solution for histological study. One other piece was used to determine fresh follicle viability, and the other specimens were frozen.
Freezing and thawing
The freezing medium, “medium B”, consisted of “medium A” without CaCl2 and supplemented with HEPES (21.8 mmol/l), glycine (50.0 mmol/l), propanediol (3.0 mol/l) and raffinose (0.05 mol/l) [38]. The “medium B” was added in 3 steps to “medium A” which contained ovarian slices, to a final dilution of 1:1 (v/v) under gentle agitation. After 15 min of equilibration at 4 °C, each slice was transferred to a sterile cryovial (Nunc, Fisher Bioblock Scientific, France) containing 1.5 ml of cryoprotective solution and loaded in a programmable freezer (Minicool 40 PC, Air Liquide, France). The cooling rate was 2 °C/min from 4 °C to −11 °C, at which temperature nucleation was induced by semi-automatic seeding. Then the temperature was lowered to −40 °C at 2 °C/min and from −40 °C to −150 °C at 10 °C/min. Finally, the cryovials were plunged into liquid nitrogen for storage.
For the thawing phase, cryovials were immersed in a 37 °C water bath for 2 min and the cryoprotective solution containing ovarian slices was diluted in two 5-min steps with the basal “medium A”, at 37 °C. Each slice was washed twice in “medium A” for 5 min at 37 °C before proceeding with quality analysis. For each patient, one fragment was fixed overnight at 4 °C in AFA for histological study. One other piece was used to determine viability of thawed follicles.
Follicle viability assessment
Follicular viability was assessed after Trypan blue staining of small enzymatically isolated follicles before freezing and immediately after thawing as previously described [36].
Developmental stage and morphological analysis of follicles
Fixed samples were embedded in paraffin and cut into 4 μm serial sections. Each set of 8 consecutive sections was mounted per slide and every second slide was deparaffinized, hydrated and stained with hematoxylin, eosin and saffron (HES). The HES sections were observed by light microscopy (BX40; Olympus Co., Germany) at ×400 magnification. Follicles were classified per developmental stage according to Gougeon’s criteria [16]. The follicles were considered atretic if they had an oocyte with eosinophilic cytoplasm, contraction and clumping of the chromatin material. In the result section, primordial and intermediary follicles have been pooled into one group and termed as “resting follicles” as previously described [34].
Morphology of follicles was evaluated on the basis of parameters previously described by Keros et al. [25]. Follicles were classified as intact if no overt signs of oocyte and GCs degeneration were noted. The oocyte had to be in contact with the surrounding GCs with the basement membrane of the follicle intact and attached to the GC layer. The follicles were regarded as degenerated if they contained an intact oocyte, but showed more than 50 % of the following signs: detachment of the oocyte from surrounding GCs and/or vacuolization of ooplasm and/or partially degenerated GCs and/or detachment of the basal membrane.
Measurement of DNA fragmentation
DNA fragmentation in follicles and stroma cells was detected by the In situ Cell Death Detection Kit (Roche, France) according to the manufacturer’s protocol. After rehydratation and permeabilization, the sections were incubated with the labeling solution containing dUTP and enzyme solution (Terminal deoxynucleotidyl transferase, Tdt) for 1 h at 37 °C. After counterstaining with Hoechst 33258 (Invitrogen, France), the tissue sections were observed by fluorescence microscopy at ×400 magnification (BX51TF; Olympus Co., Japan). A negative control was carried out by omitting Tdt from the reaction mixture. A positive control was performed by applying DNAse treatment. Follicles with positive TUNEL staining of the oocyte and/or ≥50 % of the GCs were considered as positive [48]. The proportion of TUNEL-positive stroma cells was evaluated on three fields at ×400 magnification per section. Images were captured using a digital camera Nikon DSFI-1 (Nikon, Japan).
Immunohistochemical study of blood vessels
Histology of vessel endothelium was assessed by CD31 immunostaining. Immunohistochemistry was performed using a Ventana Benchmark XT device (Ventana Medical System Inc., USA). After rehydratation, the deparaffinized sections were submitted to heat-induced antigen retrieval in the presence of a citrate buffer (CC1, Roche). The sections were then incubated with (1:20 dilution) monoclonal mouse anti-human CD31 (clone JC/70A, Dako, Denmark) followed by application of the ultraView Universal DAB Detection Kit ([45]). Hematoxylin was used as a counterstain. For the negative control the primary antibody was omitted. Inflamed human tonsil was used as positive control. As previously described, blood vessels were considered intact if the vascular endothelium was whole and without any sign of detachment [5]. Vascular anomalies were defined as follows: endothelial detachment, internal elastic membrane rupture, or smooth muscle cell bloating.
In vitro culture of thawed cortical tissues
Frozen/thawed ovarian samples from 5 selected patients were cultured in vitro. After thawing, one cortical fragment per patient was cut into small pieces ~1 × 1 mm2 in size. Tissue pieces were individually transferred to a 96-well plate coated with Ultra Low-Attachment Surface (Corning, Escolab, Belgium) in medium consisting of 100 μl/well of pre-equilibrated α-Minimum Essential Medium-GlutaMAX (Invitrogen, Belgium) supplemented with 10 % HSA (Vitrolife, Sweden), 100 μg/ml of ascorbic acid, 5 ng/ml of insulin, 5 μg/ml-5 ng/ml of transferrin-selenium as well as 25 mIU/ml of recombinant FSH (GONAL-f, Merck). Culture took place for 12 days at 37 °C in a humidified incubator with 5 % CO2 and 5 % O2 in air. The culture medium was refreshed every 3 days by replacing 50 μl of spent medium with fresh pre-equilibrated medium. Every 6 days of culture, 4 pieces were removed from each culture and fixed overnight at 4 °C in AFA for histological and immunohistochemical evaluation.
Immunohistochemical study of cell proliferation
The proliferative status of follicular cells was evaluated by immunostaining of proliferative cell nuclear antigen (PCNA). The tissue sections were deparaffinized, rehydrated and labeled with a (1:100 dilution) monoclonal mouse anti-PCNA (Novocastra, Menarini diagnostics, France) overnight at 4 °C. The second antibody, goat anti-mouse peroxidase (Amersham, France), was then applied (1:20 dilution) for 30 min at room temperature. Immunodetection was performed with diaminobenzidine (DAB, Vector NovaRED). Sections were counterstained with hematoxylin. For the negative control, the first antibody was omitted. Human mesenteric lymph node was used as positive control.
Assessment of 17β-oestradiol (E2) production during in vitro culture
At the time of culture refreshment, 50 μl of culture medium were individually collected per plate every 6 days of in vitro culture and stored at −20 °C. E2 production was measured with a direct radioimmunoassay from Clinical Assays (DiaSorin, Sorin Fueter, Belgium) that has an analytical sensitivity of 10 ng/l and a total imprecision profile <10 % (% coefficient of variation). Cross-reactions with the major steroid and metabolites mentioned in the package inserts were minimal, so that the specificity was guaranteed by the manufacturers for all methods. For each patient, E2 concentration (ng/ml) was measured from two different medium samples individually collected after 6 and after 12 days of in vitro culture. The control was a fresh culture medium (day 0).
Statistical analysis
The χ2 test was used to compare the proportion of follicles at different developmental stages in fresh and frozen/thawed tissues. A Wilcoxon signed-rank test was used to compare the mean percentages (±SEM) of viable follicles, morphologically intact follicles, and TUNEL-positive follicles and stroma cells in fresh and frozen/thawed groups. A Wilcoxon signed-rank test was also used to compare 17β-oestradiol (E2) measurements between the three periods (0 versus 6 days; 0 versus 12 days; 6 versus 12 days).
Results
Quality of frozen/thawed ovarian tissue
Follicle viability
For the 13 ovarian tissue samples collected, the average percentage of viable follicles (unstained by Trypan blue) from frozen/thawed tissue (34.9 ± 4.8 %) was not significantly different from samples isolated from fresh tissue (36.3 ± 5.2 %, p = 0.84).
Follicle distribution and morphology
The total count of analyzed follicles was respectively 978 before freezing and 537 after thawing (n = 13 patients). The distribution of follicles per growing stage is shown in Table 1. After freezing/thawing, 71.3 % of follicles were classified as resting, 22.7 % primary, 4.3 % secondary and 1.5 % atretic. In the fresh group, 73.9 % were classified as resting follicles, 20.4 % primary, 4.6 % secondary, 0.6 % preantral and 0.4 % atretic. Moreover no significant difference was found between fresh and frozen/thawed ovarian tissues in terms of distribution of follicles at different developmental stages (p > 0.05) (Table 1). Overall analysis of follicles showed that 95.6 ± 1.3 % from fresh tissue and 70.5 ± 5.2 % after freezing/thawing were morphologically intact (p = 0.04) (Table 1). Pairwise comparisons showed a significant difference in the percentage of morphologically intact resting follicles from frozen/thawed tissue (70.9 ± 6.0 %) versus fresh control (97.6 ± 0.8 %; p = 0.008), but not for the other development stages (Table 1).
Table 1.
Proportion of follicles in various developmental stages and mean percentages (± SEM) of intact follicles per developmental stage within fresh and frozen/thawed tissues
| Total follicles | Follicle distribution | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Res ting follicles | Primary follicles | Secondary follicles | Preantral follicles | Atretic follicles | |||||||
| Total | Intact | Total | Intact | Total | Intact | Total | Intact | Total | Intact | Total | |
| Fresh tissue | 978 | 95.6 ± 1.3 % | 73.9 % (723/978) | 97.6 ± 0.8 % | 20.4 % (200/978) | 93.1 ± 3.5 % | 4.6 % (45/978) | 96.9 ± 2.8 % | 0.6 % (6/978) | 100 ± 0.0 % | 0.4 % (4/978) |
| Thawed tis sue | 537 | 70.5 ± 5.2 %* | 71.3 % (383/537) | 70.9 ± 6.0 %* | 22.7 % (122/537) | 80.4 ± 6.3 % | 4.3 % (23/537) | 48.7 ± 14.6 % | 0 % | – | 1.5 % (8/537) |
*p < 0.05 versus fresh tissue
Assessment of DNA fragmentation
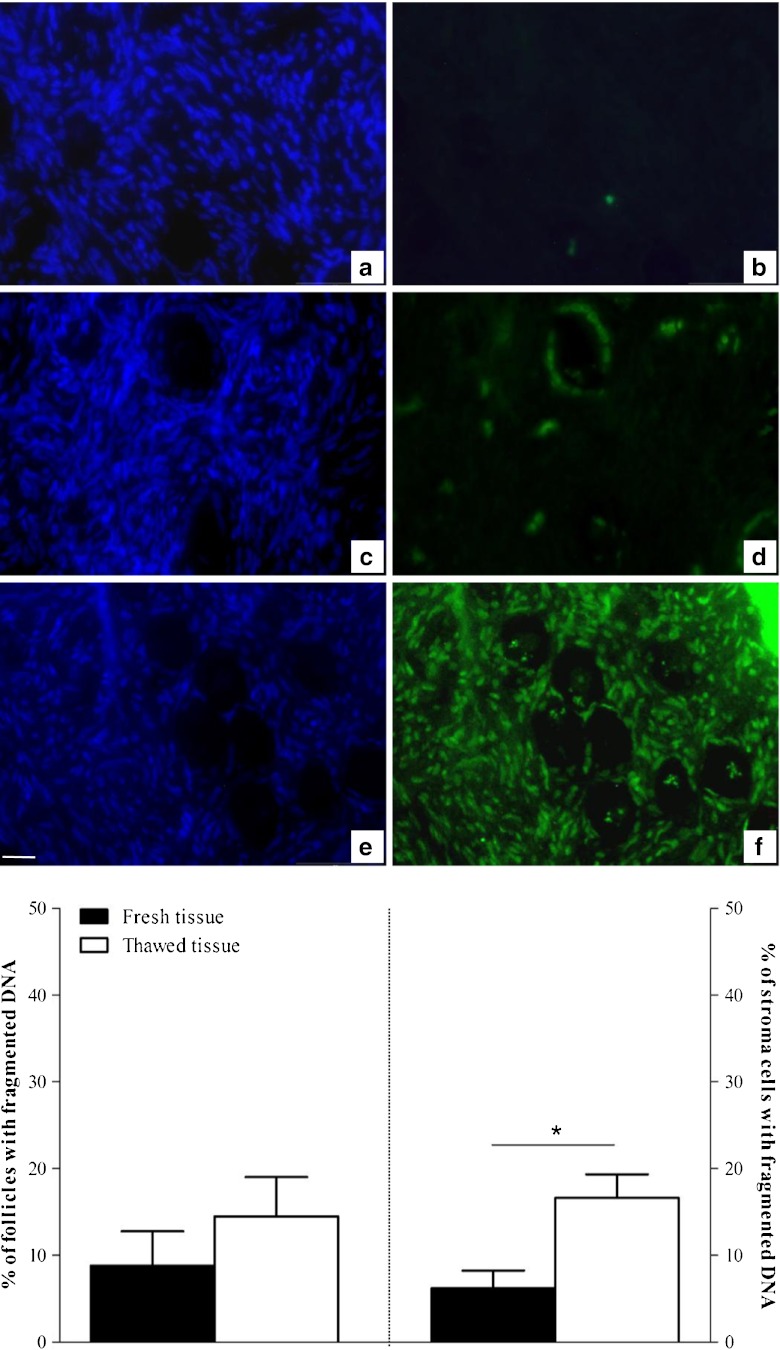
In situ analysis of DNA fragmentation using the TUNEL method was performed on ovarian follicles and stroma cells (Fig. 1). Overall, 171 and 170 follicles were observed on thawed and fresh sections respectively (n = 13 patients) for this measurement. Pairwise comparisons showed no significant difference in the percentage of follicles with DNA fragmentation in frozen/thawed (14.5 ± 4.5 %) (Fig. 1c and d) versus fresh tissue (8.8 ± 3.9 %) (p = 0.26) (Fig. 1a and b). DNA fragmentation was assessed in 2423 thawed and 3421 fresh stroma cells. A higher percentage of stroma cells with DNA fragmentation was observed in thawed tissue (16.6 ± 2.7 %) compared with fresh tissue (6.2 ± 2.0 %, p < 0.05) (Fig. 1e and f).
Fig. 1.
DNA fragmentation analysis in follicles and stroma cells. Pictures on the same line are of the same sample but stained using different methods: the Hoechst 33258 method was used to check the location of follicles (left) and the TUNEL method was used to detect DNA fragmentation (right). For each patient, this co-staining was performed on fresh ovarian tissue (a, b) and after slow freezing/thawing using our method (c, d). Cryopreservation induced a slight increase of DNA fragmentation (green fluorescence). e, f DNAse-treated section (TUNEL positive control) both after Hoechst (e) and TUNEL staining (f). Bar = 35 μm. The histograms present the mean percentages (± SEM) of TUNEL-positive follicles (left panel) and stroma cells per high power field (right panel), before (black plots) and after (white plots) cryopreservation (n = 13 patients). *p < 0.05 versus fresh tissue
Vascular system histology
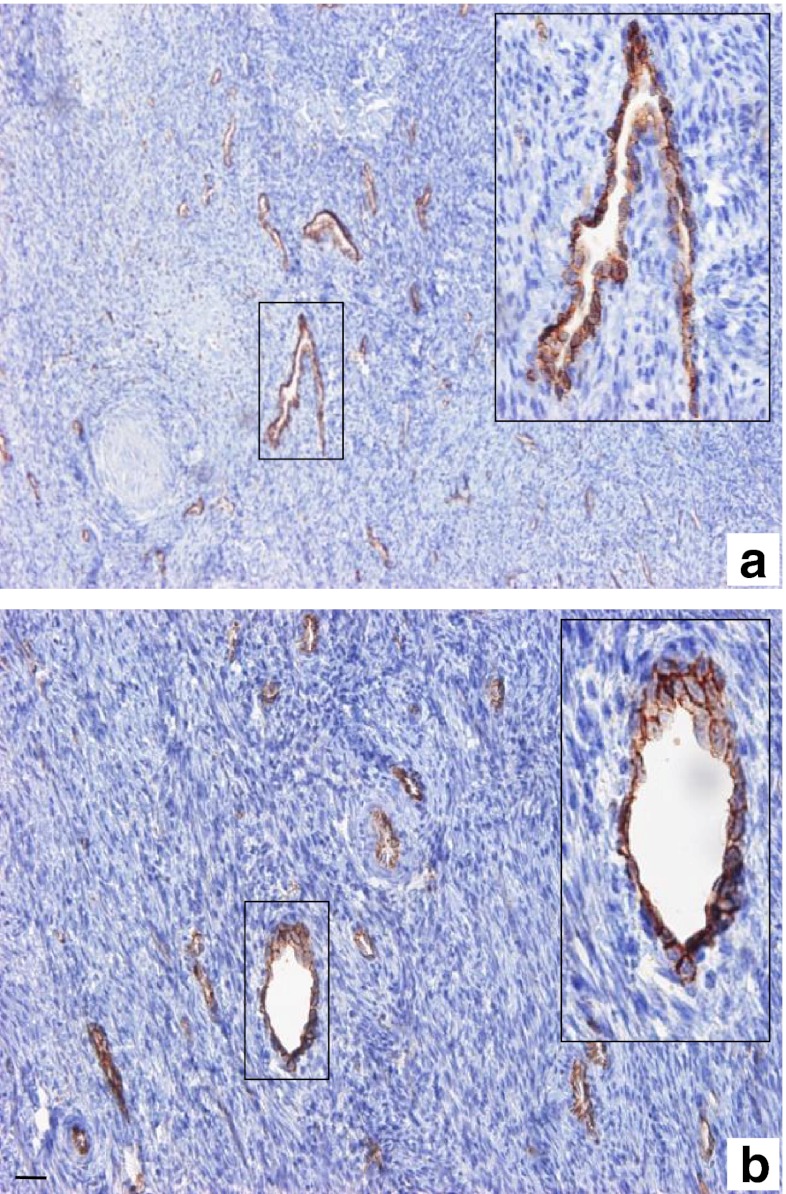
In both fresh and thawed samples, the superficial cortical stroma exhibited blood vessels with a narrow lumen (Fig. 2). No sign of histologic anomalies were found in either the fresh (Fig. 2a) or the thawed tissue (Fig. 2b).
Fig. 2.
Vascular system from superficial cortical stroma before and after freezing/thawing. Histology of the vascular system was assessed before (a) and after (b) freezing/thawing using specific immunostaining with CD31 monoclonal antibody. Fresh and thawed superficial ovarian cortex showed blood vessels with narrow lumen. The boxed regions are magnified in the inset (a, b). No major histological anomalies were found after freezing/thawing. Bar = 35 μm
Functionality of frozen/thawed human ovarian tissue
Follicular growth in culture
After 6 and 12 days of culture (n = 5 patients), the distribution of follicles in various stages was statistically different compared with day 0 control (p = 0.013). We observed a decrease in the proportion of resting (day 6: 53.6 %; day 12: 46.7 %) and primary follicles (day 6: 17.6 %; day 12: 13.3 %). Moreover, an increase in the proportion of secondary follicles (day 6: 28.6 %; day 12: 40.0 %) was shown. The percentage of atretic follicles significantly increased during in vitro culture, ranging from 1.2 % on day 0, to 62.2 % and 50.0 % respectively after 6 and 12 days of culture (p < 0.001) (Table 2).
Table 2.
Distribution of follicles in various developmental stages during in vitro culture of frozen/thawed ovarian tissue
| Day of in vitro culture | Total follicles | Follicle distribution | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Resting follicles | Primary follicles | Secondary follicles | Atretic follicles | ||||||
| n | Total | % | Total | % | Total | % | Total | % | |
| 0 | 241 | 163 | 68,5 | 46 | 19,3 | 29 | 12,2 | 3 | 1,2 |
| 6 | 74 | 15 | 53,6 | 5 | 17,9 | 8 | 28,6 | 46 | 62,2 |
| 12 | 30 | 7 | 46,7 | 2 | 13,3 | 6 | 40,0 | 15 | 50,0 |
For follicle classification, only healthy follicles (without any sign of atresia) were considered. The results are presented as total number and proportion (%) of follicles per developmental stage
Cellular proliferation during follicle growth in culture
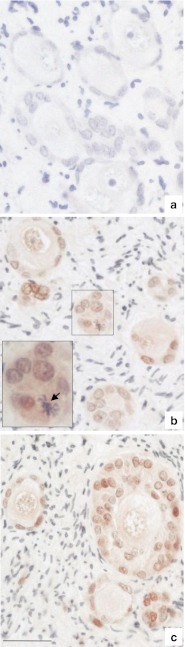
No immunostaining of PCNA was observed in the uncultured tissue control (Fig. 3a). In contrast, positive PCNA immunoreactivity was observed in the GCs of resting, primary and secondary follicles from day 6 (Fig. 3b) and after 12 days (Fig. 3c) of in vitro culture of thawed tissue (n = 5 patients). In addition, several GCs undergoing mitotic division of chromosomes were observed on HES stained slides of cultured tissue (inset Fig. 3b).
Fig. 3.
Proliferating cell nuclear antigen (PCNA) immunostaining of follicles from thawed human ovarian tissue in culture. a Unstained follicles from uncultured thawed ovarian tissue. PCNA-stained granulosa cells in follicles after 6 days (b) and 12 days (c) of in vitro culture. The boxed region in (b) is magnified in the inset; note the mitotic division of a granulosa cell (black arrow) after 6-day culture. Bar = 35 μm
E2 production in culture
Compared with day 0 control (90.3 ± 1.2 ng/ml), a significant increase of E2 concentration was observed both after 6 days (97.5 ± 7.7 ng/ml) and 12 days (106.0 ± 11.2 ng/ml) of culture (p < 0.01) (n = 5 patients). Moreover, E2 concentration was significantly higher after 12 days compared to 6 days of culture (p = 0.01), suggesting a progressive increase of E2 production throughout the culture period.
Discussion
Cryopreservation of ovarian tissue offers a possibility to preserve the fertility of young women with a high risk of POF. Improvement of the freezing/thawing procedure is of fundamental importance to optimize restoration of gonadal function after grafting. By this study, we have shown the effectiveness of an original and simple slow freezing procedure to protect ovarian tissue from cryoinjuries.
For our freezing protocol, we chose a combination of PrOH and raffinose which are respectively penetrating and non-penetrating cryoprotectants. The use of PrOH in clinical freezing protocols gives rise to the birth of healthy babies following cryopreservation of both embryos and mature oocytes [3, 26, 44]. Human ovarian tissue cryopreserved with PrOH as cryoprotectant exhibits a satisfactory morphological preservation of follicles in comparison with fresh tissue [11, 14]. Moreover, Abir et al. [1] reported better survival and development of follicles in grafts when fetal ovarian tissue was cryopreserved using PrOH compared to dimethylsulfoxide (DMSO). Raffinose was used in order to prevent cellular damage due to intracellular crystallization and avoid osmotic shock especially during the thawing process. This trisaccharide is one component of the University of Wisconsin solution, reputed to be the most used solution for perfusion and preservation of livers, kidneys and pancreases with excellent clinical and experimental preservation data [22, 29, 51]. Interestingly, a recent study reported the beneficial effect of raffinose on the post-thaw survival of metaphase II mouse oocytes [10].
Most investigators use a standard freezing protocol described more than 15 years ago for cryopreserving ovarian tissue of sheep [15]. In this protocol manual seeding is performed to induce extracellular ice formation and a very slow post-seeding freezing rate of 0.3 °C/min is applied. Later studies in sheep reported that using semi-automatic seeding and increasing the freezing rate from 0.3 to 2 °C/min was equally effective regarding follicle quality [6, 7]. Applying this freezing rate to cryopreserve human ovarian tissue would provide a significant time saving compared to the standard protocol, which would be extremely useful for routine clinical application.
Our freezing/thawing protocol allows preservation of a high number of viable follicles and allows the conservation of the different developmental stages of follicles. The morphology of follicles was well maintained (70.5 ± 5.2 %) by our original protocol. For morphological examination of follicles, we used well-defined histological criteria unlike other investigators who considered atresia as the sole morphological consequence of follicular cryoinjuries [12, 19]. This might explain the significant difference we observed between fresh and thawed morphologically intact follicles at resting stage.
Our protocol did not increase the percentage of follicles with DNA fragmentation after thawing. The follicles were thus cryopreserved without subsequent irreversible DNA damage which can be the consequence of apoptosis and/or oxidative stress activation [43, 48]. These findings show clearly the beneficial effects of our procedure, since several studies in human reported a significant increase of DNA fragmentation in follicles after ovarian cortex cryopreservation with Gosden’s procedure [12, 33, 48]. Supplementation of the collection medium as well as the freezing medium with taurine and L-glutamine, which have been found to play an antioxidant role by reducing cryopreservation-induced oxidative stress, might explain this result [4, 18, 40]. Although we observed a higher percentage of stroma cells with DNA fragmentation after thawing, this effect remains slight compared with a previous report [49] since it concerns only 16.6 % of stroma cells. The explanation for this observation is most likely linked to the higher sensitivity of these cells to cryoinjuries [11, 28]. A better understanding of the cellular pathways activated by the freezing/thawing process would enable better optimization of preservation of stroma cells, whose functions are important for follicular development [47].
In addition to follicle and stroma quality evaluation, we further investigated whether cryopreservation affects the histology of microvessels. Preservation of microvessel integrity during the freezing/thawing process is a key factor for successful neovascularization of the ovarian transplant, and indispensable for the survival of ovarian follicles [30, 46]. In our study, the histology of the vascular system in thawed tissues appeared well-preserved without any morphological sign of cryodamage. However, CD31 immunostaining provides only a global approach and is not a highly conclusive test of the vascular system quality. It would be necessary in the future to carry out a functional study using an ovarian grafting method in order to confirm the viability status of the cryopreserved vessels.
We used in vitro culture to evaluate the functionality of cryopreserved tissue, because the integrity of the tissue immediately after thawing may not reflect its true state. In vitro culture experiments were conducted using thawed ovarian samples from 5 patients, presenting the higher number of follicles according to the previous histological examination. As shown in previous studies, culture of ovarian pieces allows cellular interactions between follicles and surrounding stroma indispensable to sustain follicular growth initiation [8, 25, 34]. In order to retain the 3D structure of the follicles, a low-attachment culture system limiting stroma cell adhesion on the culture support was used. A recent study reported a positive influence of low-attachment culture conditions on cumulus cell gene expression and developmental competence of mouse oocytes [35]. To our knowledge, our study is the first which investigates the feasibility of using this low-attachment system for the culture of human ovarian tissue.
Our results demonstrate a decrease in the proportion of early stage follicles and an increase in the proportion of secondary follicles suggesting an activation of follicular growth during in vitro culture of frozen/thawed tissue. However, the decrease in the total number of secondary follicles observed over the culture period calls into question the real physiological significance of this follicular growth. Nevertheless, the enhanced 17β-oestradiol (E2) concentration in the medium during in vitro culture is an indicator of follicular growth and secondary follicle viability. Indeed, aromatase activity, which is required for the synthesis of E2, is present only from secondary follicular stages onwards [17]. Our data are in agreement with previous findings showing a correlation between E2 secretions and follicular growth in cultures of frozen/thawed tissues [2, 21, 24]. PCNA is a nuclear protein that plays an essential role in cell cycle regulation [50]. Positive PCNA staining of follicles, together with the presence of mitoses in granulosa cells provide evidence for the viability of growing follicles in culture. Taken together, these results indicate that frozen/thawed follicles can remain functional over the period of culture. Despite encouraging results regarding functionality and developmental capacity of follicles under low-attachment conditions, the increasing atresia rate suggests that conditions for in vitro culture of frozen/thawed ovarian tissues still need to be optimized. Changes in the composition of the culture medium such as a supplementation with GDF-9, BMP15 and IGF-I which have been found to improve survival rate and promote follicular growth in culture, should be considered [20, 23, 27].
In conclusion, the present study shows that after freezing/thawing according to this protocol, human ovarian cortex retains its post-thaw integrity, plus functionality markers during in vitro culture. These promising results tend to show that this freezing/thawing protocol is efficient and may be a suitable technique for ovarian tissue cryopreservation in clinical settings. Further research could be extended to optimize in vitro culture of thawed ovarian tissue.
Acknowledgments
We would like to thank all the surgical team in the department of gynecology, CHU Estaing, Clermont-Ferrand (France) for their help to recruit patients. We are grateful to Dr Wassim Essamet for his expertise regarding the histology, Emmanuel Bourgeois and Christine Artonne for their precious technical assistance and Mrs Elizabeth Petit for language revision of the manuscript. Thanks are also due to the women who donated tissue for this research.
This work is supported by an industrial PhD fellowship (Convention Industrielle de Formation par la Recherche, CIFRE) with the Centre International de Chirurgie Endoscopique (CICE), France (Grant No: 176/2009).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Capsule An original slow freezing protocol able to protect both quality and functionality of human ovarian tissue, and easily applicable in a clinical setting.
Contributor Information
Sandra Sanfilippo, Phone: +33-4-73178081, FAX: +33-4-73178077, Email: sanfilipposandra@gmail.com.
Michel Canis, Phone: +33-4-73152050, FAX: +33-4-73152079.
Sergio Romero, Phone: +32-2-4775052, FAX: +32-2-4775060.
Benoît Sion, Phone: +33-4-73178078, FAX: +33-4-73274621.
Pierre Déchelotte, Phone: +33-4-73750220, FAX: +33-4-73750221.
Jean-Luc Pouly, Phone: +33-4-73152050, FAX: +33-4-73152079.
Laurent Janny, Phone: +33-4-73178081, FAX: +33-4-73178077.
Johan Smitz, Phone: +32-2-4775052, FAX: +32-2-4775060.
Florence Brugnon, Phone: +33-4-73178081, FAX: +33-4-73178077.
References
- 1.Abir R, Orvieto R, Raanani H, Feldberg D, Nitke S, Fisch B. Parameters affecting successful transplantation of frozen-thawed human fetal ovaries into immunodeficient mice. Fertil Steril. 2003;80(2):421–428. doi: 10.1016/S0015-0282(03)00658-7. [DOI] [PubMed] [Google Scholar]
- 2.Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48(4):798–806. doi: 10.1095/biolreprod48.4.798. [DOI] [PubMed] [Google Scholar]
- 3.Borini A, Bonu MA, Coticchio G, Bianchi V, Cattoli M, Flamigni C. Pregnancies and births after oocyte cryopreservation. Fertil Steril. 2004;82(3):601–605. doi: 10.1016/j.fertnstert.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Cabrita E, Ma S, Diogo P, Martinez-Paramo S, Sarasquete C, Dinis MT. The influence of certain aminoacids and vitamins on post-thaw fish sperm motility, viability and DNA fragmentation. Anim Reprod Sci. 2011;125(1–4):189–195. doi: 10.1016/j.anireprosci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Courbiere B, Massardier J, Salle B, Mazoyer C, Guerin JF, Lornage J. Follicular viability and histological assessment after cryopreservation of whole sheep ovaries with vascular pedicle by vitrification. Fertil Steril. 2005;84(Suppl 2):1065–1071. doi: 10.1016/j.fertnstert.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 6.Demirci B, Lornage J, Salle B, Frappart L, Franck M, Guerin JF. Follicular viability and morphology of sheep ovaries after exposure to cryoprotectant and cryopreservation with different freezing protocols. Fertil Steril. 2001;75(4):754–762. doi: 10.1016/S0015-0282(00)01787-8. [DOI] [PubMed] [Google Scholar]
- 7.Demirci B, Salle B, Frappart L, Franck M, Guerin JF, Lornage J. Morphological alterations and DNA fragmentation in oocytes from primordial and primary follicles after freezing-thawing of ovarian cortex in sheep. Fertil Steril. 2002;77(3):595–600. doi: 10.1016/S0015-0282(01)03205-8. [DOI] [PubMed] [Google Scholar]
- 8.Ding CC, Thong KJ, Krishna A, Telfer EE. Activin A inhibits activation of human primordial follicles in vitro. J Assist Reprod Genet. 2010;27(4):141–147. doi: 10.1007/s10815-010-9395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43(6):437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 10.Eroglu A. Cryopreservation of mammalian oocytes by using sugars: intra- and extracellular raffinose with small amounts of dimethylsulfoxide yields high cryosurvival, fertilization, and development rates. Cryobiology. 2010;60(3 Suppl):S54–S59. doi: 10.1016/j.cryobiol.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabbri R, Pasquinelli G, Keane D, Magnani V, Paradisi R, Venturoli S. Optimization of protocols for human ovarian tissue cryopreservation with sucrose, 1,2-propanediol and human serum. Reprod BioMed Online. 2010;21(6):819–828. doi: 10.1016/j.rbmo.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Fauque P, Ben Amor A, Joanne C, Agnani G, Bresson JL, Roux C. Use of trypan blue staining to assess the quality of ovarian cryopreservation. Fertil Steril. 2007;87(5):1200–1207. doi: 10.1016/j.fertnstert.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 13.Gidoni Y, Holzer H, Tulandi T, Tan SL. Fertility preservation in patients with non-oncological conditions. Reprod Biomed Online. 2008;16(6):792–800. doi: 10.1016/S1472-6483(10)60144-7. [DOI] [PubMed] [Google Scholar]
- 14.Gook DA, Edgar DH, Stern C. Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1, 2-propanediol. Hum Reprod. 1999;14(8):2061–2068. doi: 10.1093/humrep/14.8.2061. [DOI] [PubMed] [Google Scholar]
- 15.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9(4):597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 16.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1(2):81–87. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 17.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121–155. doi: 10.1210/edrv-17-2-121. [DOI] [PubMed] [Google Scholar]
- 18.Hardikar AA, Risbud MV, Remacle C, Reusens B, Hoet JJ, Bhonde RR. Islet cryopreservation: improved recovery following taurine pretreatment. Cell Transplant. 2001;10(3):247–253. doi: 10.3727/000000001783986756. [DOI] [PubMed] [Google Scholar]
- 19.Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, et al. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11(6):1268–1272. doi: 10.1093/oxfordjournals.humrep.a019370. [DOI] [PubMed] [Google Scholar]
- 20.Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ, Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab. 2002;87(1):316–321. doi: 10.1210/jc.87.1.316. [DOI] [PubMed] [Google Scholar]
- 21.Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, et al. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction. 2009;138(2):319–327. doi: 10.1530/REP-09-0039. [DOI] [PubMed] [Google Scholar]
- 22.Jain A, Mohanka R, Orloff M, Abt P, Kashyap R, Cullen J, et al. University of Wisconsin versus histidine-tryptophan-ketoglutarate for tissue preservation in live-donor liver transplantation. Exp Clin Transplant. 2006;4(1):451–457. [PubMed] [Google Scholar]
- 23.Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T, Felz C, et al. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab. 2011;96(8):E1246–E1254. doi: 10.1210/jc.2011-0410. [DOI] [PubMed] [Google Scholar]
- 24.Kedem A, Hourvitz A, Fisch B, Shachar M, Cohen S, Ben-Haroush A, et al. Alginate scaffold for organ culture of cryopreserved-thawed human ovarian cortical follicles. J Assist Reprod Genet. 2011;28(9):761–769. doi: 10.1007/s10815-011-9605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24(7):1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 26.Konc J, Kanyo K, Varga E, Kriston R, Cseh S. Births resulting from oocyte cryopreservation using a slow freezing protocol with propanediol and sucrose. Syst Biol Reprod Med. 2008;54(4–5):205–210. doi: 10.1080/19396360802415778. [DOI] [PubMed] [Google Scholar]
- 27.Louhio H, Hovatta O, Sjoberg J, Tuuri T. The effects of insulin, and insulin-like growth factors I and II on human ovarian follicles in long-term culture. Mol Hum Reprod. 2000;6(8):694–698. doi: 10.1093/molehr/6.8.694. [DOI] [PubMed] [Google Scholar]
- 28.Marsella T, Sena P, Xella S, La Marca A, Giulini S, De Pol A, et al. Human ovarian tissue cryopreservation: effect of sucrose concentration on morphological features after thawing. Reprod Biomed Online. 2008;16(2):257–267. doi: 10.1016/S1472-6483(10)60583-4. [DOI] [PubMed] [Google Scholar]
- 29.Muhlbacher F, Langer F, Mittermayer C. Preservation solutions for transplantation. Transplant Proc. 1999;31(5):2069–2070. doi: 10.1016/S0041-1345(99)00265-1. [DOI] [PubMed] [Google Scholar]
- 30.Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000;74(1):122–129. doi: 10.1016/S0015-0282(00)00548-3. [DOI] [PubMed] [Google Scholar]
- 31.Oktem O, Urman B. Options of fertility preservation in female cancer patients. Obstet Gynecol Surv. 2010;65(8):531–542. doi: 10.1097/OGX.0b013e3181f8c0aa. [DOI] [PubMed] [Google Scholar]
- 32.Poirot C, Schubert B. Fertility preservation in prepubertal children. Bull Cancer. 2011;98(5):489–499. doi: 10.1684/bdc.2011.1362. [DOI] [PubMed] [Google Scholar]
- 33.Rimon E, Cohen T, Dantes A, Hirsh L, Amit A, Lessing JB, et al. Apoptosis in cryopreserved human ovarian tissue obtained from cancer patients: a tool for evaluating cryopreservation utility. Int J Oncol. 2005;27(2):345–353. doi: 10.3892/ijo.27.2.345. [DOI] [PubMed] [Google Scholar]
- 34.Sadeu JC, Smitz J. Growth differentiation factor-9 and anti-Mullerian hormone expression in cultured human follicles from frozen-thawed ovarian tissue. Reprod Biomed Online. 2008;17(4):537–548. doi: 10.1016/S1472-6483(10)60242-8. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez F, Romero S, Albuz FK, Smitz J. In vitro follicle growth under non-attachment conditions and decreased FSH levels reduces Lhcgr expression in cumulus cells and promotes oocyte developmental competence. J Assist Reprod Genet. 2012;29(2):141–152. doi: 10.1007/s10815-011-9690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanfilippo S, Canis M, Ouchchane L, Botchorishvili R, Artonne C, Janny L, et al. Viability assessment of fresh and frozen/thawed isolated human follicles: reliability of two methods (Trypan blue and Calcein AM/ethidium homodimer-1) J Assist Reprod Genet. 2011;28(12):1151–1156. doi: 10.1007/s10815-011-9649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt KT, Larsen EC, Andersen CY, Andersen AN. Risk of ovarian failure and fertility preserving methods in girls and adolescents with a malignant disease. BJOG. 2010;117(2):163–174. doi: 10.1111/j.1471-0528.2009.02408.x. [DOI] [PubMed] [Google Scholar]
- 38.Schubert B, Canis M, Darcha C, Artonne C, Pouly JL, Dechelotte P, et al. Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod. 2005;20(7):1786–1792. doi: 10.1093/humrep/dei002. [DOI] [PubMed] [Google Scholar]
- 39.Schubert B, Canis M, Darcha C, Artonne C, Smitz J, Grizard G. Follicular growth and estradiol follow-up after subcutaneous xenografting of fresh and cryopreserved human ovarian tissue. Fertil Steril. 2008;89(6):1787–1794. doi: 10.1016/j.fertnstert.2007.03.101. [DOI] [PubMed] [Google Scholar]
- 40.Shiva Shankar Reddy N, Jagan Mohanarao G, Atreja SK. Effects of adding taurine and trehalose to a tris-based egg yolk extender on buffalo (Bubalus bubalis) sperm quality following cryopreservation. Anim Reprod Sci. 2010;119(3–4):183–190. doi: 10.1016/j.anireprosci.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18(2):59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 42.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16(4):395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sordet O, Khan QA, Pommier Y. Apoptotic topoisomerase I-DNA complexes induced by oxygen radicals and mitochondrial dysfunction. Cell Cycle. 2004;3(9):1095–1097. doi: 10.4161/cc.3.9.1086. [DOI] [PubMed] [Google Scholar]
- 44.Tjer GC, Chiu TT, Cheung LP, Lok IH, Haines CJ. Birth of a healthy baby after transfer of blastocysts derived from cryopreserved human oocytes fertilized with frozen spermatozoa. Fertil Steril. 2005;83(5):1547–1549. doi: 10.1016/j.fertnstert.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Touraine P, Beau I, Gougeon A, Meduri G, Desroches A, Pichard C, et al. New natural inactivating mutations of the follicle-stimulating hormone receptor: correlations between receptor function and phenotype. Mol Endocrinol. 1999;13(11):1844–1854. doi: 10.1210/me.13.11.1844. [DOI] [PubMed] [Google Scholar]
- 46.Weissman A, Gotlieb L, Colgan T, Jurisicova A, Greenblatt EM, Casper RF. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol Reprod. 1999;60(6):1462–1467. doi: 10.1095/biolreprod60.6.1462. [DOI] [PubMed] [Google Scholar]
- 47.Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;14(8 Suppl):6–10. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Z, Wang Y, Li L, Li SW. Cryopreservation of the human ovarian tissue induces the expression of Fas system in morphologically normal primordial follicles. Cryo-Letters. 2010;31(2):112–119. [PubMed] [Google Scholar]
- 49.Xiao Z, Wang Y, Li L, Luo S, Li SW. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil Steril. 2010;94(6):2323–2328. doi: 10.1016/j.fertnstert.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Xiong Y, Connolly T, Futcher B, Beach D. Human D-type cyclin. Cell. 1991;65(4):691–699. doi: 10.1016/0092-8674(91)90100-D. [DOI] [PubMed] [Google Scholar]
- 51.Zheng JH, Min ZL, Li YL, Zhu YH, Ye TJ, Li JQ, et al. A modified CZ-1 preserving solution for organ transplantation: comparative study with UW preserving solution. Chin Med J (Engl) 2008;121(10):904–909. [PubMed] [Google Scholar]