Abstract
Purpose
To evaluate whether psychological stress, as well as changes in hypothalamus-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS) at different time points during a first in vitro fertilization (IVF) cycle, correlates with the reproductive outcome.
Methods
A prospective study was conducted in 264 women undergoing IVF or intracytoplasmic sperm injection (ICSI) treatment between January 2009 and March 2010. Standardized psychological questionnaires were used to assess anxiety and depression. Norepinephrine and cortisol in serum were measured with specific assays.
Results
The non-pregnant women reported higher anxiety and depression scores at the pregnancy detection day compared with the pregnant group. Lower levels of norepinephrine and cortisol at the time of oocyte retrieval and lower levels of cortisol at the time of pregnancy test were found in women with successful treatment. Significant increases in serum norepinephrine and cortisol values were observed during ovarian stimulation. State Anxiety scores were negatively correlated with live birth rate, and positively associated with serum norepinephrine and cortisol values.
Conclusions
State anxiety is associated with both pregnancy rate and live birth rate in IVF patients, an effect that is partly mediated by activities in the HPA and SNS.
Keywords: Cortisol, In vitro fertilization, Live birth rate, Norepinephrine, Pregnancy rate, Stress
Introduction
In vitro fertilization (IVF) is psychologically and emotionally stressful. Stress before, during and/or after the IVF treatment is multidimensional. There is the chronic source of stress caused by the threat of permanent infertility and loss of hope. Another source of stress is the threat of the treatment itself, such as daily injections, blood draws, ultrasound, oocyte retrieval, and the possibility of failure at any of the various phases. The third source of stress is the risk of spontaneous abortion [1]. Oocyte retrieval and pregnancy test proved to constitute the most stressful stages of the IVF cycle [2–5].
The perception that psychological stress may prevent a woman from attaining and maintaining a pregnancy has become widely accepted, although a few studies have been unable to confirm this link [6, 7]. The variability is largely determined by differences in the time points of the assessments, and methodological issues such as the use of different standardized psychometric self-report instruments and the use of different threshold scores [8]. Until now, there are few studies that measure both the physiological and psychological aspects of psychiatric disorders during IVF and relate it to pregnancy outcome.
Psychological stressors involve the reciprocal and differential reactions of the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic nervous system (SNS). Patients hospitalized for an acute major depressive episode often exhibit signs of HPA system dysregulation like hypercortisolemia [9]. There is also evidence of increased SNS outflow in these patients, e.g., tachycardia [10] or increased norepinephrine appearance in extravascular and vascular compartments [11]. So far, no clear picture emerges on the exact relationship between psychosocial stress and the release of adrenal hormones in relation to IVF/ICSI outcome. Smeenk et al. have shown that anxiety and especially depression before IVF/ICSI treatment were positively associated with urine adrenaline levels during treatment. They also observed the association of a lower adrenalin level at oocyte retrieval with an increased pregnancy chance [12]. Harlow et al. [13] showed that levels of state anxiety and concentrations of prolactin and cortisol rose during IVF treatment. However, there was no relationship between increased anxiety, hormone concentrations and pregnancy outcome. In an earlier study, we have reported that lower concentrations of norepinephrine and cortisol, both in serum and follicular fluid, were found in women with successful treatment. We propose that norepinephrine and cortisol might be an important factor in the complex relationship between psychosocial stress and outcome after IVF/ICSI [14].
Since the majority of studies reported clinical pregnancy as the endpoint, data relating to spontaneous abortion and premature delivery are scarce. Klonoff-Cohen et al. [5] reported that live birth rate was negatively influenced by baseline stress, but not by procedural stress during IVF or gamete intrafallopian transfer (GIFT). Epidemiological evidence provides complementary findings of positive correlations between various pregnancy failure outcomes with pre-conception negative life events and daily urinary cortisol [15].
For future prospective studies we recommend more exploration of the use of biomarkers to measure stress exposure in addition to perceived stress assessments. The purpose of the current study is to assess both psychological and physiological markers of stress at different time points during IVF and determine if they are related to pregnancy outcome.
Materials and methods
Patients
The data used were from women who came for the first cycle of IVF or ICSI treatment at the Centre of Reproduction, the First Affiliated Hospital of Harbin Medical University in China between January 2009 and March 2010. Only women with regular menstrual cycles and using no hormonal contraceptives could participate. Smokers, subjects with acute or chronic hormonal dysregulations, ovarian endometriosis, psychosomatic, and psychiatric diseases were excluded. All subjects should have sufficient knowledge of the Chinese language to fill out the questionnaires. Before entering the study all participants received an information sheet, provided written consent, and underwent a comprehensive medical examination. The study was approved by the clinical ethics committee of Harbin Medical University.
Psychological assessment
Women, who agreed to participate, were asked to complete questionnaires on four time points as follows: before the start of treatment (T1), the day of oocyte retrieval (T2), the day of pregnancy detection (about two weeks following embryo transfer) (T3) and, for the patients who became pregnant, 5–8 weeks of gestation(T4). Two psychological dimensions of stress were assessed in this study: anxiety and depression. State anxiety was measured by means of the Chinese version of the State and Trait Anxiety Inventory (STAI) [16]. Depression was measured using the Chinese version of the Beck Depression Index (C-BDI-II) [17]. Both questionnaires have shown satisfactory reliability and validity.
Measurement of hormones
Blood samples were collected (between 8 and 9 a.m.) at four timepoints during their treatment cycle. Five ml was drawn each time into test tubes and the serum was separated by centrifugation and stored at −20°C for the assessment of hormones.
Levels of norepinephrine (3H-NE, Tiangen Corp., Beijing, China) were determined by fluorometric determination after high performance liquid chromatography (intra- and interassay coefficient of variation <6 %) [18]. Cortisol was measured by radioimmunoassay (intra- and interassay coefficient of variation <10 %) after breaking protein binding with absolute ethanol using the method of Vecsei et al. [19].
Multiple variables can impact serum NE and cortisol levels (e.g., fasting state, medications, E2 levels, etc.). To reduce these variables, the following steps were taken: all patients had serum levels checked before 8 A.M. as is the standard in our clinic and were fasting; all patients received gonadotropins and GnRH agonist in the standard IVF protocol as stated [14]. Data were collected, stored, and then analyzed after all patients’ birth outcomes were recorded, approximately 12 months from start of study.
Statistical analysis
Continuous variables were compared by the use of independent t-tests and are presented as mean ± SD. Frequencies were compared between groups using the chi-square test. Multivariate logistic regression analyses were used to estimate the predictive effect of psychological scores and steroid measurements on the live birth rate. The Spearman correlation coefficient was used to express the association between psychological and hormonal measurements (two-tailed). All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 14.0). A p-value of <0.05 was considered statistically significant.
Results
A total of 353 out of 412 participants (86 %) returned the questionnaires and were willing to collect blood specimens. For 39 women, the treatment was cancelled before oocyte retrieval. Fifty women failed to fill out one or more psychological questionnaires and were therefore excluded from analysis. Stress hormones were measured in the remaining 264 patients at T1, T2, T3 and, for the 92 women(36 %) who became pregnant, at T4.
Table 1 presents characteristics on demographic and psychological variables of the participants. Pregnant and non-pregnant women demographics were consistent, which are in keeping with our previously presented studies [14]. At the pregnancy detection day (T3), the non-pregnant women reported higher anxiety and depression scores compared with the pregnant group. There was an increase in anxiety and depression scores at T2, then a slight decrease at T3 and an increase at T4, especially in pregnant group, but the level did not reach significance.
Table 1.
Demographic and psychological characteristics of pregnant and non-pregnant women
| Pregnant (n = 92) | Non-pregnant (n = 172) | p-value | |
|---|---|---|---|
| Age (years) | 33.1 (4.1) | 33.4 (3.9) | 0.11 |
| Duration of infertility (years) | 6.8 (3.3) | 7.0 (3.5) | 0.67 |
| Anxiety T1 | 36.1 (8.8) | 37.6 (10.0) | 0.21 |
| Anxiety T2 | 38.7 (6.7) | 39.7 (7.4) | 0.31 |
| Anxiety T3 | 36.7 (10.1) | 39.6 (7.6) | 0.03 |
| Anxiety T4 | 37.5 (9.1) | ||
| Depression T1 | 1.5 (1.3) | 1.6 (1.5) | 0.72 |
| Depression T2 | 1.6 (1.6) | 1.9 (1.8) | 0.33 |
| Depression T3 | 1.4 (1.4) | 1.9 (1.5) | 0.04 |
| Depression T4 | 1.5 (1.4) |
Data are presented as Mean (SD), SD standard deviation, T1: before the start of treatment, T2: day of oocyte retrieval, T3: day of pregnancy detection
Next, hormonal levels between successfully and unsuccessfully treated women were compared (Table 2). NE and cortisol levels at T2 were significantly lower in pregnant group (p < 0.001). While the cortisol levels at T3 were higher in the non-pregnant group (p = 0.04), but the significance was weaker.
Table 2.
Concentrations of norepinephrine (NE) and cortisol in serum
| Pregnant, Mean (SD) (n = 92) | Non-pregnant, Mean (SD) (n = 172) | p-value | |
|---|---|---|---|
| NE T1 (ng/l) | 167.8 (45.0) | 178.0 (44.5) | 0.08 |
| NE T2 (ng/l) | 214.3 (46.4) | 238.3 (51.5) | <0.001 |
| NE T3 (ng/l) | 180.6 (55.3) | 189.5 (53.4) | 0.34 |
| Cortisol T1 (nmol/l) | 251.4 (87.3) | 238.7 (78.2) | 0.23 |
| Cortisol T2 (nmol/l) | 325.2 (84.1) | 369.5 (63.2) | <0.001 |
| Cortisol T3 (nmol/l) | 345.5 (91.0) | 378.4 (77.8) | 0.04 |
Data are presented as Mean (SD), SD standard deviation, T1: before the start of treatment, T2: day of oocyte retrieval, T3: day of pregnancy detection
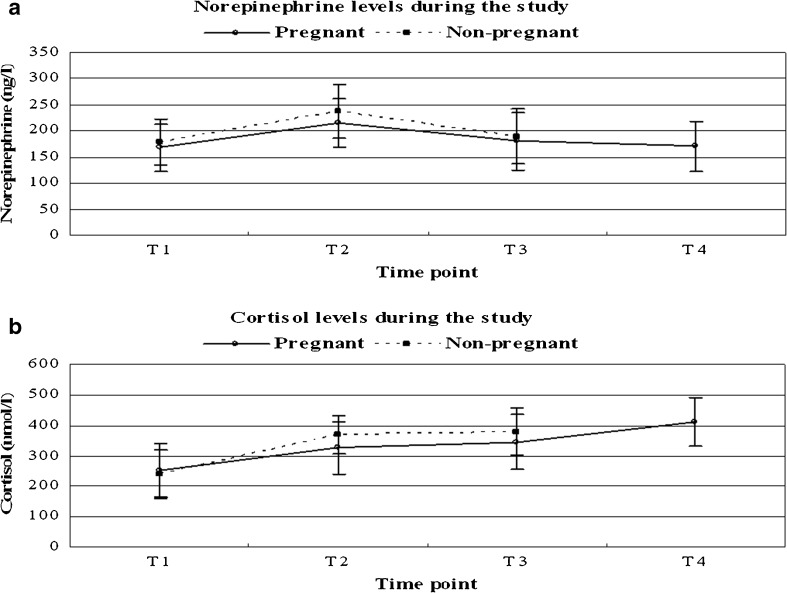
When serum data were analyzed during the IVF cycles, significantly increase in serum norepinephrine (p = 0.03) and cortisol (p = 0.02) values were observed during ovarian stimulation. Maximum values were recorded on the day of oocyte retrieval, and then showed a downward trend in pregnant women, as well as in non-pregnant women, but the difference was not statistically significant (Fig. 1). In comparing the different time points of the pregnant women, significantly higher levels of cortisol were found at T4 in comparison with T2 (t = 4.8; P < 0.01) and T3 (t = 2.6; P = 0.01). Even though the norepinephrine value decreased at T4, it remained higher than baseline, but the difference was not statistically significant.
Fig. 1.
The variation of serum norepinephrine and cortisol during the study. T1: before the start of treatment, T2: day of oocyte retrieval, T3: day of pregnancy detection, T4: 5–8 weeks of gestation for 92 pregnant women
We constructed a multivariable logistic regression model for the prediction of live birth rate with the measures at T4 (Table 3). In all models we adjusted for potential biological confounders: female age, BMI, previous miscarriage, endometrial thickness and number of transferred embryos. State anxiety was significantly associated with live birth rate, even when controlling for other medical factors related to the patient or the IVF treatment (OR: 0.855, p = 0.03). There was no influence of depression, as well as serum norepinephrine and cortisol values, on the live birth rate. BMI, previous miscarriage, endometrial thickness and number of transferred embryos also had no influence on the live birth rate. With higher female age, there was a trend towards a decreased chance of live birth (OR: 0.955, p = 0.07).
Table 3.
Multivariate analyses of the effects of stress measures at T4 on live birth rate among women in the pregnant group. (n = 92)
| Measures | Coefficient (B) | Wald | Odds Ratio | 95%CI | P |
|---|---|---|---|---|---|
| Age | −0.032 | 3.270 | 0.955 | 0.903–1.002 | 0.07 |
| BMI | 0.004 | 0.934 | 1.002 | 0.996–1.012 | 0.32 |
| Previous miscarriage | −0.157 | 0.454 | 0.823 | 0.483–1.407 | 0.50 |
| Endometrial thickness | 0.289 | 1.576 | 0.754 | 0.499–1.158 | 0.21 |
| Number of transferred embryos | 0.005 | 2.081 | 1.023 | 0.991–1.067 | 0.14 |
| State anxiety* | −0.181 | 4.285 | 0.855 | 0.810–1.006 | 0.03 |
| Depression* | 0.009 | 0.898 | 1.166 | 0.832–1.630 | 0.38 |
| Norepinephrine* | 0.020 | 0.688 | 1.104 | 0.748–1.651 | 0.63 |
| Cortisol* | −0.133 | 1.307 | 0.960 | 0.894–1.043 | 0.30 |
BMI body mass index, NE norepinephrine
*The odds ratio for the psychological variables corresponds to the relative change in odds on outcome when the variable is increased by 1 SD
Table 4 shows the correlation between the State Anxiety Inventory and the Beck Depression Inventory scores and endocrine variables at different time points. State Anxiety scores were positively associated with serum norepinephrine and cortisol values. In particular, a significant positive association was found at T1 between the State Anxiety scores and norepinephrine (r = 0.37, p = 0.01) or cortisol (r = 0.20, p = 0.001) levels in serum. Norepinephrine levels at the day of pregnancy detection (r = 0.22, p = 0.02) and cortisol concentrations at the early pregnancy (r = 0.19, p = 0.04) had significant correlation with State Anxiety scores. Interestingly, there were no correlations between BDI scores and these two stress hormones.
Table 4.
Spearman correlations between questionnaire findings and steroid measurements
| STAI score | BDI score | |||
|---|---|---|---|---|
| r | P value | r | P value | |
| NE T1 (ng/l) | 0.37 | 0.01 | 0.01 | 0.89 |
| NE T2 (ng/l) | 0.10 | 0.12 | 0.08 | 0.18 |
| NE T3 (ng/l) | 0.22 | 0.02 | 0.14 | 0.24 |
| NE T4 (ng/l) | 0.01 | 0.56 | −0.06 | 0.50 |
| Cortisol T1 (nmol/l) | 0.20 | 0.001 | 0.05 | 0.46 |
| Cortisol T2 (nmol/l) | 0.09 | 0.12 | 0.07 | 0.27 |
| Cortisol T3 (nmol/l) | 0.11 | 0.65 | −0.09 | 0.34 |
| Cortisol T4 (nmol/l) | 0.19 | 0.04 | 0.10 | 0.12 |
BDI Beck Depression Index, NE norepinephrine, STAI State and Trait Anxiety Inventory, T1: before the start of treatment, T2: day of oocyte retrieval, T3: day of pregnancy detection, T4: 5–8 weeks of gestation for 92 pregnant women
Discussion
The present study demonstrates psychological and endocrinological differences before, during and/or several weeks after the IVF treatment. Additionally, the psychological findings and hormonal levels at various stages of treatment were compared between successfully and unsuccessfully treated women.
In this study, we did not find an impact of baseline psychological factors or procedural anxiety on the pregnancy rate of IVF, although stress and distress have been found to be associated with a poorer ovarian functional response to IVF-treatment, and reduced fertilization, implantation and live birth rates [9]. One possible reason for this could be a lack of sensitivity of the instruments used. Another explanation could be that patients’ answers were more positive than what they actually experienced. It has been reported that infertility patients suppress their feelings of stress because they want to show the clinic that they are functioning well both socially and psychologically [20].
Several studies have assessed women’s anxiety and general distress during the course of one treatment cycle. In one study, distress levels did not show remarkable changes in the first segment of the treatment cycle, then increased significantly at the end of the cycle, just before the pregnancy test [3]. Overall, oocyte retrieval and pregnancy test proved to constitute the most stressful stages of the IVF cycle [2, 4, 5]. In the current study, anxiety and depression levels did not show remarkable changes during the treatment cycle. Somewhat surprisingly, a slight decrease of distress was found on the pregnancy test day. This indicates a positive effect of pregnancy on emotional status for women undergoing IVF treatment. The initial serum HCG measurement was made two weeks following embryo transfer to confirm pregnancy, but before that, most patients might have known the result by a urine pregnancy test at home. When IVF results in a pregnancy, negative emotions tend to disappear immediately, indicating that the stress of the treatment is predominantly determined by the threat of failure.
Pathophysiological changes in response to stress are extremely complex and current research endeavours focus on identifying the impact and hierarchy of individual markers involved. Previously, we have reported a relationship between low levels of norepinephrine and cortisol, both in serum and follicular fluid, and the establishment of clinical pregnancies in women undergoing IVF/ICSI treatment [14]. In the current study, We found that successful treatment was associated with significantly lower levels of norepinephrine and cortisol at the time of oocyte retrieval and lower levels of cortisol at the time of pregnancy test. So far, studies encompassing both norepinephrine and cortisol in this particular field are extremely rare. Cortisol was studied far more often over the years, thus resulting in more hypotheses. Stress-like concentrations of cortisol may block or delay follicular development and embryo implantation [21]. Furthermore, a direct effect on granulosa cells affecting steroidogenesis and an influence on oocyte quality was proposed by Michael and Cooke [22]. Demyttenaere et al. [20] indicated that women with high anticipatory state anxiety levels and high cortisol concentrations have lower pregnancy rates in IVF. This is in line with our finding. However, in most studies merely an association is presented since optimal research is hardly possible under ‘in vivo’ circumstances in this field.
Norepinephrine is a well-known neurotransmitter and hormone, highly elevated during stress, and plays an essential role in basic developmental processes such as embryogenesis and morphogenesis, regulating cell proliferation, differentiation, and migration [23]. Moreover, changes in the sympathetic outflow of the ovary could participate in the control of follicular development, and, thus, changes in the sympathetic input (by stress or steroidal hormones) could modify ovary function [24]. Recently, Li et al. have shown that norepinephrine levels in follicular fluid were negatively associated with the percentage of good quality embryos [25]. It was partially supported by our previous data, which indicate that norepinephrine accumulates in follicular fluid, supporting the physiological significance of norepinephrine in the local regulation of human ovarian functions.
In addition, the present work was to study the variation of serum concentrations of norepinephrine and cortisol during IVF treatment as well as explore how a possible norepinephrine or cortisol variation returned to the pre-stimulation values. As our study demonstrates, both serum norepinephrine and cortisol values increased during ovarian stimulation, while maximum values were recorded on the day of oocyte retrieval. A slightly lower, not significant, reduction in norepinephrine was found on the pregnancy test day both in the conception and non-conception group. This finding is in contrast with Smeenk et al. who didn’t find any significant changes for the urinary concentrations of noradrenaline or cortisol during treatment [26]. Some of the differences could be explained by the methodology. Assays measuring cortisol in blood and urine are not necessarily measuring the same thing. Furthermore, serum cortisol values increased significantly, while norepinephrine values decreased slightly, but didn’t return to baseline levels, during early pregnancy. This indicates a longterm psychological adjustment after successful IVF treatment. However, pregnancy could have introduced a bias, as it is known that serum concentrations of cortisol are increased during gestation [27].
Successful IVF was also found to be associated with increased levels of anxiety in comparison with women who had conceived naturally [28]. High stress perception is a risk factor for pregnancy outcomes include failed implantation, placentation, spontaneous abortion and preterm delivery [29]. New multi-disciplinary research on brain–body interactions triggered by stress in early pregnancy has shown that maternal biological responses, including localized inflammation in uterine tissue and sustained depression of progesterone production, challenge the endocrine–immune steady state during pregnancy, leading to serious consequences for the fetal environment [30]. Animal experiments have convincingly demonstrated that prenatal maternal stress can significantly affect the pregnancy outcome [31]. Some results suggest that stress-triggered norepinephrine could directly affect embryo development in the oviduct via adrenergic receptors and support the opinion that maternal stress can influence the embryo even in very early pregnancy [32]. In humans, it has been demonstrated that pregnancies characterized by increased maternal cortisol (commonly used stress marker) during the first three weeks after conception are more likely to result in spontaneous abortion [33].
A statistically significant association between state anxiety and live birth rate in IVF-assisted pregnancies was found in our multivariate analysis, when adjusted for potential biological confounders: female age, BMI, previous miscarriage, endometrial thickness and number of transferred embryos. Previous studies also have identified a relationship between perceived infertility-related stress and IVF outcomes [34]. Milad et al. have shown that women with a positive serum β-HCG concentration following IVF often report very high levels of anxiety and stress. However, it does not appear that high levels of anxiety and stress result in an adverse pregnancy outcome [35].
In our study, State Anxiety scores were positively associated with serum norepinephrine and cortisol values. In particular, a positive association was found between the State Anxiety scores and norepinephrine or cortisol levels in serum before IVF treatment. A similar association was found between State Anxiety scores and norepinephrine levels at the day of pregnancy detection, as well as cortisol concentrations at the early pregnancy. Interestingly, there were no significant correlations between BDI score and these two stress hormones. Present evidence indicates that stress may result in psychological indicators that could correlate with biological changes but not necessarily do so. Sanders and Bruce [36] established a relationship between psychosocial stress and fertility in women, independent of stress hormone levels. This complication asks for generally agreed upon parameters on how and when psychological factors should be measured and at what levels they should be considered relevant for IVF outcome.
In conclusion, State anxiety is associated with both pregnancy rate and live birth rate in IVF patients, an effect that is partly mediated by activities in the HPA and SNS. Oocyte retrieval proved to constitute the most stressful stage of the IVF cycle. We propose that the norepinephrine and cortisol concentration may negatively influence the clinical pregnancy rate of IVF treatment. Therefore, interventions aimed at reducing the influence of stress on reproductive outcome, should be part of treatment protocol.
Acknowledgments
The authors would like to thank all of the women who participated in the study. This study was supported by the “Doctor fund” of the First Affiliated Hospital of Harbin Medical University(grant no. 2011BS019), as well as the scientific project of Health Bureau in Heilongjiang Province(grant no. 2012–538).
Footnotes
Capsule
State anxiety might influence both pregnancy rate and live birth rate in IVF patients, an effect that is partly mediated by activities in the HPA axis and SNS.
References
- 1.Verhaak CM, Smeenk JM, van Minnen A, Kremer JA, Kraaimaat FW. A longitudinal, prospective study on emotional adjustment before, during and after consecutive fertility treatment cycles. Hum Reprod. 2005;20:2253–60. doi: 10.1093/humrep/dei015. [DOI] [PubMed] [Google Scholar]
- 2.Ardenti R, Campari C, Agazzi L, La-Sala GB. Anxiety and perceptive functioning of infertile women during in-vitro fertilization: Exploratory survey of an Italian sample. Hum Reprod. 1999;14:3126–32. doi: 10.1093/humrep/14.12.3126. [DOI] [PubMed] [Google Scholar]
- 3.Boivin J, Takefman JE, Tulandi T, Brender W. Reactions to infertility based on extent of treatment failure. Fertil Steril. 1995;63:801–7. [PubMed] [Google Scholar]
- 4.Yong P, Martin C, Thong J. A comparison of psychological functioning in women at different stages of in vitro fertilization treatment using the mean affect adjective checklist. J Assist Reprod Genet. 2000;17:553–6. doi: 10.1023/A:1026429712794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolonoff-Cohen H, Chu E, Natarajan L, Sieber W. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian transfer. Fertil Steril. 2001;76:675–87. doi: 10.1016/S0015-0282(01)02008-8. [DOI] [PubMed] [Google Scholar]
- 6.Klonoff-Cohen H. Female and male lifestyle habits and IVF: What is known and unknown. Hum Reprod Update. 2005;11:179–203. doi: 10.1093/humupd/dmh059. [DOI] [PubMed] [Google Scholar]
- 7.de Klerk C, Hunfeld JAM, Heijnen EMEW, Eijkemans MJC, Fauser BCJM, Passchier J, Macklon NS. Low negative affect prior to treatment is associated with a decreased chance of live birth from a first IVF cycle. Hum Reprod. 2008;23:112–6. doi: 10.1093/humrep/dem357. [DOI] [PubMed] [Google Scholar]
- 8.Williams KE, Marsh WK, Rasgon NL. Mood disorders and fertility in women: A critical review of the literature and implications for future research. Hum Reprod Update. 2007;13:607–16. doi: 10.1093/humupd/dmm019. [DOI] [PubMed] [Google Scholar]
- 9.Klonoff-Cohen H, Natarajan L. The concerns during assisted reproductive technologies (CART) scale and pregnancy outcomes. Fertil Steril. 2004;81:982–8. doi: 10.1016/j.fertnstert.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 10.Lintsen AM, Verhaak CM, Eijkemans MJ, Smeenk JM, Braat DD. Anxiety and depression have no influence on the cancellation and pregnancy rates of a first IVF or ICSI treatment. Hum Reprod. 2009;24:1092–8. doi: 10.1093/humrep/den491. [DOI] [PubMed] [Google Scholar]
- 11.Deuschle M, Schweiger U, Weber B, Gotthardt U, Körner A, Schmider J, Standhardt H, Lammers CH, Heuser I. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82:234–8. doi: 10.1210/jc.82.1.234. [DOI] [PubMed] [Google Scholar]
- 12.Lake CR, Pickar D, Ziegler MG, Lipper S, Slater S, Murphy DL. High plasma norepinephrine levels in patients with major affective disorder. Am J Psychiatry. 1982;139:1315–8. doi: 10.1176/ajp.139.10.1315. [DOI] [PubMed] [Google Scholar]
- 13.Harlow CR, Fahy UM, Talbot WM, Wardle PG, Hull MG. Stress and stress-related hormones during in-vitro fertilization treatment. Hum Reprod. 1996;11:274–9. doi: 10.1093/HUMREP/11.2.274. [DOI] [PubMed] [Google Scholar]
- 14.An Y, Wang Z, Ji H, Zhang Y, Wu K. Pituitary-adrenal and sympathetic nervous system responses to psychiatric disorders in women undergoing in vitro fertilization treatment. Fertil Steril. 2011;96:404–8. doi: 10.1016/j.fertnstert.2011.05.092. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Sheps S, Arck PC. Stress and reproductive failure: Past notions, present insights and future directions. J Assist Reprod Genet. 2008;25(2–3):47–62. doi: 10.1007/s10815-008-9206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spielberger CD. Manual for the State-trait anxiety scale. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 17.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the Beck Depression Inventory for primary care. Behav Res Ther. 1997;35:785–91. doi: 10.1016/S0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 18.Willemsen JJ, Ross HA, Jacobs MC, Lenders JW, Thien T, Swinkels LM, Benraad TJ. Highly sensitive and specific HPLC with fluorometric detection for determination of plasma adrenaline and noradrenaline applied to kinetic studies in humans. Clin Chem. 1995;41:1455–60. [PubMed] [Google Scholar]
- 19.Vecsei P, Penke B, Katzy R, Baek L. Radioimmunological determination of plasma cortisol. Experientia. 1972;28:1104–5. doi: 10.1007/BF01918701. [DOI] [PubMed] [Google Scholar]
- 20.Demyttenaere K, Bonte L, Gheldof M, Vervaeke M, Meuleman C, Vanderschuerem D, D’Hooghe T. Coping style and depression level influence outcome in in vitro fertilization. Fertil Steril. 1998;69:1026–33. doi: 10.1016/S0015-0282(98)00089-2. [DOI] [PubMed] [Google Scholar]
- 21.Michael AE. Life after liquorice: The link between cortisol and conception. Reprod Biomed Online. 2003;7:683–90. doi: 10.1016/S1472-6483(10)62091-3. [DOI] [PubMed] [Google Scholar]
- 22.Michael AE, Cooke BA. A working hypothesis for the regulation of steroidogenesis and germ cell development in the gonads by glucocorticoids and 11 beta-hydroxysteroid dehydrogenase (11 beta HSD) Mol Cell Endocrinol. 1994;100:55–63. doi: 10.1016/0303-7207(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 23.Buznikov GA, Shmukler YB, Lauder JM. From oocyte to neuron: Do neurotransmitters function in the same way throughout development? Cell Mol Neurobiol. 1996;16:537–59. doi: 10.1007/BF02152056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barria A, Leyton V, Ojeda SR, Lara HE. Ovarian steroidal response to gonadotropins and beta-adrenergic stimulation is enhanced in polycystic ovary syndrome: Role of sympathetic innervation. Endocrinology. 1993;133:2696–703. doi: 10.1210/en.133.6.2696. [DOI] [PubMed] [Google Scholar]
- 25.Li XH, Ma YG, Geng LH, Qin L, Hu H, Li SW. Baseline psychological stress and ovarian norepinephrine levels negatively affect the outcome of in vitro fertilisation. Gynecol Endocrinol. 2011;27:139–43. doi: 10.3109/09513590.2010.501871. [DOI] [PubMed] [Google Scholar]
- 26.Smeenk JM, Verhaak CM, Vingerhoets AJ, Sweep CG, Merkus JM, Willemsen SJ, van Minnen A, Straatman H, Braat DD. Stress and outcome success in IVF: The role of self-reports and endocrine variables. Hum Reprod. 2005;20:991–6. doi: 10.1093/humrep/deh739. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay JR, Nieman LK. The hypothalamic-pituitary-adrenal axis in pregnancy: Challenges in disease detection and treatment. Endocr Rev. 2005;26:775–99. doi: 10.1210/er.2004-0025. [DOI] [PubMed] [Google Scholar]
- 28.Hjelmstedt A, Widstrom AM, Wramsby H, Matthiesen AS, Collins A. Personality factors and emotional responses to pregnancy among IVF couples in early pregnancy: A comparative study. Acta Obstet Gynecol Scand. 2003;82:152–61. doi: 10.1034/j.1600-0412.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 29.Orr ST, James SA, Prince CB. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore. Maryland Am J Epidemiol. 2002;156:797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 30.Arck P, Hansen PJ, Jericevic BM, Piccinni MP, Szekeres-Bartho J. Progesterone during pregnancy: Endocrine-immune cross talk in mammalian species and the role of stress. Am J Reprod Immunol. 2007;58:268–79. doi: 10.1111/j.1600-0897.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 31.Mulder EJ. Robles de Medina PG, Huizink AC, Van den Bergh BR, Buitelaar JK, Visser GH. Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Hum Dev. 2002;70:3–14. doi: 10.1016/S0378-3782(02)00075-0. [DOI] [PubMed] [Google Scholar]
- 32.Cikos S, Rehák P, Czikková S, Veselá J, Koppel J. Expression of adrenergic receptors in mouse preimplantation embryos and ovulated oocytes. Reproduction. 2007;133:1139–47. doi: 10.1530/REP-07-0006. [DOI] [PubMed] [Google Scholar]
- 33.Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassman BI, England BG. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci USA. 2006;103:3938–42. doi: 10.1073/pnas.0511183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper BC, Gerber JR, McGettrick AL, Johnson JV. Perceived infertility-related stress correlates with in vitro fertilization outcome. Fertil Steril. 2007;88:714–7. doi: 10.1016/j.fertnstert.2006.11.158. [DOI] [PubMed] [Google Scholar]
- 35.Milad MP, Klock SC, Moses S, Chatterton R. Stress and anxiety do not result in pregnancy wastage. Hum Reprod. 1998;13:2296–300. doi: 10.1093/humrep/13.8.2296. [DOI] [PubMed] [Google Scholar]
- 36.Sanders KA, Bruce NW. Psychosocial stress and treatment outcome following assisted reproductive technology. Hum Reprod. 1999;14:1656–62. doi: 10.1093/humrep/14.6.1656. [DOI] [PubMed] [Google Scholar]