Abstract
Background
Polycystic ovary syndrome (PCOS) is an endocrine disorder exhibiting variable age at onset of clinical features allied with complex diseases in the later life. ACE is a pleiotropic molecule associated with various pathophysiological functions. The present study was aimed to establish the frequency of ACE I/D gene polymorphism in patients and controls and to assess the influence of this polymorphism on anthropometric and various clinical features of the condition.
Methods
ACE I/D genotyping was carried out in 259 PCOS patients and 315 healthy ultrasound scanned women of South Indian origin.
Results
The distribution of DD, ID and II genotypes in patients was 39, 37 and 24 %, whereas in the controls it was 31, 51 and 18 % respectively. Significant difference was observed in the genotypic frequency distributions between the patients and controls, however the allelic frequencies did not vary between the groups (p > 0.05). Quartile analysis revealed preponderance of DD genotype in the first two quartiles and a linear increase of II genotype from first to the last quartiles. Further, Multiple Logistic regression analysis revealed significant association of ACE I/D gene polymorphism with acanthosis and age at onset (AAO) of the syndrome (p < 0.05).
Conclusion
The present study is the first report to highlight the predisposing role of DD and protective role of ID genotype towards PCOS. Patients with single or double dose of D allele may develop PCOS symptoms at an early age and also significantly associated with acanthosis, a marker of insulin resistance.
Keywords: Angiotensin converting enzyme, Age at onset, Polycystic Ovary Syndrome, Polymorphism
Introduction
Polycystic Ovary Syndrome (PCOS) is a common endocrine disorder affecting 4–12 % of women of reproductive age worldwide and is a major cause of female infertility [1–3]. The syndrome is characterized by hyperandrogenism, polycystic ovaries and chronic anovulation along with insulin resistance, hyperinsulinemia, abdominal obesity and dyslipidemia as frequent metabolic traits. Inter-individual variation is commonly observed with respect to clinical features changing throughout the life span, starting from adolescence to postmenopausal age. Thus, predisposing the individual to serious long term consequences such as type 2 diabetes, endometrial hyperplasia, thyroid dysfunction and cardiovascular diseases [4–8]. Wild et al. (2000) reported that women with previous history of PCOS have a higher prevalence of hypertension and other cardiovascular diseases later in life than the controls [9]. Our epidemiological data suggested that PCOS women with familial background of complex diseases are associated a significant genetic background for the susceptibility to develop this multifactorial disorder [10]. Some of the PCOS complications are associated with high Angiotensin II and low bradykinin levels in rennin-angiotensin system (RAS) pathway [11]. Angiotensin converting enzyme (ACE), a key factor involved in the conversion of Angiotensin I to Angiotensin II, is expressed in multiple tissues including ovaries. Apart from regulating blood pressure and fluid balance, ACE and its products play an essential role in the regulation of ovarian function all the way through follicular development, oocyte maturation, ovulation and follicular atresia [12]. Yashimura in the year 1997 suggested the involvement of RAS in the development of PCOS [13].
Inter-individual variability of the plasma ACE concentration is associated with an insertion (I)/deletion (D) polymorphism involving a 287-bp DNA sequence situated in intron 16 of the ACE gene [14]. To date, most of the studies conducted on PCOS patients from various ethnic groups differ in their reports [15]. Controversial reports of this polymorphism with the clinical manifestations such as insulin resistance and testosterone levels among the PCOS patients were also reported [16–18]. There are no studies available from Indian population pertaining to ACE I/D gene polymorphism in relation to PCOS susceptibility and its clinical manifestations. Therefore, the present study was focused to investigate whether ACE I/D polymorphism is associated in the predisposition of PCOS in South Indian women.
Materials and methodology
Study population
The study was carried out in 571 women comprising of 256 patients and 315 normal Ultrasound Scanned Controls. Samples were obtained from Government Maternity Hospital, Petlaburz, Hyderabad, India. Patients were selected based on Rotterdam criteria proposed by ESHRE (2003). Informed consent was taken from subjects prior sample collection. Ethical clearance was obtained from local ethical committee (Osmania University, Hyderabad, India). Detailed information on clinical and anthropometric measures was collected through proforma. Obesity was measured by calculating body mass index (BMI); waist/hip ratio (W/R) was used as a marker for abdominal obesity and a value of ≥0.8 was considered as obese. The two markers acanthosis nigricans for insulin resistance; acne hirsutism, alopecia and premature pubarche for hyperandrogenism were considered as important clinical features of PCOS. Ferriman- Gallway (FG) score of ≥7 was used to determine hirsutism. The inclusion criteria for controls was healthy, ultrasound scanned normal fertile women with no signs of menstrual dysfunction or history of infertility.
Analysis of clinical features
We compared the distribution of the ACE I/D genotypes in relation to factors such as BMI, W/H ratio, age at onset (AAO), hirsutism, acne, alopecia, premature pubarche and acanthosis. The patients were invariably asked about the onset of menstrual dysfunction/development of clinical symptoms either at adolescence or at adulthood and the duration of the symptoms.
Molecular analysis
Five milliliters of blood sample was collected from all the participants using EDTA as anticoagulant. Genomic DNA was extracted by standard protocol routinely used in our laboratory [19]. For each subject, the ACE genotyping was performed by polymerase chain reaction using forward and reverse primers. PCR amplification was carried out in a total volume of 10 μl containing 50 ng template DNA, 0.15 μl of each primer (Bioserve, India) (Forward: 5′-CTG GAG ACC ACT CCC ATC CTT TCT-3′; Reverse: 5′-GAT GTG GCC ATC ACA TTC GTC AGA T-3′), 1.00 μl PCR buffer with Mgcl2 and 1U of Taq DNA polymerase [Labpro, India].
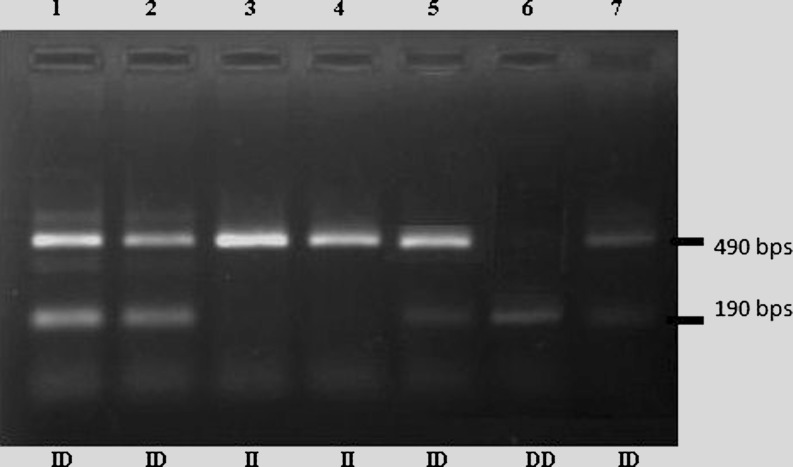
PCR was performed in a thermocycler (Eppendorf) with following conditions : an initial denaturation step for 5 min at 95 °C, then 30 cycles consisting of 30 s of denaturation at 94 °C, 45 s of annealing at 59 °C and a final extension for 5 min at 72 °C. The products were run on 2 % agarose gel and analyzed in gel documentation system (UV tech). A product of 490 bp indicates a genotype homozygous for insertion (II), 190 bp homozygous for DD and the presence of 490 and 190 bp products indicate heterozygous genotype (Fig. 1).
Fig. 1.
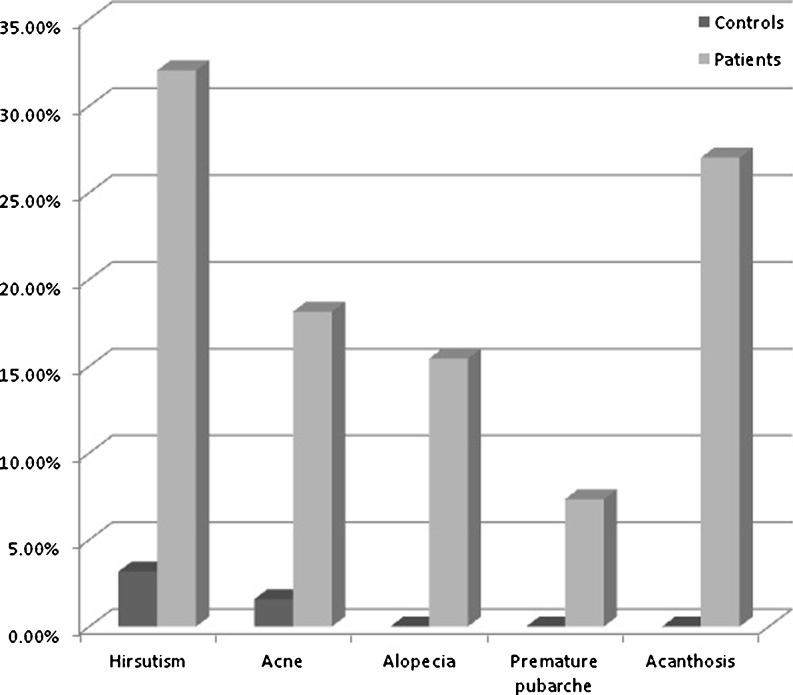
Clinical characteristics of PCOS probands and control group
Statistical analysis
Statistical analysis was done using SPSS, version 16. The clinical and anthropometric measures were compared between patients and controls by using Student’s t test. Genotypic, allelic frequencies and Hardy-Weinberg equilibrium were calculated using chi-square analysis. Multiple Logistic Regression (MLR) analysis was performed for all the anthropometric and clinical measures with respect to ACE I/D genotypes. The association between genotypes and PCOS risk was evaluated by calculating the odds ratios (OR) at 95 % confidence interval. A two-tailed value of p < 0.05 was regarded as statistically significant.
Results
Data analysis on a total cohort of 571 individuals revealed that the mean age of the patients and controls at the time of sample collection was 24.52 ± 4.47 years and 25.95 ± 5.37 years respectively. The mean age at onset (AAO) of the clinical symptoms in the patients was 16.30 ± 4.91 years. Anthropometric measurements and clinical features of patients and the controls were presented in Table 1 and Fig. 1 respectively. A significant difference between patients and controls with respect to BMI and W/H ratio was observed (p < 0.05).
Table 1.
Anthropometric measurements and clinical features of the study group
| Variables | Total controls | Total patients | p-value |
|---|---|---|---|
| N = 315 | N = 259 | ||
| BMI (kg/m2) | 22.05 ± 3.45 | 25.79 ± 5.02 | 0.0001* |
| W/H Ratio | 0.76 ± 0.04 | 0.79 ± 0.05 | 0.0001* |
| AAM | 12.37 ± 0.95 | 12.57 ± 1.81 | 0.09 |
| AAO | – | 16.30 ± 4.91 | – |
Quantitative data are presented X ± SD
*significant at 5% level of significance (p<0.05)
Table 2 represents the distribution of ACE I/D genotypes in patients and controls. The percentage distribution of DD, ID and II genotypes was 39, 37 and 24 in patients and 31, 51 and 18 in the controls correspondingly. The genotypic frequencies between the patients and controls differed significantly (χ2 = 11.31; df = 2; p = 0.003). Individuals with ID genotype predominated in the controls with an OR of 0.56 (CI 0.404–0.790, p = 0.0009). Frequencies of D and I alleles did not vary significantly between the groups (p > 0.05). The distribution of ACE genotypes was in agreement with Hardy Weinberg equilibrium in controls (χ2 = 0.675; p = 0.411) however, it deviated in patients (χ2 = 14.24; p = 0.0002).
Table 2.
Distribution of ACE I/D gene polymorphism in patients and controls
| Category | DD | ID | II | D | I | Comparison of groups | OR(95 % CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||||||
| Patients (259) | 100(39) | 97 (37) | 62 (24) | 0.57 | 0.43 | D vs I | 1 | 1 |
| Controls (315) | 97 (31) | 162(51) | 56 (18) | 0.57 | 0.43 | DD vs ID + II | 1.41 | 0.05 |
| ID vs DD + II | 0.56 | 0.0009* | ||||||
| II vs DD + ID | 0.89 | 0.57 |
X2 = 11.31; df = 2; p = 0.003
*significant at 5% level of significance (p<0.05)
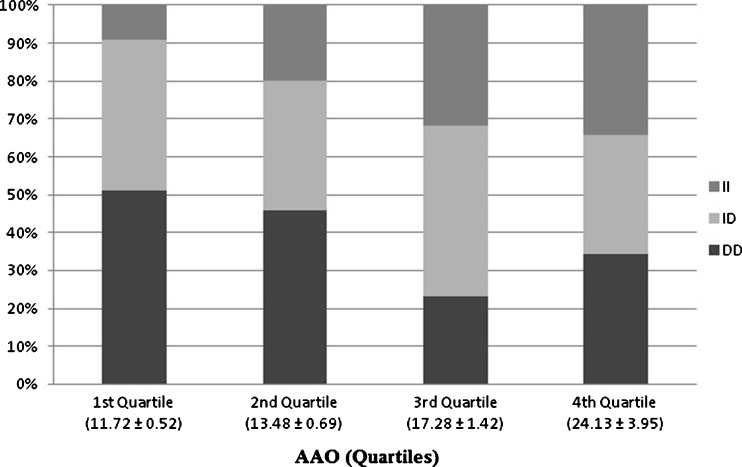
The whole patient data was categorized into quartiles to see whether the genotypes have a role to play in the age at onset of the disease (Fig. 2). Quartile data analysis showed preponderance of DD genotype in the first two quartiles and a linear increase of II genotype from first to the last quartiles. Multiple Logistic Regression analysis of I/D polymorphism with respect to risk factors such as BMI, W/H ratio, AAO and clinical features was carried out and we observed a considerable association with AAO and acanthosis (Table 3). Patients with D allele whether in single (ID vs. II) or double dose (DD vs. II), showed significant association with AAO and acanthosis (Table 3). The noticeable results with respect to AAO, a quantitative parameter in relation to genotypes, lead us to further pool the first two and the last two quartiles into two groups for the categorical analysis, and the cumulative mean AAO of the first two quartiles was 13.90 ± 2.34 years and the last two was 18.41 ± 6.07 years. Based on these observations, we defined the first two quartiles as ‘early age at onset group’ (early AAO) and the last two as ‘late age at onset group’ (late AAO). Interestingly, an elevated frequency of DD genotype was identified in the early AAO group (48 % vs. 27 %), and II genotype in the late AAO group (33 % vs. 15 %). However, the ID genotype did not vary between the groups (Table 4).
Fig. 2.
Distribution of ACE I/D polymorphism according to age at onset
Table 3.
Multiple logistic regression analysis (ACE I/D polymorphism vs risk factors)
| Variables | DD vs II OR (95 % CI) | p-value | ID vs IIOR (95 % CI) | p-value |
|---|---|---|---|---|
| BMI | 1.14 (0.47–2.77) | 0.78 | 1.08(0.58–2.00) | 0.81 |
| W/H ratio | 1.28 (0.98–1.69) | 0.07 | 0.73(0.47–1.14) | 0.16 |
| AAO | 3.34 (1.68–6.66) | 0.001* | 2.71(1.36–5.38) | 0.004* |
| Hirsutism | 1.26 (0.62–2.53) | 0.521 | 1.55(0.76–3.16) | 0.23 |
| Acne | 1.21 (0.50–2.90) | 0.66 | 0.87(0.37–2.04) | 0.87 |
| Alopecia | 0.62 (0.23–1.64) | 0.33 | 0.79(0.29–2.15) | 0.79 |
| Premature puberche | 0.77 (0.24–2.47) | 0.66 | 1.40(0.38–5.15) | 0.61 |
| Acanthosis | 2.40 (0.12–5.17) | 0.025* | 2.26(1.04–4.88) | 0.03* |
II genotype has been considered as referral group
*significant at 5% level of significance (p<0.05)
Table 4.
Distribution of ACE I/D gene polymorphism in early and late onset patients
| Category | DD | ID | II | D | I | Comparison of groups | OR(95 % CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||||||
| Early onset (130) | 63(48) | 48 (37) | 19 (15) | 0.65 | 0.35 | D vs I | 2.011 | 0.015* |
| Late onset (129) | 37 (27) | 49(38) | 43 (33) | 0.48 | 0.52 | DD vs ID + II | 1.81 | 0.022* |
| ID vs DD + II | 1.27 | 0.358 | ||||||
| II vs DD + ID | 0.336 | 0.0004* |
OR for genotype was calculated with DD vs ID, II and ID vs II. OR for allele was calculated with D vs I
*significant at 5% level of significance (p<0.05)
Discussion
Angiotensin-converting enzyme (ACE) plays an important role in rennin-angiotensin system (RAS) that regulates blood pressure, angiogenesis of ovarian endothelium, follicular growth, steroidogenesis and inflammation. The production angiotensin II of the RAS can make follicle atresia in every period of the follicle development and promote the formation of polycystic ovary and hyperandrogenism [20]. ACE is expressed in multiple tissues including ovaries and also implicated in various diseases [21–25]. To date more than 76 polymorphisms of ACE gene have been identified, the most common is the ACE insertion/deletion (I/D) polymorphism associated with plasma ACE concentration (Fig. 3). The existence of an association between ACE I/D polymorphism and PCOS is controversial. Several studies reported no contribution of this polymorphism in susceptibility to PCOS, however, few studies did find a relationship with insulin resistance, hyperandrogenism and aggravated clinical manifestations of PCOS [16–18]. A recent meta-analysis by Jia Hongxia et al. (2012) reported a significant relation between this polymorphism and PCOS risk in Caucasians but produced a null result for Asians [15]. Since no studies were reported from India, the present study was carried out in South Indian women in order to establish the role of ACE I/D polymorphism in susceptibility to PCOS. To the best of our knowledge, this is the first study to assess the possible role of ACE I/D polymorphisms in the aetiopathogenesis of PCOS.
Fig. 3.
Gel picture showing different genotypes of ACE gene
In our study, the presence of slight elevation of DD genotype in the patients and appreciably high frequency of ID in the controls suggests the protective role of ID towards PCOS. O. Celik et al. (2010) and Karabulet et al. (2010) proposed lack of association of DD genotype with PCOS but with insulin resistance. Contrary to these studies, Koika et al. from Greece reported increased frequency of ID genotype in PCOS women with hyperandrogenism, but not in non-hyperandrogenic PCOS women [16, 18, 26].
A wide variation has been reported in the distribution of D and I alleles in various ethnic groups. Previous studies within the Caucasians and Asians revealed the association of D allele with hyperandrogenism, insulin resistance, obesity and enhanced RAS activity [17, 18, 27, 28]. In our study the frequency of D allele did not differ between patients and controls.
When our results were evaluated from the aspect of clinical features and anthropometric measures between patients and controls; acanthosis, an insulin resistance feature was significantly associated with PCOS women. In addition, BMI and W/H ratio were also higher in the patient group than controls. Our study results correspond to the findings by Karabulut et al. and Bayram et al. [18, 20].
MLR analysis revealed an association of ACE I/D polymorphism with acanthosis and AAO of the syndrome (Table 3). To our surprise, although there was no significant variation in the distribution of D and I allele between the patients and controls, frequency of D allele and DD genotype were significantly higher in the early AAO group than the late AAO group. High frequency of DD genotype in the early AAO is suggestive of its predisposing role in the early onset of the disease symptoms. The linear increase in the frequency of II genotype starting from first quartile to the last quartile, indicating II genotype confers protection and influences physiologically delayed expression of the clinical symptoms of the disease. However, differential influence of ACE I/D genotype disappeared in the fourth quartile. These results support the presumption that D allele could have played a modifying role in the development of clinical symptoms of PCOS at an early age and I allele in delaying the onset of the condition.
Conclusion
The three main findings of our study are; first, ACE ID genotype with intermediate levels of angiotensin converting enzyme confers protection towards developing PCOS than the other two homozygotes in our population. Second, PCOS women with DD genotype might be the high risk individuals who may develop symptoms at an early age, than the II genotype, indicating the modifying effect of D/I alleles on AAO of PCOS. Finally, PCOS women with D allele either in single or double dose may develop diabetes in later life, as acanthosis, a marker for insulin resistance is significantly associated with D allele in our study. Additionally, the current study supports the hypothesis that DD genotype contribute to the onset of the clinical symptoms at an early age.
In summary, currently available data from genetic association studies do not provide positive evidence for a role of ACE I/D polymorphism in the causation of PCOS. Large studies in subjects from different ethnic background are required before considering it as a molecular marker for susceptibility to PCOS and onset of the clinical symptoms.
Acknowledgments
We thank all the subjects for their co-operation in giving consent for blood sample and the clinical information. And we would also like to thank all the doctors and nursing staff of various Endocrinological Clinics of Hyderabad, (India) for supporting the implementation of the study and assisting with data collection. We further thank Indian Council of Medical Research (ICMR), India for providing financial assistance to M.L.N Deepika.
Disclosure statement
The authors declare that no competing financial interests exist.
Author’s contribution
Deepika M.L.N performed the experiment and drafted the manuscript; K. Ranjith worked on initial analysis of data generated, Dr. Usha Rani helped in sampling, Dr. N Balakrishna carried out the statistical analysis, Dr. Parveen Jahan and K. Prasanna Latha contributed to the preparation of manuscript.
Abbreviations
- PCOS
Polycystic Ovary Syndrome
- ACE
Angiotensin converting enzyme
- AAO
Age at onset
- BMI
Body mass index
- W/R
Waist and Hip ratio
- OR
Odds ratio
- RAS
Renin Angiotensin System
- I/D
insertion/deletion
Footnotes
Capsule
The ACE ID genotype confer protection to PCOS in our population. The D allele either in single or double dose appears to influence the age at onset of the disease.
Contributor Information
M. L. N. Deepika, Phone: +91-988-5617014, Email: mlndeepika@gmail.com
K. Ranjith Reddy, Phone: +91-900-0120345, Email: kranjithreddy@yahoo.com.
V. Usha Rani, Phone: +91-984-8318181, Email: vitals@gmail.com.
N. Balakrishna, Phone: +91-949-0701485, Email: dr_nbk@yahoo.com
K. Prasanna Latha, Phone: +91-949-3389288, Email: p_komaravalli@yahoo.com.
Parveen Jahan, Phone: +91-40-27682335, FAX: +91-40-27095178, Email: dr.pjahan@gmail.com.
References
- 1.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of polycystic ovary syndrome in unselected black and white women of south eastern United States: a prospective study. J Clin Endcrinol Metab. 1998;83(9):3078–82. doi: 10.1210/jc.83.9.3078. [DOI] [PubMed] [Google Scholar]
- 2.Farah L, Lazenby AJ, Boots LR, Azziz R. Prevalence of polycystic ovary syndrome in women seeking treatment from community electrologists. Alabama Professional Electrology Association Study Group. J Reprod Med. 1999;44:870–4. [PubMed] [Google Scholar]
- 3.Han S, Tan S, Elsenbruch S, Quadbeck B, Herrmarnn BL, Mann K, et al. Clinical and biochemical characterization of women with polycystic ovary syndrome in North Rhine-Westphalia. Horm Metab Res. 2005;37:438–44. doi: 10.1055/s-2005-870236. [DOI] [PubMed] [Google Scholar]
- 4.Balen AH, Dunger D. Pubertal maturation of the internal genitalia. Ultrasound Obstet Gynecol. 1995;6:164–5. doi: 10.1046/j.1469-0705.1995.06030164.x. [DOI] [PubMed] [Google Scholar]
- 5.Elting MW, Korsen TJM, Rekers-Mombarg LTM, Schoemaker J. Women with polycystic ovary syndrome gain regular menstrual cycles when aging. Hum Reprod. 2000;15:24–8. doi: 10.1093/humrep/15.1.24. [DOI] [PubMed] [Google Scholar]
- 6.Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome and type 2 diabetes mellitus. Fertil Steril. 2002;77(6):1095–105. doi: 10.1016/S0015-0282(02)03111-4. [DOI] [PubMed] [Google Scholar]
- 7.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24(3):302–12. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 8.Hardiman P, Pillay OS, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361(9371):1810–2. doi: 10.1016/S0140-6736(03)13409-5. [DOI] [PubMed] [Google Scholar]
- 9.Wild S, Pierpont T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of 31 year follow-up study. Hum Fertil (Camb) 2000;3(2):101–5. doi: 10.1080/1464727002000198781. [DOI] [PubMed] [Google Scholar]
- 10.Deepika MLN, Ranjith K, Usha Rani V, Ishaq M, Jahan P. Familial background of complex diseases in PCOS probands of South Indian population. Asian J Epidemiol. 2012;5(2):50–5. doi: 10.3923/aje.2012.50.55. [DOI] [Google Scholar]
- 11.Giaccchetti G, Sechi LA, Rillis S. The rennin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab. 2005;16(3):120–6. doi: 10.1016/j.tem.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Schwentner L, Wöckel A, Herr D, Wulff C. Is there a role of the local tissue RAS in the regulation of physiologic and pathophysiologic conditions in the reproductive tract? J Renin-Angiotensin-Aldosterone Syst. 2011;12(4):385–93. doi: 10.1177/1470320311418140. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura Y. The ovarian renin-angiotensin system in reproductive physiology. Front Neuroendocrinol. 1997;18:247–91. doi: 10.1006/frne.1997.0152. [DOI] [PubMed] [Google Scholar]
- 14.Rigat B, Hubert C, Alence-Gelas F. An insetion/deletion polymorphism in the angiotensin I converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–6. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia H, Wang B, Yu L, Jiang Z. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with polycystic ovary syndrome: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2012;0(0):1–8. doi: 10.1177/1470320312452768. [DOI] [PubMed] [Google Scholar]
- 16.Celika O, Yesiladab E, Hascalika S, Celikc N, Sahind I, Keskind L, Ozerole E. Angiotensin-converting enzyme gene polymorphism and risk of insulin resistance in PCOS. Reprod Biomed Online. 2010;20(4):492–8. doi: 10.1016/j.rbmo.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Fan H, Che Y, Cao Y, Wu X, Sun H, Liang F, Yi L, Wang Y. Association between ACE gene I/D polymorphisms and hyperandrogenism in women with Polycystic Ovary Syndrome (PCOS) and controls. BMC Med Genet. 2009;10:64. doi: 10.1186/1471-2350-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karabulut A, Turgut S, Turgut G. Angiotensin converting enzyme gene insertion/deletion polymorphism in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2010;26(6):393–8. doi: 10.3109/09513591003632167. [DOI] [PubMed] [Google Scholar]
- 19.Tippisetty S, Ishaq M, Komaravalli PL, Jahan P. Angiotensin converting enzyme (ACE) gene polymorphism in vitiligo: protective and predisposing effects of genotypes in disease susceptibility and progression. Eur J Dermatol. 2011;21(2):173–7. doi: 10.1684/ejd.2011.1279. [DOI] [PubMed] [Google Scholar]
- 20.Bayram B, et al. Association of angiotensin converting enzyme (ACE) gene I/D polymorphism and polycystic ovary syndrome (PCOS) Gene. 2011;489(2):86–8. doi: 10.1016/j.gene.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Van Sande ME, Scharpe SL, Neels HM, Van Camp KO. Distribution of Angiotensin converting enzyme in human tissues. Clin Chim Acta. 1985;147(3):255–60. doi: 10.1016/0009-8981(85)90207-4. [DOI] [PubMed] [Google Scholar]
- 22.Plendl J, Neumiiller C, Vollmar A, Auerbach R, Sinowatz F. Isolation and characterization of endothelial cells from different organs of fetal pigs. Anat Embryol. 1996;194:445–56. doi: 10.1007/BF00185992. [DOI] [PubMed] [Google Scholar]
- 23.Acosta TJ, Berisha B, Ozawa T, Sato K, Schams D, Miyamoto A. Evidence for a local endothelin-angiotensin-atrial natriuretic peptide systemin bovine mature follicles in vitro: effects on steroid hormones and prostaglandin secretion. Biol Reprod. 1999;61(6):1419–25. doi: 10.1095/biolreprod61.6.1419. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen AH, Hagemann A, Svenstrup B, Nielsen J, Poulsen K. Angiotensin II receptor density in bovine ovarian follicles relates to tissue renin and follicular size. Clin Exp Pharmacol Physiol. 1994;21(6):463–9. doi: 10.1111/j.1440-1681.1994.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 25.Koh WP, Yuan JM, Sun CL, et al. Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer Res. 2003;63:573–8. [PubMed] [Google Scholar]
- 26.Koika V, Georgopoulos NA, Piouka A, Roupas ND, Karela A, Armeni AK, Katsantoni E, Panidis D. Increased frequency of the DI genotype of the angiotensin-I converting enzyme and association of the II genotype with insulin resistance in polycystic ovary syndrome. Eur J Endocrinol. 2012;166(4):695–702. doi: 10.1530/EJE-11-0894. [DOI] [PubMed] [Google Scholar]
- 27.Javaid A, Mansoor Q, Bilal N, Bilal A, Shaukat U, Ismail M. ACE gene DD genotype association with obesity in Pakistani population. Int J Bioautom. 2011;15(1):49–56. [Google Scholar]
- 28.Cao Y, Wang Y, Zhou P et al. Relationship between ACE gene polymorphism and the renin-angiotensin system in PCOS patients. Chinese Journal of Practical Gynecology and Obstetrics; 2002–11. doi:CNKI:SUN:ZGSF.0.2002-11-012.