Abstract
The plant vascular system, composed of xylem and phloem, evolved to connect plant organs and transport various molecules between them. During the post-embryonic growth, these conductive tissues constitutively form from cells that are derived from a lateral meristem, commonly called procambium and cambium. Procambium/cambium contains pluripotent stem cells and provides a microenvironment that maintains the stem cell population. Because vascular plants continue to form new tissues and organs throughout their life cycle, the formation and maintenance of stem cells are crucial for plant growth and development. In this decade, there has been considerable progress in understanding the molecular control of the organization and maintenance of stem cells in vascular plants. Noticeable advance has been made in elucidating the role of transcription factors and major plant hormones in stem cell maintenance and vascular tissue differentiation. These studies suggest the shared regulatory mechanisms among various types of plant stem cell pools. In this review, we focus on two aspects of stem cell function in the vascular cambium, cell proliferation and cell differentiation.
Keywords: cambium, secondary development, stem cell, vascular tissue
Introduction
A stem cell is a self-renewing cell whose progeny has a competence to differentiate into diverse ranges of specialized cell types. By classical definition, a stem cell is characterized by asymmetric cell division in which one daughter cell retains the characteristics of an undifferentiated mother cell and the other acquires a specific cell fate, which is triggered by intrinsic and extrinsic signals. Stem cells maintain their pluripotent status in special microenvironments, called ‘stem cell niche’, together with niche cells (Spradling et al, 2001). Niche cells send out short-range signals that are required for stem cell maintenance. The concept of stem cell niche was first proposed in mammalian haematopoietic tissue and has been extended to various types of stem cells (Fuchs et al, 2004; Li and Li, 2006).
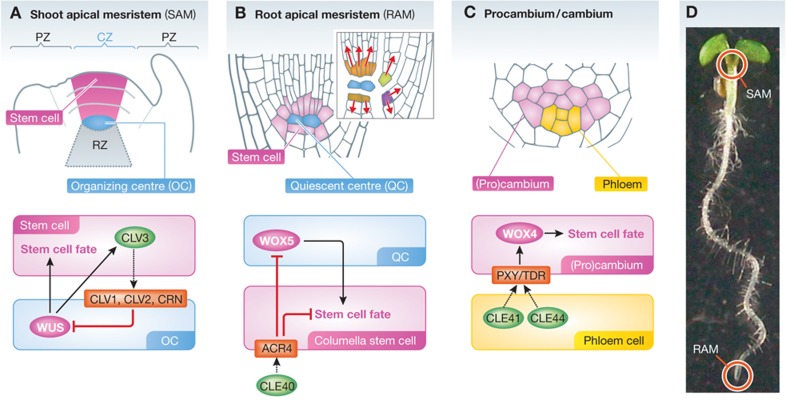
Plants continue to grow in apical and lateral directions, forming new cells and organs throughout their life cycle. Cell division that promotes these growth activities is concentrated in meristems, specialized domains that maintain actively dividing undifferentiated cells. Meristems provide a microenvironment that protects the stem cell niche, thereby acts as a central place for growth and development (reviewed by Scheres, 2007). The shoot apical meristem (SAM) and the root apical meristem (RAM), located at the apices of main and lateral shoots and roots, produce cells for lateral organ formation and tip growth (Figure 1A, B and D). The number of stem cells within SAM and RAM is tightly maintained by mechanisms that balance between cell division and cell differentiation. The SAM is divided into three different zones: the peripheral zone (PZ), the central zone (CZ) and the rib zone (RZ) (Figure 1A). In the CZ, pluripotent stem cells and the organizing centre (OC) together form a stem cell niche (Figure 1A). These stem cells continuously produce daughter cells by asymmetric cell division, and their daughter cells, subsequently displaced to the PZ, are recruited into lateral organ primordia (Tucker and Laux, 2007). In the RAM, the stem cells are present as a single cell layer that surrounds the quiescent centre (QC), and their daughter cells are committed into specific cell fates depending on their position (Figure 1B; Van den berg et al, 1995). In apical meristems, the OC and QC act as niche cells that maintain adjacent stem cells (Laux et al, 1996; Mayer et al, 1998; Fletcher et al, 1999; Schoof et al, 2000; Sarkar et al, 2007; Stahl et al, 2009).
Figure 1.
Regulation of stem cells and their niches in Arabidopsis. (A–C) The organization of stem cell niche in shoot apical meristem (SAM) (A), root apical meristem (RAM) (B) and vascular cambium (C), and their WUS-CLV regulatory mechanism in each meristem. In SAM and RAM, the stem cells are adjacent to the niche cells, which maintain their pluripotency (A, B). In RAM, daughter cells of stem cells are committed into specific cell fates depending on their position (B). (D) The location of SAM and RAM is indicated in 5-day-old Arabidopsis seedling.
In addition to stem cells in the SAM and RAM, vascular stem cells, or more commonly called procambium and cambium are formed in vascular plants (Figures 1C and 2A). This pool of stem cells continuously produces xylem and phloem, major plant vascular tissues. Plant vascular tissues provide physical strength to plant bodies and transport water, nutrients and other substances required for growth and defense. They interconnect all the plant body parts by their conductive function, from the root tip to the various organs in the shoot. Xylem is the main tissue for transporting water and solute minerals, whereas phloem is the route for distributing photosynthetic products and various signalling molecules. Vascular stem cells generate these two conductive tissues via asymmetric periclinal cell division (Eames and MacDaniels, 1947; Esau, 1965).
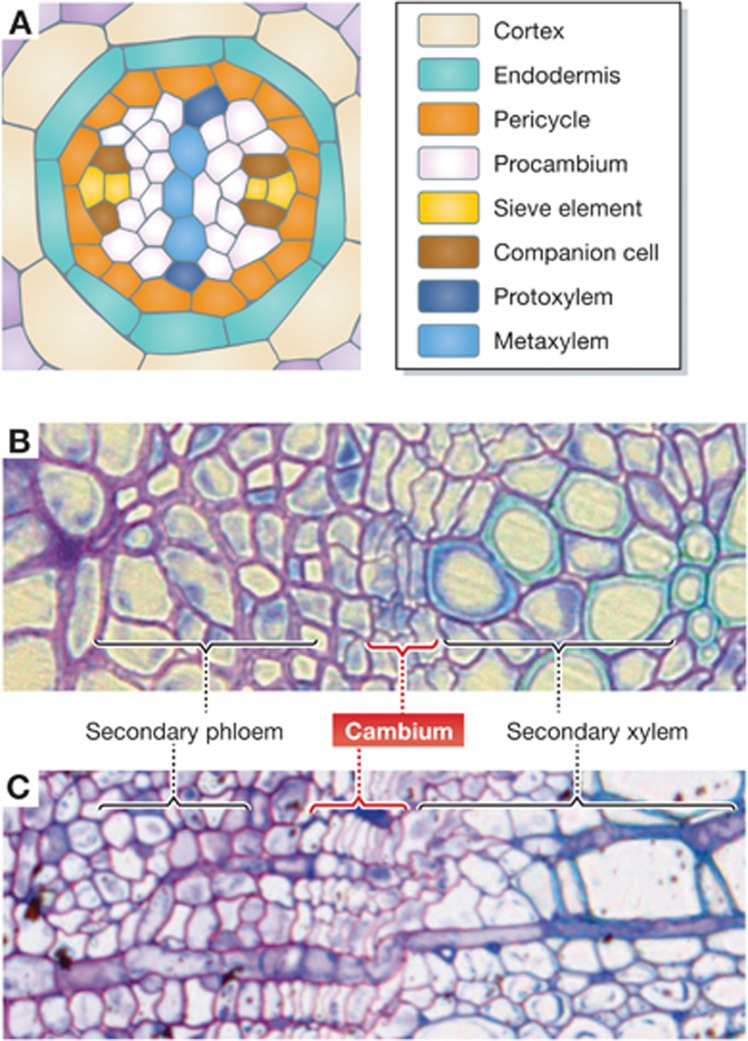
Figure 2.
Organization of vascular tissues in Arabidopsis root and Populus stem. (A) A schematic cross-section of Arabidopsis root showing the vascular organization during the primary development. (B, C) Cross-sections of Arabidopsis root (B) and Populus stem (C) during the secondary development.
Gymnosperms and many dicotyledons undergo two distinct phases of growth and development (Eames and MacDaniels, 1947; Esau, 1965). The primary growth increases plant biomass mainly in the apical direction while the secondary growth does in the lateral direction. During primary growth, xylem and phloem differentiate from cells that are derived from intervening procambium. Once the primary vascular tissues are established, cambium is generated from procambium and its neighbouring cells in stems and roots where it promotes the secondary growth. Cell proliferation in the cambium is usually more active than the one in the procambium (Eames and MacDaniels, 1947; Esau, 1965). An increase in the amount of vascular tissues mediated by cambium is one of the characteristics that distinguish dicotyledons and gymnosperms from monocots. In most monocots, all the procambial cells appear to differentiate into vascular tissues during primary growth. As a result, the cambium does not form and the secondary growth does not occur in these plants (Esau, 1965). The procambium and the cambium may be considered as the same set of vascular stem cells at two developmental stages, because their basic characteristics are very similar. For instance, both procambium and cambium are the source of xylem and phloem. Furthermore, the morphology of procambial cells gradually becomes similar to cambial cells’ as both become vacuolated (Esau, 1965). Currently, however, we lack an understanding of the precise developmental transition from procambium to cambium.
Cell division and differentiation in the cambium lead to the thickening of stems and roots and thereby increase the biomass. Since the secondary growth massively occurs especially in woody plants, its research has been traditionally focusing on tree species. However, the secondary growth is also observed in many herbaceous plants, and it results in the tissue organization very similar to that in tree species (Figure 2B and C). With several advantages such as genomic resources, Arabidopsis has emerged as a useful model for investigating the secondary growth. In particular, Arabidopsis root is an excellent model system for studying vascular development in the primary and the secondary growth because it is simpler and more predictable than the vascular development in other organs (Mahonen et al, 2000, 2006a; Matsumoto-Kitano et al, 2008; Carlsbecker et al, 2010).
With the advancement in genomics and other molecular tools, our knowledge regarding the vascular development, such as the formation of vascular stem cells and subsequent differentiation into xylem and phloem, has rapidly expanded in recent years. Among them, significant progress has been made in studies on the role of phytohormones during the vascular development. Several phytohormones, such as auxin, cytokinin, gibberellins (GAs), ethylene and brassinosteroids, have been shown to regulate various aspects of vascular morphogenesis (Tuominen et al, 1997; Scarpella et al, 2006; Nilsson et al, 2008; Matsumoto-Kitano et al, 2008; Ibañes et al, 2009; Mauriat and Moritz, 2009). Recent molecular genetic studies in Arabidopsis and Populus and cellular studies with Zinnia xylogenic cell culture have started to reveal the molecular mechanisms underlying phytohormone action in vascular development (Ohashi-Ito et al, 2002; Schrader et al, 2004b; Mahonen et al, 2006a; Scarpella et al, 2006). In addition, several transcription factors involved in the vascular stem cell maintenance have been identified. These findings suggest the existence of conserved regulatory programs among different plant stem cell pools (reviewed by Sablowski, 2011).
Vascular stem cells: initiation and primary development
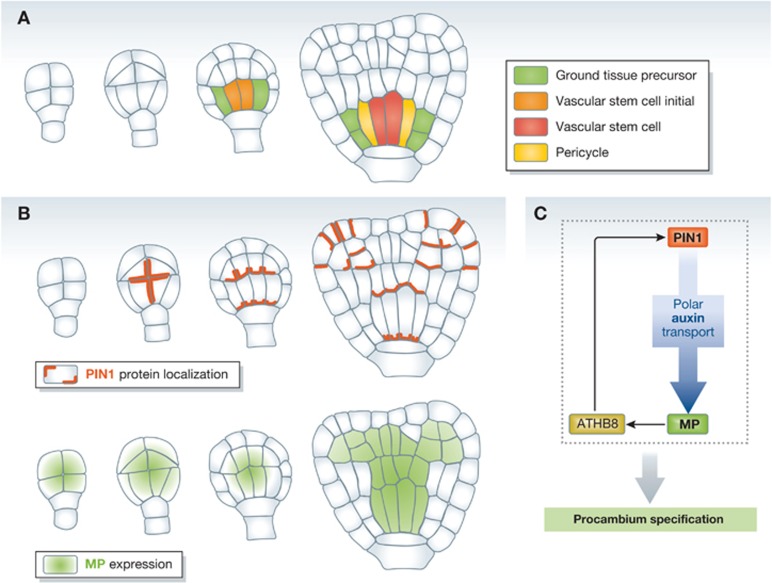
The initiation of vascular stem cells during embryogenesis is well characterized in Arabidopsis. At the early globular stage of Arabidopsis embryos, cells inside the protoderm divide into distinct layers, the ground tissue precursors and vascular stem cell initials (procambium) (Figure 3A; Mansfield and Briarty, 1991; Lau et al, 2010). At the late globular stage, four procambial cells divide periclinally, giving rise to the pericycle and vascular stem cells. During the late globular, heart and torpedo stages, the number of cell files in the procambium continues to increase by further periclinal cell divisions resulting in the radial vascular pattern identical to that of a post-embryonic primary root (Scheres et al, 1994; Mahonen et al, 2000).
Figure 3.
The initiation and formation of procambium cells is regulated by the auxin-mediated positive feedback loop. (A) Schemes of longitudinal median sections during early embryogenesis in Arabidopsis. (B) The polar localization of PIN1 and the expression pattern of MP during early Arabidopsis embryogenesis. (C) The auxin-mediated regulatory loop controlling the vascular initiation during leaf vein growth.
Simultaneously, a network of vascular stem cells arises in cotyledons as well. Although a continuous procambial tissue extends in an apical-basal axis without exhibiting any feature of differentiated vascular elements in a mature embryo, several vascular tissue-specific markers are reported to be expressed at this developmental stage, indicating that vascular cell fate decisions are already made during embryogenesis (Bonke et al, 2003; Mahonen et al, 2006a).
Over the years numerous genetic studies have revealed several genes involved in the radial patterning during Arabidopsis embryogenesis (reviewed by Jenik et al, 2007 and Lau et al, 2012). Among them, a pair of LRR receptor-like kinases, RECEPTOR-LIKE PROTEIN KINASE1 (RPK1) and TOADSTOOL2 (TOAD2), seems to be involved in the determination of the procambium position during early embryogenesis (Table I; Nodine et al, 2007). Both of these genes start to be expressed in the early globular stage. Embryo development in the rpk1 toad2 double mutant is arrested at the late globular stage. Interestingly, in the rpk1 toad2 embryo, the expression of procambial marker genes expands beyond procambium cells into ground tissue initials and protoderm, while the expression of markers for protoderm and ground tissue is severely attenuated. This indicates that these two LRR receptor-like kinases regulate positioning and patterning of the early procambium in the embryo (Nodine et al, 2007).
Table 1. List of genes that regulate vascular stem cells.
| Gene name | Description | Function | References |
|---|---|---|---|
| RECEPTOR-LIKE PROTEIN KINASE1 (RPK1) | LRR-receptor kinase | Determination of procambium position in Arabidopsis embryo | Nodine et al (2007) |
| TOADSTOOL2 (TOAD2) | LRR-receptor kinase | Determination of procambium position in Arabidopsis embryo | Nodine et al (2007) |
| MONOPTEROS (MP) | Auxin responsive transcription factor | Auxin response, vascular stem cell initiation | Hardtke and Berleth (1998); Scarpella et al (2006); Donner et al (2009) |
| PIN-FORMED1 (PIN1) | Auxin efflux carrier | Polar auxin transport, vascular stem cell initiation | Hardtke and Berleth (1998); Scarpella et al (2006); Donner et al (2009) |
| ARABIDOPSIS THALIANA HOMEOBOX 8 (ATHB8) | Class III homeodomain-leucine zipper | Xylem formation, targeted by microRNA165/166 | Baima et al (2001); Carlsbecker et al (2010); Donner et al (2009); Hardtke and Berleth (1998); Scarpella et al (2006) |
| SCARFACE (SFC) | ARF-GAP protein | PIN protein recycling; affects vein growth | Deyholos et al (2000); Koizumi et al (2005); Sieburth et al (2006) |
| Xylogen (XYP1/2) | Proteoglycan-like protein | Affects the continuity of xylem networks | Motose et al (2004) |
| OCTOPUS (OPS) | Membrane localized protein | Affects the continuity of phloem networks | Truernit et al (2012) |
| PHABULOSA (PHB) | Class III homeodomain-leucine zipper | Xylem formation, targeted by microRNA165/166 | Carlsbecker et al (2010) |
| PHAVOLUTA (PHV) | Class III homeodomain-leucine zipper | Xylem formation, targeted by microRNA165/166 | Carlsbecker et al (2010) |
| REVOLUTA (REV) | Class III homeodomain-leucine zipper | Formation of interfascicular fibres, xylem formation, targeted by microRNA165/166 | Carlsbecker et al (2010) |
| CORONA (CNA) | Class III homeodomain-leucine zipper | Xylem formation, targeted by microRNA165/166 | Carlsbecker et al (2010) |
| KANADI (KAN) | GARP family protein, putative transcription factor | Antagonistic role to class III homeodomain-leucine zipper | Emery et al (2003); Ilegems et al (2010) |
| SHORT ROOT (SHR) | GRAS type transcription factor | Endodermis formation, regulatin microRNA165/166 in root | Carlsbecker et al (2010) |
| SCARECROW (SCR) | GRAS type transcription factor | Endodermis formation, regulatin microRNA165/166 in root | Carlsbecker et al (2010) |
| VASCULAR-RELATED NAC-DOMAIN 6/7 | NAC transcription factor | Vessel formation | Kubo et al (2005) |
| ALTERD PHLOEM DEVELOPMENT (APL) | MYB coiled-coil-type transcription factor | Phloem differentiation | Bonke et al (2003) |
| HIGH CAMBIAL ACTIVITY 2 (HCA2) | Dof type transcription factor | Cell proliferation and phloem formation in cambium | Guo et al (2009) |
| Pta LBD1 | LBD/ASL family protein | Cell proliferation in cambium | Yordanov et al (2010) |
| ARK1/2 | Class I KNOTTED1-like homeobox (KNOX) transcription factor | Cell proliferation in cambium | Du et al (2009); Groover et al (2006) |
| WUSCHEL-like HOMEOBOX 4 (WOX4) | Homeobox transcription factor | Stem cell maintenance in cambium | Hirakawa et al (2010b); Suer et al (2011) |
| CLAVATA3/ESR-related 41/44 (CLE41/44) | Peptide ligand | Stem cell maintenance in cambium | Hirakawa et al (2008); Whitford et al (2008) |
| PHLOEM INTERCALATED WITH XYLEM (PXY)/TDIF RECEPTOR (TDR) | LRR-receptor kinase | Stem cell maintenance in cambium | Fisher and Turner (2007); Hirakawa et al (2008) |
| MORE LATERAL GROWTH 1 (MOL1) | LRR-receptor kinase | Regulating cell proliferation in interfascicular cambium | Agusti et al (2011b) |
| REDUCED IN LATERAL GROWTH 1 (RUL1) | LRR-receptor kinase | Regulating cell proliferation in interfascicular cambium | Agusti et al (2011b) |
| CYTOKININ RESPONSE 1 (CRE1)/WOODEN LEG (WOL)/ARABIDOPSIS HISTIDINE KINASE (AHK) | Histidine kinase, cytokinin receptor | Cytokinin receptor, stem cell maintenance in procambium/cambium by inhibiting xylem differentiation | Mahonen et al (2000) |
| AHP6 | A pseudo-phosphotransfer protein | Cytokinin signalling, protoxylem specification | Mahonen et al (2006a) |
| ARR1, 10 and 12 | Type B ARRs | Cytokinin signalling, inhibit the differentiation of procambium into xylem | Argyros et al (2008); Hutchison et al (2006); Ishida et al (2008); Yokoyama et al (2007) |
| ISOPENTENYLTRANSFERASE (IPT) | ISOPENTENYLTRANSFERASE | Cytokinin biosynthesis, promote cambium activity | Matsumoto-Kitano et al (2008) |
| BRI1, BRL1-3 | LRR-receptor kinase | Brassinosteroid receptor, promote xylem development | Cano-Delgado et al (2004); Nakamura et al (2006); Yamamoto et al (2001, 2007) |
| GA 20-oxidase | GIBBERELLIN 3-OXIDASE | Gibberellin biosynthesis, promote a cambium activity and the formation of xylem fibres | Israelsson et al (2005) |
| GA 3-oxidase | GIBBERELLIN 20-OXIDASE | Gibberellin biosynthesis, promote a cambium activity and the formation of xylem fibres | Israelsson et al (2005) |
| JASMONATE ZIM-DOMAIN (JAZ) 7 and 10 | Jasmonate ZIM-domain protein | Jasmonates signalling, jasmonates receptor, repress cambial activities by inhibiting MYC2 | Chini et al (2007); Sehr et al (2010); Thines et al (2007) |
| CORONATINE INSENSITIVE1 (COI1) | F-box protein | Jasmonates signalling, jasmonates receptor | Chini et al (2007); Sehr et al (2010); Thines et al (2007) |
| MYC2 | MYC related transcription factor | Induced by jasmonates, promotes cambial activities | Chini et al (2007); Sehr et al (2010); Thines et al (2007) |
| ERF104 | ETHYLENE RESPONSE FACTOR | Promote interfascicular cambium initiation | Bethke et al (2009) |
The importance of auxin in vascular stem cell initiation has been demonstrated in several studies using Arabidopsis as a model system (Table I; reviewed by Reinhardt, 2003). An auxin responsive transcription factor, MONOPTEROS (MP)/AUXIN RESPONSE FACTOR5 (ARF5), plays a critical role in the specification of vascular stem cells (Hardtke and Berleth, 1998). During early embryogenesis, MP is expressed in the procambial cells and its expression is rapidly upregulated by auxin (Figure 3B). In mp knockout mutant embryos, procambium completely fails to form and weak alleles display irregular vascular development (Berleth and Jurgens, 1993; Hardtke and Berleth, 1998). Such a defect seems to be related to the lack of polar auxin transport during embryogenesis. During early embryogenesis, PIN-FORMED1 (PIN1) proteins, major auxin efflux carriers, are polarly localized in the inner cells of pro-embryo before they turn into procambial cells (Figure 3B; Friml, 2003). PIN1 expression is dramatically reduced in a loss-of-function mp mutant, suggesting that MP might regulate its transcription (Wenzel et al, 2007).
In early stages of leaf development, preprocambial cells, from a sub-epidermal layer of leaf primordia, are organized into continuous strands to form veins, networks of vascular tissues (Eames and MacDaniels, 1947). Unlike stem cells in the SAM or RAM, vascular stem cells in developing leaves are constantly formed to produce vein networks. Therefore, the proper vein growth requires the same molecular processes involved in the vascular stem cell formation in an iterative manner. One key question in the vein growth is how procambial cells signal from one to the other to form continuous vascular strands. The auxin-canalization hypothesis has been proposed to explain this (Sachs, 1981; Rolland-Lagan and Prusinkiewicz, 2005). According to this hypothesis, the vein growth is promoted via a positive feedback regulation of auxin transport. More specifically, the auxin maxima established in procambial cells enhance the machinery that transports auxin to adjacent cells in a polar manner. The adjacent cells that subsequently perceive a high level of auxin turn into procambium and repeat the same process to the next neighbours.
The auxin canalization theory was supported by the findings of the auxin efflux carrier protein, PIN1. In pin1 mutant stems, the arrangement of vascular bundles is abnormal, similar to when the auxin transport inhibitor is applied (Gälweiler et al, 1998). PIN1 expression was further characterized in developing leaves (Scarpella et al, 2006). This study showed that PIN1 starts to be expressed in the vascular stem cells before they become morphologically discernible. Then, expression domains of PIN1 expanded to the future veins as leaf primordia emerged and grew. When the veins were about to bifurcate, PIN1 localization became bipolar in the bifurcating cell. These suggest that polar auxin transport is vital for the proper organization of vascular stem cells.
The auxin canalization theory proposes that the positive feedback regulation of auxin transport is required for the auxin maxima to be established in the preprocambial cells of growing veins. Recent genetic studies revealed the molecular basis of such regulation (reviewed by Scarpella et al, 2010). One of the earliest expressing transcriptional regulators in the preprocambial cells is the ARABIDOPSIS THALIANA HOMEOBOX8 (ATHB8), a class III homeo-domain leucine zipper (HD-ZIP III) transcription factor family gene (Table I; Donner et al, 2009). It turned out that the transcription of ATHB8 is activated directly by MP. ATHB8 subsequently directs the formation of preprocambial cells and induces the expression of PIN1 (Figure 3C; Scarpella et al, 2006). These processes establish a positive feedback loop of auxin-MP-ATHB8-PIN1 in the initiation and specification of vascular stem cells. When this positive feedback regulation was disrupted in the athb8 mutant, the expression domain of a preprocambium marker (J1721) expanded. This supports the importance of positive feedback regulation for spatially restricting preprocambial cell specification (Donner et al, 2009).
PIN proteins cycle between plasma membranes and endosomes via the activity of ARF–GEF protein, GNOM (Geldner et al, 2003). The importance of polar auxin transport in vein growth and patterning was further evident from mutations in components of PIN localization machinery. For example, scarface (sfc)/van3 generates dense but fragmented vein islands on leaves and cotyledons. SFC encodes an ADP ribosylation factor GTPase activating protein (ARF–GAP), a modulator of ARF–GEF, involved in the vesicle trafficking (Deyholos et al, 2000; Koizumi et al, 2005; Sieburth et al, 2006). In the sfc, intracellular trafficking of PIN1 was abnormal, suggesting that SFC might affect the endosomal cycling of PIN1 (Sieburth et al, 2006). Other factors involved in vascular continuity have also been reported (Table I). Among them, Xylogen, a large proteoglycan-like protein (Motose et al, 2004), is polarly localized in the cell walls of differentiating tracheary elements to function as an intercellular signal molecule. The Arabidopsis genome contains two genes encoding Xylogen, AtXYP1 and AtXYP2. When these two are knocked out xylem strands develop in a discontinuous manner (Motose et al, 2004). OCTOPUS, a polarly localized membrane-associated protein in the phloem, has been reported to regulate phloem continuity in Arabidopsis (Truernit et al, 2012).
Besides ATHB8, the Arabidopsis genome contains four other HD-ZIP III genes PHABULOSA (PHB), PHAVOLUTA (PHV), REVOLUTA/INTERFASCICULAR FIBERLESS1 (REV/IFL1), CORONA (CNA)/ATHB15 (Table I) (Talbert et al, 1995; Baima et al, 2001; McConnell et al, 2001; Green et al, 2005; Prigge et al, 2005). All of them are expressed in vascular tissues in somewhat overlapping fashion, suggesting a likely redundancy in their function (Prigge et al, 2005; Carlsbecker et al, 2010). A quadruple knockout mutant for ATHB8, CNA, PHB and PHV was reported to exhibit enhanced cell proliferation in procambium (Carlsbecker et al, 2010). Furthermore, a quintuple mutant, athb8-11 cna-2 phb-13 phv-11 rev-6, completely lacked xylem, highlighting their involvement in the vascular cell type specification (Carlsbecker et al, 2010). The KANADI (KAN) genes, a subclass of GARP family transcription factors, have been shown to play an antagonistic role to HD-ZIP III genes in dorsoventral patterning of lateral organs (Emery et al, 2003). In the vascular tissue of a quadruple loss-of-function KANADI mutant (kan1 kan2 kan3 kan4), the cell proliferation in procambium was strikingly enhanced (Ilegems et al, 2010). On the contrary, ectopic expression of KAN1 under the CNA/ATHB15 promoter suppressed PIN1 expression in procambium during embryogenesis and thereby repressed the vascular stem cell initiation (Ilegems et al, 2010). This finding indicates that KAN genes might antagonize HD-ZIP III genes through the modulation of PIN1 expression. Interestingly, KAN genes are expressed in the phloem, thus they might control vascular stem cell development in a non-cell autonomous manner (Emery et al, 2003). In summary, a positive feedback loop of auxin-MP-PIN1 mediated by HD-ZIP III and KAN genes primarily controls the initiation and specification of vascular stem cells.
Establishment of vascular cambium during the secondary development
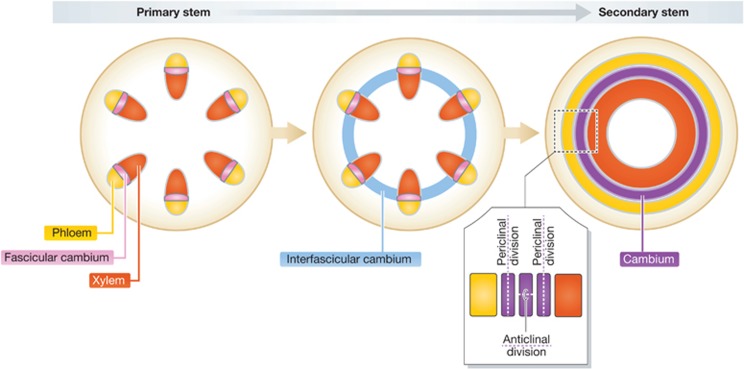
The cambium or vascular cambium represents a pool of stem cells arranged in radial series and gives rise to xylem and phloem cells during the secondary phase of plant development (Figures 2 and 4; Eames and MacDaniels, 1947; Esau, 1965). In stems, the procambium derived from apical meristem usually resides within the bundles (Eames and MacDaniels, 1947). During the secondary growth phase, the procambial cells become the ‘fascicular cambium’, the cambium derived from a vascular bundle of primary development (Figure 4). The clusters of fascicular cambium become interconnected by the ‘interfascicular cambium’, which is formed from parenchyma cells between the vascular bundles (Eames and MacDaniels, 1947), thereby completing the radial arrangement of cambium (Figure 4). The development of xylem in Arabidopsis can be divided into two distinct phases, based on which cell types differentiate from cambial cells. In an early phase, only vessel elements differentiate, and the rest of the xylem cells remain as parenchyma. At a later phase, both vessel elements and lignified fibre cells are produced from cambial cells, resulting in a stem anatomy comparable to wood of an angiosperm tree (Chaffey et al, 2002). Although numerous histological analyses in several plant species have revealed the dynamic morphological changes during the secondary development (Esau, 1965; Eames and MacDaniels, 1947), little is known about the molecular mechanisms underlying these processes. A loss-of-function mutation in REV leads to the disrupted development of xylem fibres and vessel elements in Arabidopsis, indicating a role for REV during the secondary phase of vascular development (Zhong and Ye, 1999).
Figure 4.
Schematic illustration of the primary and secondary stem anatomy in Arabidopsis. The primary stem exhibits disconnected vascular bundles with procambium. In the secondary developmental phase, this procambium turns into a fascicular cambium and the cells between bundles become an interfascicular cambium. Fascicular and interfascicular cambia interconnect to each other and establish a cambium in a circular form.
The vascular cambium enlarges its circumference through anticlinal cell divisions (occur perpendicular to an adjacent cell layer), while it continues to undergo periclinal cell divisions (occur parallel to an adjacent cell layer) to produce mother cells for xylem and phloem (Figure 4). The vascular cambium contains two types of cells: elongated cells with tapering ends, the fusiform initials (spindle-shaped initials), and the ray initials, which are nearly isodiametric and relatively small in size. The fusiform initials upon differentiation give rise to xylem and phloem, while the ray initials give origin to the radial strands of cubical cells that play a role in nutrient transport and storage (Vanbel, 1990).
Just before the formation of cambium, there is a transitional period during which cell divisions occur in multiple planes in the procambial cells (Eames and MacDaniels, 1947). In Arabidopsis root, periclinal cell divisions first take place in the procambium during the transitional period. This occurs even before the primary vessel members complete their differentiation (Baum et al, 2002). This along with sequential periclinal divisions in the procambial derivatives lead to an oblong shape stele with constrictions at xylem poles (Baum et al, 2002). The periclinal cell divisions in the pericycle adjacent to the xylem pole extend the pericycle away from xylem poles and produce cells that eventually form a continuous cylinder of cambium cells (Baum et al, 2002). The pericycle cells then keep dividing periclinally to form cork cambium, the second layer of lateral meristem that produces the protective outer layer or cork (Baum et al, 2002). During this process, the epidermis and ground tissue are gradually stretched and peeled off from the expanding root.
As described above, a stem cell niche is a cellular microenvironment providing intercellular signals that maintain pluripotency of stem cells. Several recent studies have revealed the molecular regulatory mechanisms underlying stem cell maintenance in SAM and RAM (Figure 1A and B; Sablowski, 2011). Even though substantial progress has been made in understanding stem cell functions in apical meristems, our knowledge about vascular stem cells and their niche in lateral meristem is still limited. The current opinion holds cambium includes undifferentiated ‘initial cells’ (stem cells), with the first committed derivatives on each side being phloem and xylem mother cells (Esau, 1965). Although the existence of pluripotent stem cells in cambium is generally acknowledged, the spatial distribution and identity of niche cells in cambial zone is still unclear. The initial cells in cambium are the only cells in cambial zone that can give rise to both phloem and xylem by periclinal divisions and produce new cell files by anticlinal divisions. It has been shown that anticlinal cell divisions in cambial zone are concentrated in a few cell layers in the vascular cambium in Populus (Schrader et al, 2004b). This implies that the identity of initial cells might be restricted to these cell layers in the cambial zone. The subdivision of cambium into initial cells and mother cells has mainly been a conceptual one. This is largely due to the failure in distinguishing these two cell types under a microscope as they are anatomically more or less uniform. Furthermore, there are no molecular markers currently available that can define the initial cells in the cambial zone.
In order to establish the molecular tools, which mark a stem cell population in cambial zone, a high-resolution transcription profile analysis was performed in poplar cambium using tangential thin sections. This study provides evidence that anatomically indistinguishable cells in the cambial zone actually differ in their transcription profiles (Schrader et al, 2004b). Interestingly, several homologues of known SAM regulators showed cambium-specific expression patterns (PttCLV1, PttANT and PttKNOXs), suggesting the existence of similar regulatory networks in the poplar cambium and SAM. Although this transcription map provided molecular support for a compartmentalization of the cambial zone and identified several distinct layers in the poplar cambium, the resolution is not high enough to differentiate molecular events at the level of a single cell layer (Birnbaum et al, 2003; Brady et al, 2007). However, these data serve as a rich source for developing new molecular cambium markers.
Transcriptional and post-transcriptional regulations in the specification and proliferation of vascular tissues
Soon after the germination, xylem and phloem strands differentiate in the defining domains in an organ-specific manner. In Arabidopsis post-embryonic roots, 4–5 cell files of xylem vessels develop in the central axis, and the two poles of phloem develop perpendicular to the xylem axis (Figure 2A; Scheres et al, 1994; Mahonen et al, 2000). Two types of xylem vessels develop in the root. Protoxylem strands with lignins deposited in a spiral pattern develop in the periphery of xylem axis and metaxylem strands with lignins in a reticulate pattern develop in the centre (Figure 2A). Between xylem and phloem, vascular stem cells maintain their undifferentiated state and later give rise to cambium at the secondary development stage (Figure 2A and B) (reviewed by Elo et al, 2009). As mentioned above, all the HD-ZIP III genes are expressed in Arabidopsis vascular tissues. In Zinnia mesophyll cell culture, the expression of HD-ZIP III genes (ZeHB-10, -11 and -12) is induced during xylogenesis (Demura et al, 2002; Ohashi-Ito et al, 2002; Ohashi-Ito and Fukuda, 2003), suggesting their involvement in xylem differentiation. In Arabidopsis root, a loss of all the five HD-ZIP IIIs results in the failure of xylem vessel development (Carlsbecker et al, 2010). On the contrary, overexpression of AHTB8 causes enhanced xylem differentiation (Baima et al, 2001), indicating that HD-ZIP IIIs are de novo regulators of the xylem vessel formation.
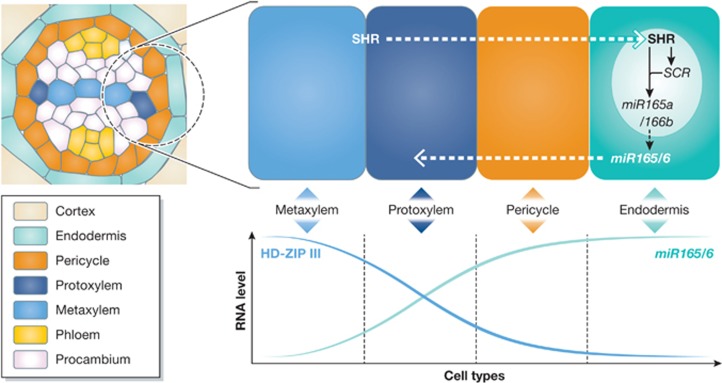
Levels of HD-ZIP III transcription factors are post-transcriptionally controlled by microRNA 165/6 (Emery et al, 2003; Mallory et al, 2004). Recently, it was discovered that this microRNA-mediated post-transcriptional regulation is a critical process in specifying and patterning proto- and metaxylem vessels in roots (Figure 5; Table I; Carlsbecker et al, 2010; Miyashima et al, 2011). HD-ZIP IIIs specify these two xylem vessel types in a dosage-dependent manner. A high level of HD-ZIP IIIs specifies metaxylem and a low level does protoxylem. For xylem vessels pattern properly in the root, HD-ZIP IIIs should be differentially distributed in a way that their levels are high in the centre of xylem axis but low in the periphery. Such a differential distribution of HD-ZIP IIIs in the xylem precursors turned out to be regulated by SHORT ROOT (SHR) and SCARECROW (SCR), two GRAS family transcription factors, in a non cell-autonomous manner. SHR proteins are generated in the vascular cylinder and then move into the endodermis layer and QC where they activate SCR (Cui et al, 2007; Nakajima et al, 2001). In the endodermis, it was found that SHR and SCR together activate two genes that encode microRNA 165/6. When either SHR or SCR was knocked out, all the xylem strands were specified and differentiated into metaxylem. Conversely, microRNA 165/6 expressed in the ground tissue layer in shr or scr mutant was able to recover the gradient distribution of HD-ZIP IIIs and wild-type xylem patterning. These results suggest that microRNA 165/6 acts as mobile signals that establish and facilitate the dosage-dependent regulation of HD-ZIP IIIs. Further supporting the role of HD-ZIP IIIs in determining xylem cell types, the gain-of-function mutants phb-d (phb-1d and -7d), in which the microRNA 165/6 target site is disrupted, exhibited ectopic metaxylem vessel formation at the position where protoxylem would normally differentiate (Carlsbecker et al, 2010).
Figure 5.
Cell type patterning in Arabidopsis roots is controlled by the bidirectional cross-talk mediated by SHR and microRNA 165/166 that are mobile between cells. SHR, generated in the stele, travels to the endodermis and activates the expression of microRNA 165/6. MicroRNA 165/6 from the endodermis travels to the vascular cylinder and degrades mRNAs of HD-ZIP III transcription factors in the endodermis and periphery of vascular cylinder, resulting in a graded distribution of HD-ZIP IIIs. A high level of HD-ZIP IIIs specifies metaxylem and a low level specifies protoxylem.
A study in Populus also supports that HD-ZIP III transcription factors are key regulators of vascular stem cell proliferation and xylem formation. When a microRNA-resistant Populus REVOLUTA was ectopically expressed, ectopic cambium formed in cortex layers (Robischon et al, 2011). In addition, these transgenic plants formed xylem towards the stem periphery, further highlighting the importance of relative levels and spatial distribution of HD-ZIP IIIs in vascular tissue patterning.
NAC domain transcription factors VASCULAR-RELATED NAC-DOMAIN6 (VND6) and VND7 also play an essential role in xylem vessel differentiation in both Arabidopsis and Populus (Table I). It has been first reported in Arabidopsis that VND6 promotes the differentiation of metaxylem and VND7 induces protoxylem fate (Kubo et al, 2005). Further analyses including ectopic expression studies in leaves demonstrated that VND6 and VND7 respectively act as central regulators of metaxylem and protoxylem vessel fates (reviewed by Ohashi-Ito and Fukuda, 2010).
In summary, pathways that integrate transcriptional and post-transcriptional regulation and cell-to-cell communication govern the xylem specification and patterning. Furthermore, factors involved in the xylem formation are evolutionally conserved among various vascular plants (Floyd and Bowman, 2004; Prigge and Clark, 2006; Floyd and Bowman, 2007), raising the possibility that aforementioned xylem regulatory programs might have arisen in early lineages of vascular plants.
Unlike xylem development, our knowledge about the molecular control of phloem formation is still relatively poor. An MYB transcription factor, ALTERD PHLOEM DEVELOPMENT (APL), which is expressed in developing phloem cells, has been identified as a key regulator of phloem development. Knocking out APL gene results in the absence of phloem sieve elements and companion cells (Table I; Bonke et al, 2003). In addition, xylem vessel-like cells are formed where phloem cells normally develop. On the contrary, ectopic expression of APL under the control of WOL promoter inhibits xylem development, suggesting that APL possesses a dual role: one is to promote phloem differentiation and the other is to repress xylem differentiation during root vascular development (Bonke et al, 2003).
Recently, two other transcription factors that also affect vascular stem cell activities and phloem formation were identified. HCA2 (HIGH CAMBIAL ACTIVITY2) was isolated among activation tagging lines in Arabidopsis (Table I; Guo et al, 2009). This activation tagging line promoted cambium formation and phloem proliferation. The phenotype was found to be due to ectopic expression of At5g62940, a gene that encodes a Dof family transcription factor. HCA2 is highly expressed in the cambium, phloem and interfascicular parenchyma in wild-type background, suggesting that it might promotes the formation of cambium and asymmetric cell division that forms phloem cells at the very early stage. This idea was further supported by the finding that the ectopic expression of HCA2 upregulates phloem and cambium marker genes, but downregulates xylem marker genes. HCA2 seems to function mainly as a transcriptional activator to promote the formation of interfascicular cambium. When plants were introduced with modified HCA2, which was fused with EAR-motif repressor domain, they exhibited phenotypes opposite to the ectopic expression lines. In these transgenic plants, the formation of interfascicular cambium was significantly inhibited; however, no major effect on the phloem development was observed.
Another transcription factor that is actively involved in the cambial activities is a Populus homologue of LBD1 (Pta LBD1). Pta LBD1 promotes the lateral stem growth, by increasing phloem proliferation (Yordanov et al, 2010). In parallel, Pta LBD1 was found to repress two important cambium regulators, ARBORKNOX1 (ARK1) and ARK2, class I KNOTTED1-like homeobox (KNOX) transcription factors. ARK1 is an orthologue of SHOOT MERISTEMLESS (STM) and ARK2 is an orthologue of BREVIPEDICELLUS (BP) in Arabidopsis. These two proteins were also suggested to promote vascular stem cell maintenance in Populus (Groover et al, 2006; Du et al, 2009). The repression of ARK2 by Pta LBD1 is reminiscent of the repression of BP by ASYMMETRIC LEAVES2 (AS2), an LOB domain transcription factor, in Arabidopsis. In Arabidopsis, AS2 (Iwakawa et al, 2002) and AS1 (Byrne et al, 2000) form a complex and repress BP expression (Guo et al, 2008). These again suggest the existence of conserved regulatory programs between Arabidopsis and Populus.
Peptide-receptor signalling pathways in the specification and proliferation of vascular tissues
Stem cell populations are maintained by balancing cell proliferation and differentiation. A key mechanism in the plant stem cell maintenance is the signalling contributed by the interaction between small mobile peptides and receptor like kinases (Sablowski, 2011). In the SAM, dodecapeptide CLAVATA3 (CLV3) (Fletcher et al, 1999; Kondo et al, 2011) diffuses from the dividing stem cells to the underlying cell layer and interacts with CLV1 (Clark et al, 1997; Ogawa et al, 2008), a Leucine-Rich Repeat (LRR) Receptor-Like Kinase (RLK), or a receptor complex composed of CLV2, a receptor-like protein, and CORYNE, a membrane localized Serine/Threonine kinase (Figure 1A; Jeong et al, 1999; Muller et al, 2008). A signal produced by this interaction represses the expression of WUSCHEL (WUS), a homeobox transcription factor that specifies niche cells and maintains the stem cell population through cell-to-cell movement (Mayer et al, 1998; Yadav et al, 2011). Recently a similar regulatory program was also identified in the RAM (Figure 1B; Sarkar et al, 2007; De Smet et al, 2008). Analogous to SAM, this signalling module also comprises a small peptide ligand, CLE40 (CLV3 homologue) and an LRR-RLK protein ARABIDOPSIS CRINKLY4 (ACR4) (Stahl et al, 2009). CLE40 regulates distal stem cell differentiation in a dose-dependent manner with high concentrations promoting differentiation. Like WUS in the SAM, WOX5 acts in the QC to maintain the stem cell pool while the CLE40-ACR4 ligand-receptor complex restricts its function and triggers distal stem cell differentiation.
Like in the SAM and RAM, the interaction between CLEs and RLKs controls vascular stem cells (Figure 1C; Table I; Hirakawa et al, 2010a). In a study using the xylogenic culture system of Zinnia mesophyll cells, CLV3 promoted the differentiation of tracheary elements (or xylem vessels) whereas another peptide, named as TDIF (Tracheary Element Differentiation Inhibitory Factor), which is identical to CLE41/44 in Arabidopsis, inhibited this process (Ito et al, 2006). Consistent with the finding in Zinnia, overexpressing CLE41 or 44 in Arabidopsis also inhibited the differentiation of tracheary elements and resulted in the discontinuous xylem strand formation. Furthermore, it promoted division of vascular stem cells in the hypocotyl (Hirakawa et al, 2008; Whitford et al, 2008). Therefore, CLE41 and 44 have two roles: one is to act as positive signals for cell proliferation and the other is to act as negative signals for xylem differentiation. These signals were found to be perceived via the interaction between CLE41/44 and PXY (PHLOEM INTERCALATED WITH XYLEM), also named as TDR (TDIF RECEPTOR) (Figure 1C; Hirakawa et al, 2008). PXY was originally identified from an Arabidopsis mutant with an aberrant organization of xylem and phloem in the stem (Fisher and Turner, 2007). In the absence of PXY/TDR, plants became insensitive to high levels of CLE41/44 in the cell proliferation and xylem differentiation.
Similarly to the CLV3-CLV1 regulation in the SAM, CLE41/44 and PXY/TDR are expressed in different cell types that are adjacent to each other. CLE41 and 44 are produced in the phloem and diffuse towards the vascular stem cells where PXY/TDR is expressed. Further investigation suggests that the spatial restriction of CLE41/44 in phloem cells is important for proper vascular tissue patterning in the stem (Figure 1C; Etchells and Turner, 2010). When CLE41 was expressed ubiquitously or on the xylem side, the organization of vascular tissues in the hypocotyl was perturbed dramatically and formed mixed populations of xylem and phloem. Furthermore, ectopic expression of CLE41 suppressed the PXY/TDR expression. When such a negative feedback regulation was completely broken by the ectopic expression of both PXY and CLE41, a dramatic increase in the size of vascular stem cell population was observed. This suggests that the vascular stem cell population is maintained by the negative feedback regulation of PXY/TDR, which is triggered by the interaction between CLE41/44 and PXY/TDR.
An interaction between CLE41/44/TDIF and PXY/TDR induces the expression of WOX4, a homologue of WUS, in vascular stem cells (Hirakawa et al, 2010b; Ji et al, 2010). WOX4 expression was rapidly induced when a high dose of TDIF was treated to Arabidopsis. However, such an induction failed to happen in the absence of PXY/TDR. This positive regulation of WOX4 by TDIF-PXY/TDR complex is opposite to what CLV3-CLV1 complex does to WUS. Further investigation suggested that although WOX4 regulates the proliferation of vascular stem cells, it does not influence the differentiation of tracheary elements in response to TDIF–TDR interaction. In wox4 mutant, exogenously applied TDIF still caused the intermittent xylem strand formation, but did not promote vascular stem cell proliferation any more. When wox4 mutant plants were grown for a long term, the secondary growth of hypocotyls was significantly reduced, suggesting that WOX4 is required to maintain the cell proliferation activity of vascular stem cells (Hirakawa et al, 2010b). In addition, a recent study has reported the positive effect of auxin on WOX4 expression in the cambium, suggesting that various inputs integrated into WOX4 are controlling the cambium activity (Suer et al, 2011).
TDIF-TDR-WOX4 pathway seems to be an evolutionarily conserved regulatory program. Transcription profiling in the Populus cambial zone suggested that Ptt-HB3, a homologue of WOX4, and Ptt-RLK3, a homologue of CLV1, are highly expressed in the vascular stem cells (Schrader et al, 2004b). Ectopic expression of WOX4 orthologue in tomato, which has bicollateral vascular organization in stems (phloem is formed in both inside and outside the xylem), also promoted the vascular cell proliferation (Ji et al, 2010).
In addition to TDR/PXY, two LRR-RLKs were shown to regulate vascular stem cell activities (Agusti et al, 2011b). In this study, cells that are in the process of interfascicular cambium formation were isolated from Arabidopsis stem segments in a time course using the laser capture micro-dissection technique and global mRNA expression was profiled in those cells. Two genes encoding LRR-RLK, MOL1 (MORE LATERAL GROWTH1) and RUL1 (REDUCED IN LATERAL GROWTH1), are highly induced during the interfascicular cambium formation (Table I). As their names indicate, the cell proliferation related to interfascicular cambium activity was enhanced in the mol1 mutant, whereas it was suppressed in rul1. Interestingly, the mol1 mutations enhance the expression of WOX4 and TDR/PXY. Thus, MOL1 seems to regulate cell proliferation in the interfascicular cambium through the TDR/PXY-WOX4 pathway.
Vascular stem cell regulation by plant hormones
Auxin
Auxin plays a crucial role in the specification of vascular stem cells (procambium) as explained above and so it does in the cambium activities (Figure 7). Analysis of the auxin distribution across the cambial region in hybrid aspen trees showed a radial auxin gradient reaching a peak level in the cambial zone or at the border between the cambial zone and the expansion zone towards the xylem (Uggla et al, 1996; Tuominen et al, 1997). This auxin distribution pattern is very similar to what was found in a gymnosperm, Pinus sylvestris, suggesting an evolutionary conserved function of auxin in cambium activities (Uggla et al, 1996). When a stem is wounded, the orientation of xylem growth changes and grain patterns are altered. It turned out that such changes correlate with changes in the auxin distribution in response to wounding, which subsequently affect the orientation of cambial initials and derived cells of secondary xylem (Kramer et al, 2008). Auxin function in the secondary growth was further tested in Populus by perturbing auxin signalling. In this study, a stabilized form of PttIAA3, which has a mutation in an amino acid that is important for the auxin-mediated degradation of IAA, was ubiquitously expressed (Moyle et al, 2002; Nilsson et al, 2008). Suppressing auxin responsiveness significantly reduced cambial cell division activities and the radial growth. Such reduction in cambial cell divisions affected the growth of xylem more significantly than the phloem. Recently, strigolactones, a carotenoid-derived group of plant hormones were shown to act downstream of auxin-dependent activation of cambium activities and secondary growth in Arabidopsis (Agusti et al, 2011a).
Figure 7.
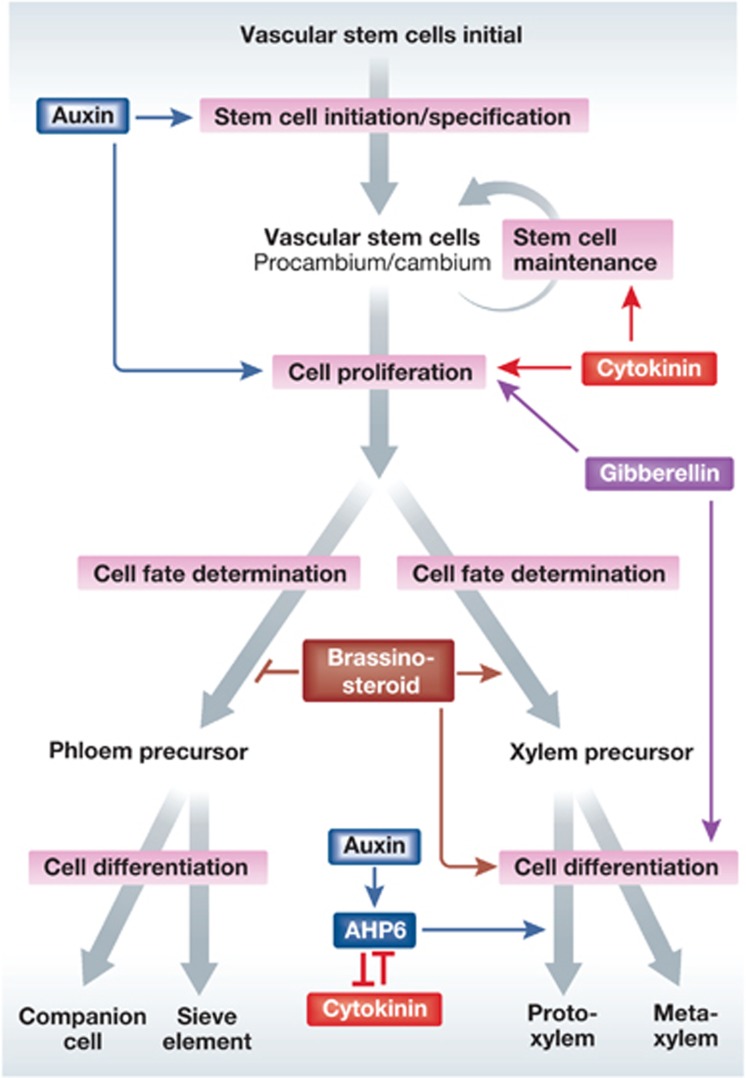
Hormonal regulation in the vascular stem cells.
In trees, rates of radial stem growth change seasonally. When the day length becomes shorter than the one that permits growth, cell division stops in the cambium (dormant). Consistently, cambium recovers cell division activities if plants are exposed to long days again. Comparison of genome-wide expression profiles in the active and dormant Populus cambia revealed significant repression of auxin efflux carriers PttPIN1 and PttPIN2 in the dormant cambium (Schrader et al, 2003, 2004a). During active phases of growth, expression of these polar auxin transporters was reactivated in response to the increase in auxin level via the positive feedback loop mechanism (Schrader et al, 2003; Vieten et al, 2005). A recent study suggests that auxin signalling pathway is significantly disrupted during cambium dormancy. This seems to be due to the perturbation of upstream auxin signalling pathways, one of which is the SCFTIR activity and ARF regulation (Baba et al, 2011). Dormancy of cambial activity and other growth responses to seasonal changes have been shown to be signalled through FLOWERING LOCUS T (FT), which is regulated by CONSTANS and PHYA (Böhlenius et al, 2006). In Populus, two FT gene duplicates were found to be sub-functionalized and induced under different environmental conditions (Hsu et al, 2011). An FT gene that is induced under conditions of a warm temperature and long day promotes the radial stem growth. It remains to be identified whether the growth regulation of FT is mediated via auxin signalling pathways.
Cytokinins
Cytokinins, a key phytohormone along with other factors, maintain stem cell pools by regulating cell differentiation (Figures 6 and 7; Table I). The role of cytokinin in vascular stem cell maintenance first emerged from a finding of the wooden leg (wol) mutant that has a severely reduced number of procambial cell files in embryos and roots (Scheres et al, 1995). In addition, the vascular cylinder of the wol primary root consists solely of protoxylem (Mahonen et al, 2000). The WOL gene encodes a two-component histidine kinase protein, one of the three cytokinin receptors in Arabidopsis (Mahonen et al, 2000). The wol mutation mimics the cytokinin-unbound state and confers constitutive phosphatase activity, removing phosphate from histidine-containing phosphotransfer (HPt) protein. This blocks the total phospho-load and cytokinin signalling (Mahonen et al, 2006b). Similarly to the wol mutant, a triple loss-of-function mutant of cytokinin receptors (ahk2 ahk3 ahk4) exhibited ectopic protoxylem formation, accompanied by a reduction in vascular cell files (Mahonen et al, 2006a). AHP6, a pseudo-phosphotransfer protein, has been reported to act as a negative regulator of cytokinin signalling by interacting with the phosphorelay machinery (Mahonen et al, 2006a). AHP6 is specifically expressed in the protoxylem cells, and its loss-of-function mutation results in sporadic differentiation of protoxylem (Mahonen et al, 2006a). The expression analysis of ARR15 as cytokinin signalling reporter revealed that cytokinin signalling is ectopically activated at sites where protoxylem would normally differentiated in ahp6 mutant. This suggests that AHP6 counteracts cytokinin signalling and thereby allows cells to differentiate into protoxylem identity (Mahonen et al, 2006a). Therefore, the pluripotency of vascular stem cells and identity of protoxylem seem to be determined by spatially distributed regulatory interactions between cytokinin signalling and its inhibitor AHP6 in the Arabidopsis root.
Figure 6.
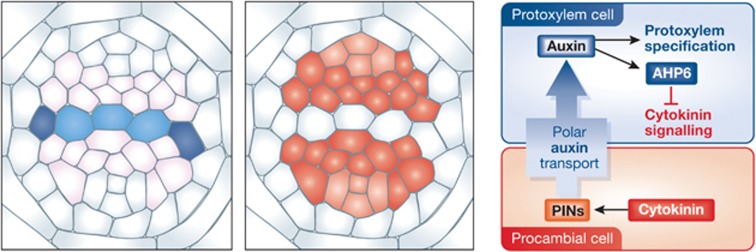
The regulatory loop between auxin and cytokinin controls vascular patterning in the Arabidopsis primary root. Cytokinin signalling restricts the auxin signalling maximum to the xylem axis by promoting the bisymmetric localization of PINs. Consequently, auxin activates the expression of AHP6 in the protoxylem position, and in turn AHP6 counteracts cytokinin signalling, thereby allowing cells to differentiate into protoxylem.
Recently, it has been reported that a mutually inhibitory feedback loop between auxin and cytokinin regulates the vascular patterning in Arabidopsis primary root (Figure 6; Bishopp et al, 2011). In the post-embryonic Arabidopsis root, vascular tissues are organized in a bisymmetric pattern, in which the first plane of symmetry runs through a central xylem axis and the second plane through the phloem poles. Along with this bisymmetric vascular pattern, the signalling maxima of two major phytohormones, cytokinin and auxin, is established in a bisymmetric manner. Cytokinin signalling maximum is detected in the intervening procambium cell files. On the other hand, auxin signalling maximum is observed in the xylem axis, overlapping with AHP6 expression domain which is positioned in the protoxylem pole. AHP6 expression is promoted by auxin through ARF binding sites in the AHP6 promoter, suggesting that AHP6 is targeted as a primary auxin response gene. When plants are treated with the auxin transport inhibitor 1-naphthylpthalamic acid (NPA), both auxin signalling maximum in the xylem axis and AHP6 expression in the protoxylem position disappear. Consistently, a loss-of-protoxylem phenotype is observed in NPA-treated plants similar to that of ahp6 mutant. This suggests that the active auxin transport facilitates the formation of auxin signalling maximum in the xylem axis, which in turn promotes AHP6 expression in the protoxylem position. Among eight PIN genes, the expression of PIN3 and PIN7 exhibits a non-radially symmetric pattern in the root stele. PIN3 is predominantly expressed in the pericycle cells touching the protoxylem pole and PIN7 is present in all the intervening procambium cells and phloem initials. In pin3 pin7 double mutant, the robust bisymmetric expression patterns of DR5 and AHP6 become more random than in the wild type, suggesting that these PINs are required for creating a stable auxin maximum in the xylem axis. Interestingly, the expression of these PINs is significantly downregulated in the wol mutant where the cytokinin signalling is reduced. In contrast, exogenous cytokinin treatment results in the expansion of PIN7 domain into the protoxylem position and the shift of PIN3 domain away from the protoxylem-associated pericycle cells. These suggest that cytokinin signalling might regulate the expression of PINs in Arabidopsis vascular tissues. Furthermore, auxin signalling maximum is expanded throughout the vascular cylinder in the wol mutant. Taken together, cytokinin signalling restricts the auxin signalling maximum to the xylem axis by regulating the bisymmetric localization of PINs. Auxin then promotes the expression of AHP6 in the protoxylem precursor so that it can counteract cytokinin signalling and allow for cells to differentiate into protoxylem.
Other genetic studies also suggested that cytokinin is required to maintain the vascular stem cells by preventing differentiation process. When the cytokinin signalling was abolished via mutations in AHPs or type-B ARABIDOPSIS RESPONSE REGULATORs (B-ARRs), vascular stem cells underwent differentiation into xylem (Table I; Hutchison et al, 2006; Yokoyama et al, 2007; Argyros et al, 2008; Ishida et al, 2008). In mutants of ARR1, 10 and 12, or AHP 1, 2, 3, 4 and 5 all the vascular cells in Arabidopsis primary roots differentiated into xylem. Recently, exogenous application of CLE peptides was reported to inhibit protoxylem formation in Arabidopsis roots through a CLV2-dependent pathway (Kondo et al, 2011). These CLEs were found to enhance cytokinin signalling through the repression of A-ARRs, and thereby suppress the protoxylem cell fate.
Cytokinins are also required for promoting cell proliferation activities in the vascular stem cells during the secondary growth. When the cytokinin level was significantly reduced in the quadruple mutant of IPTs (ipt1 3 5 7), secondary growth did not occur (Matsumoto-Kitano et al, 2008). A similar approach was made by expressing cytokinin oxidase, an enzyme that breaks down an active form of cytokinin, in the vascular stem cells of Populus (Nieminen et al, 2008). The low level of cytokinin significantly reduced the secondary growth of Populus stems. Consistent with the effects of a cytokinin level on the vascular stem cell maintenance, perturbing the cytokinin signalling also affected the secondary growth of Arabidopsis stems. Mutating two cytokinin receptors, AHK2 and AHK3, caused significant reduction in the secondary growth of vascular tissues (Hejatko et al, 2009). Similar effects were also observed when modified CKI1, which has a mutation in Histidine residue, was ectopically expressed (Hejatko et al, 2009). CKI1 does not bind cytokinin in vitro, however, possesses the phospho-transfer activity-like AHKs. Mutation in Histidine residue of CKI1 perturbs phospho-transfer activity and results in phenotypes similar to cytokinin receptor mutants. This and other aforementioned studies suggest that balancing phospho-transfer activities in the cytokinin signalling plays a crucial role for controlling downstream regulators in the maintenance and activities of vascular stem cells.
Brassinosteroids
During xylogenesis in Zinnia, brassinosteroid (BR) promotes the formation of tracheary elements by triggering the secondary cell wall formation and cell death (Figure 7; Yamamoto et al, 1997). Consistent with its involvement in the differentiation of tracheary elements, an increase in the BR levels and expression of genes involved in BR biosynthesis was observed during in vitro xylogenesis (Yamamoto et al, 2001, 2007). When BR synthesis was inhibited by Brassinazole treatment, dramatic decrease in the secondary xylem proliferation was observed in Cress plants. This suggests that BR also regulates the cell proliferation activity of cambium (Nagata et al, 2001).
BR is perceived by BRI1, a membrane-localized LRR-RLK (Table I). There are three BRI1 homologues in Arabidopsis (BRL1–3) and one of them, VASCULAR HIGHWAY 1 (VH1) or BRL2, regulates the procambial cell formation in developing leaves (Clay and Nelson, 2002). However, this protein does not functionally substitute BRI1 or bind BR. By contrast, BRL1 and 3 bind to BR and complement bri1 when they are expressed under the BRI1 promoter. Unlike BRI1, which is expressed broadly in Arabidopsis, BRL1 and 3 are only expressed in the vascular tissues. When BRL1 was knocked out, phloem domain expanded at the expense of xylem. This suggests that BR positively regulates the xylem development (Cano-Delgado et al, 2004). BRI1, BRL1 and BRL3 seem to have diverged before the divergence of monocot and eudicot species. Putative orthologues of these three genes are present in rice and like in Arabidopsis, phloem formation was promoted in rice when BR signalling was perturbed (Nakamura et al, 2006).
In addition to being involved in xylem development, BR coordinates with auxin to spatially regulate the formation of vascular bundles (Ibañes et al, 2009). Mathematical modelling combined with mutant analyses suggests that auxin maxima generated by the auxin efflux transporters (PINs) may promote vascular bundle formation. However, it is the BR signalling pathway that seems to regulate the spacing of auxin maxima and vascular bundles, likely by influencing cell division activities.
Gibberellins
Analysis of the distribution of GAs in the main stems of Populus showed that GA levels are usually low in the vascular cambium but high in the region where xylem cells expand (Figure 7; Table I; Israelsson et al, 2005). Similar patterns were observed for the expression of GA 20-oxidase1 and GA 3-oxidase, genes that are involved in generating biologically active GAs. Consistent with these data, two GA responsive genes, DELLA-like1 and GIP-like1, were found highly induced in expanding xylem cells.
However, when GA levels were increased in transgenic Populus by ectopically expressing GA 20 oxidase, there was a significant increase in plant growth rate (Eriksson et al, 2000). These transgenic plants also exhibited an enhanced vascular stem cell activity and an increase in the xylem fibre number and length. Consistent with this study, an increase in cambial cell division activity was observed when GA was locally applied to the stems of other angiosperm tree species (Funada et al, 2008). Interestingly, xylem fibres generated under a high level of GA showed characteristics of the tension wood that is formed under the mechanical stress. Therefore, GAs might not be the main driver of cell division activities of vascular cambium under an unstressed condition, but might be under the mechanical stress. In a more recent study, GA was found to acts as a mobile signal that promotes xylem expansion in Arabidopsis hypocotyls (Ragni et al, 2011).
Jasmonate and ethylene
Jasmonate (JA) and its signalling are known to regulate several aspects of plant stress responses (Wasternack, 2007). During the establishment of interfascicular cambium, cells in that region undergo dramatic reorganization. A recent study suggests that genes in JA signalling pathways are actively involved in the formation and activity of interfascicular cambia (Sehr et al, 2010). In JA signalling pathway, JASMONATE ZIM-DOMAIN (JAZ) proteins act as repressor of JA signalling by directly binding to a key transcriptional regulator MYC2 (Table I; Chini et al, 2007; Thines et al, 2007). Once JA binds to its receptor, CORONATINE INSENSITIVE1 (COI1), JA-COI1 elicits the degradation of JAZ proteins, by which MYC2 is released from JAZ and becomes transcriptionally active. Among 12 genes that encode JAZ proteins in Arabidopsis, the expression of JAZ7 and 10 is induced by JA. Genome-wide expression profiling of stem segments undergoing interfascicular cambia formation showed upregulation of JAZ7 and 10 and several other genes in JA signalling pathway (Sehr et al, 2010). In the knockout mutant of JAZ10, the radial growth and interfascicular cambium formation in stems became more active than in the wild type. Consistently, the stem radial growth was reduced by about 25% when the COI1 or MYC2 was knocked out.
Similarly, ethylene, which is also involved in plant stress responses, plays an important role in vascular stem cell activities that forms tension wood, which has a high level of cellulose and a low level of lignins and microfibrils, in Populus (Love et al, 2009). When Populus was treated with ethylene, xylem growth significantly increased in comparison to non-treated plants. This increase in xylem growth was from the enhancement in the cambial cell division rate, not in the cell expansion. Transgenic plants that cannot sense ethylene properly failed to form tension wood in the leaning stem, suggesting that ethylene is a key signalling molecule that initiates tension wood formation in the cambium. In addition, transcriptional profiling indicated alterations in the touch-inducible ethylene signalling component ETHYLENE RESPONSE FACTOR 104 expression (Table I; Bethke et al, 2009). Further analysis of its knockout line revealed a decrease in the interfascicular cambium initiation in the acropetal direction. Taken together, these findings suggest active involvement of stress-related hormones such as JAand ethylene in the interfascicular cambium formation.
Conclusions and future perspectives
Extensive progress has been achieved in understanding the molecular mechanisms governing the formation and maintenance of vascular cambium during this decade (Figure 8). However, in comparison with apical meristems, our knowledge of the vascular cambium and its regulation are still very poor.
Figure 8.
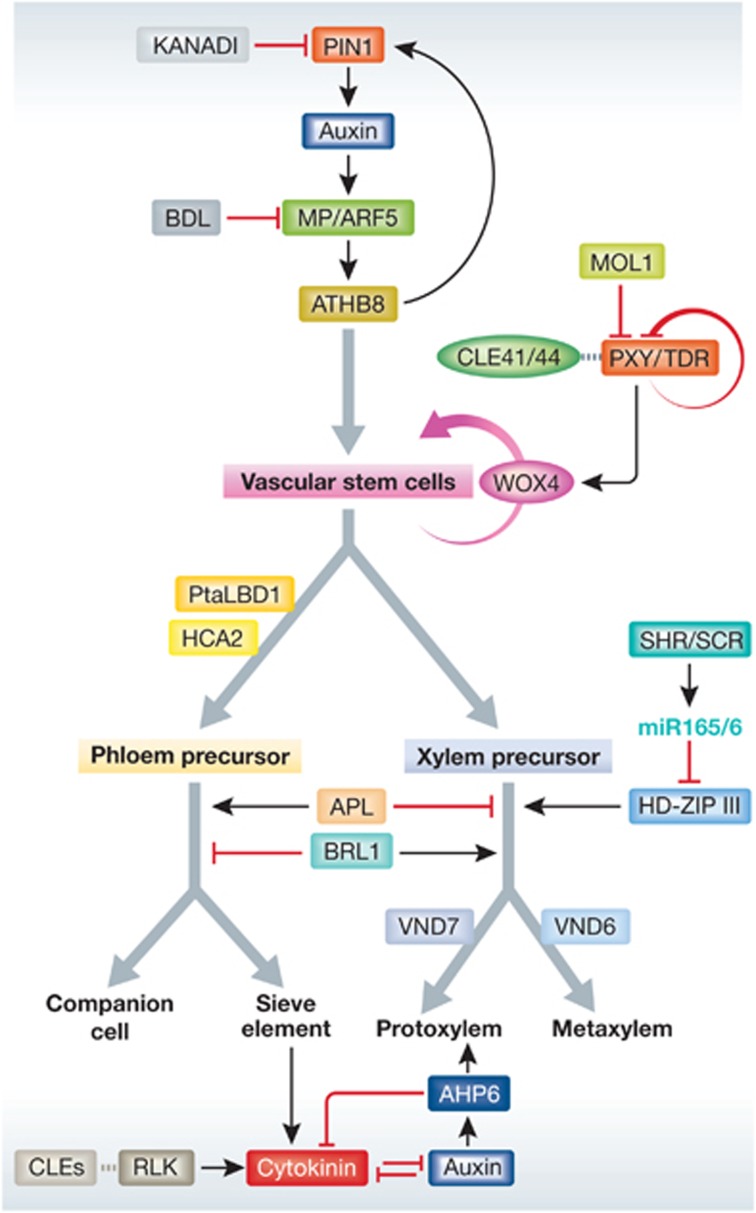
Regulatory networks in the control of vascular stem cell activities.
In Arabidopsis apical meristems, the position of stem cells and their niche has been determined with molecular markers that are specifically expressed in the stem cells or their niche cells. On the contrary, the position of the stem cells and accompanying niche cells in the cambium is still unclear. The main obstacle in answering these questions has been the lack of reliable molecular makers, which will enable us to define the stem cells and associated niche cells. A genome-wide expression analysis combined with technologies that can isolate an individual cell will aid in establishing a reliable gene expression map of the vascular cambium. In addition, such an approach will provide the marker genes that can differentiate the collectively called ‘daughter cells’ into cell identities with distinctive functions. Furthermore, a clonal analysis of the secondary cambium can aid in generating the precious cell lineage map, which permits defining the location and identity of the stem cells and the niche cells.
In Arabidopsis roots, the radially symmetric cambium is formed from the bisymmetrically organized procambial cells, indicating that the bisymmetric pattern is broken and radial symmetric pattern is established during the transition phase. Based on the anatomical analyses, the pericycle cells adjacent to protoxylem pole also seem to be recruited into cambial zone during transition phase, suggesting that the radially symmetric cambial tissue is derived from two distinct types of cells, procambial cells and pericycle cells. As described above, cytokinin is the key regulator of the initiation of the secondary vascular development. However, it is completely unknown how this symmetry conversion is regulated during the transition from primary to secondary development. A forward genetic screen with the appropriate gene marker will allow uncovering the molecular basis of this process.
Recent works started to reveal various transcription factors that regulate several aspects of vascular development including the cell proliferation and differentiation. However, little is known about how these transcription factors construct the regulatory networks during vascular development. The expression patterns of these transcription factors are tightly controlled; however, our knowledge about the upstream regulators of these transcription factors is limited. Recently, in an attempt to delineate the gene regulatory networks, comprehensive yeast one-hybrid and two-hybrid assays have been performed against a large number of transcription factors that are expressed in a tissue-specific manner in Arabidopsis stele (Brady et al, 2011). A similar approach will provide us with the vital information regarding the transcriptional networks in the secondary cambium.
Overall plant growth and development depends heavily on environmental factors. Different environmental and physiological conditions such as resource availability, temperature and stresses have a profound impact on the activities of vascular stem cells. Recently in Pinus radiata, it was reported that xylem transcriptome exhibits seasonal reorganizations in accordance with various physiological changes and tree age (Li et al, 2010). Vascular stem cell activity also shows plasticity in response to mechanical loads. In angiosperm trees, a local increase in cambial cell division in the leaning stems results in the formation of tension wood. Therefore, understanding how gene regulation and signaling in vascular stem cells is created and rewired in response to ever-changing environments is critical for predicting and maintaining plant growth and yields in the future.
Footnotes
The authors declare that they have no conflict of interest.
References
- Agusti J, Herold S, Schwarz M, Sanchez P, Ljung K, Dun EA, Brewer PB, Beveridge CA, Sieberer T, Sehr EM, Greb T (2011a) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci USA 108: 20242–20247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J, Lichtenberger R, Schwarz M, Nehlin L, Greb T (2011b) Characterization of transcriptome remodeling during cambium formation identifies MOL1 and RUL1 as opposing regulators of secondary growth. PLoS Genet 7: e1001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyros RD, Mathews DE, Chiang Y-H, Palmer CM, Thibault DM, Etheridge N, Argyros DA, Mason MG, Kieber JJ, Schaller GE (2008) Type B response regulators of arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell 20: 2102–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba K, Karlberg A, Schmidt J, Schrader J, Hvidsten TR, Bako L, Bhalerao RP (2011) Activity-dormancy transition in the cambial meristem involves stage-specific modulation of auxin response in hybrid aspen. Proc Natl Acad Sci USA 108: 3418–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, Ruberti I, Morelli G (2001) The Arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol 126: 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum SF, Dubrovsky JG, Rost TL (2002) Apical organization and maturation of the cortex and vascular cylinder in Arabidopsis thaliana (Brassicaceae) roots. Am J Bot 89: 908–920 [DOI] [PubMed] [Google Scholar]
- Berleth T, Jurgens G (1993) The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118: 575–587 [Google Scholar]
- Bethke G, Unthan T, Uhrig JF, Pöschl Y, Gust AA, Scheel D, Lee J (2009) Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc Natl Acad Sci USA 106: 8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN (2003) A gene expression map of the Arabidopsis root. Science 302: 1956–1960 [DOI] [PubMed] [Google Scholar]
- Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, Benková E, Mähönen Ari P, Helariutta Y (2011) A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr Biol 21: 917–926 [DOI] [PubMed] [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Bonke M, Thitamadee S, Mahonen AP, Hauser M-T, Helariutta Y (2003) APL regulates vascular tissue identity in Arabidopsis. Nature 426: 181–186 [DOI] [PubMed] [Google Scholar]
- Brady SM, Orlando DA, Lee J-Y, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN (2007) A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318: 801–806 [DOI] [PubMed] [Google Scholar]
- Brady SM, Zhang L, Megraw M, Martinez NJ, Jiang E, Yi CS, Liu W, Zeng A, Taylor-Teeples M, Kim D, Ahnert S, Ohler U, Ware D, Walhout AJM, Benfey PN (2011) A stele-enriched gene regulatory network in the Arabidopsis root. Mol Syst Biol 7: 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408: 967–971 [DOI] [PubMed] [Google Scholar]
- Cano-Delgado A, Yin Y, Yu C, Vafeados D, Mora-Garcia S, Cheng J-C, Nam KH, Li J, Chory J (2004) BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131: 5341–5351 [DOI] [PubMed] [Google Scholar]
- Carlsbecker A, Lee J-Y, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vatén A, Thitamadee S, Campilho A, Sebastian J, Bowman JL, Helariutta Y, Benfey PN (2010) Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffey N, Cholewa E, Regan S, Sundberg B (2002) Secondary xylem development in Arabidopsis: a model for wood formation. Physiol Plant 114: 594–600 [DOI] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, Garcia-Casado G, Lopez-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM (1997) The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89: 575–585 [DOI] [PubMed] [Google Scholar]
- Clay NK, Nelson T (2002) VH1, a provascular cell-specific receptor kinase that influences leaf cell patterns in Arabidopsis. Plant Cell 14: 2707–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Levesque MP, Vernoux T, Jung JW, Paquette AJ, Gallagher KL, Wang JY, Blilou I, Scheres B, Benfey PN (2007) An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 316: 421–425 [DOI] [PubMed] [Google Scholar]
- De Smet I, Vassileva V, De Rybel B, Levesque MP, Grunewald W, Van Damme D, Van Noorden G, Naudts M, Van Isterdael G, De Clercq R, Wang JY, Meuli N, Vanneste S, Friml J, Hilson P, Jürgens G, Ingram GC, Inzé D, Benfey PN, Beeckman T (2008) Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322: 594–597 [DOI] [PubMed] [Google Scholar]
- Demura T, Tashiro G, Horiguchi G, Kishimoto N, Kubo M, Matsuoka N, Minami A, Nagata-Hiwatashi M, Nakamura K, Okamura Y, Sassa N, Suzuki S, Yazaki J, Kikuchi S, Fukuda H (2002) Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells. Proc Natl Acad Sci USA 99: 15794–15799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyholos M, Cordner G, Beebe D, Sieburth L (2000) The SCARFACE gene is required for cotyledon and leaf vein patterning. Development 127: 3205–3213 [DOI] [PubMed] [Google Scholar]
- Donner TJ, Sherr I, Scarpella E (2009) Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 136: 3235–3246 [DOI] [PubMed] [Google Scholar]
- Du J, Mansfield SD, Groover AT (2009) The Populus homeobox gene ARBORKNOX2 regulates cell differentiation during secondary growth. Plant J 60: 1000–1014 [DOI] [PubMed] [Google Scholar]
- Eames AJ, MacDaniels LH (1947) An Introduction to Plant Anatomy 2nd edn. New York: McGraw-Hill, [Google Scholar]
- Elo A, Immanen J, Nieminen K, Helariutta Y (2009) Stem cell function during plant vascular development. Semin Cell Dev Biol 20: 1097–1106 [DOI] [PubMed] [Google Scholar]
- Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL (2003) Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol 13: 1768–1774 [DOI] [PubMed] [Google Scholar]
- Eriksson ME, Israelsson M, Olsson O, Moritz T (2000) Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat Biotech 18: 784–788 [DOI] [PubMed] [Google Scholar]
- Esau K (1965) Vascular Differentiation in Plants New York: Holt, Rinehart, and Winston, [Google Scholar]
- Etchells JP, Turner SR (2010) The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 137: 767–774 [DOI] [PubMed] [Google Scholar]
- Fisher K, Turner S (2007) PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr Biol 17: 1061–1066 [DOI] [PubMed] [Google Scholar]
- Fletcher JC, Brand U, Running MP, Rd Simon, Meyerowitz EM (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911–1914 [DOI] [PubMed] [Google Scholar]
- Floyd SK, Bowman J (2007) The ancestral developmental tool kit of land plants. Int J Plant Sci 168: 1–35 [Google Scholar]
- Floyd SK, Bowman JL (2004) Gene regulation: ancient microRNA target sequences in plants. Nature 428: 485–486 [DOI] [PubMed] [Google Scholar]
- Friml J (2003) Auxin transport -- shaping the plant. Curr Opin Plant Biol 6: 7–12 [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116: 769–778 [DOI] [PubMed] [Google Scholar]
- Funada R, Miura T, Shimizu Y, Kinase T, Nakaba S, Kubo T, Sano Y (2008) Gibberellin-induced formation of tension wood in angiosperm trees. Planta 227: 1409–1414 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Green KA, Prigge MJ, Katzman RB, Clark SE (2005) CORONA, a member of the class III homeodomain leucine zipper gene family in Arabidopsis, regulates stem cell specification and organogenesis. Plant Cell 17: 691–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groover A, Mansfield S, DiFazio S, Dupper G, Fontana J, Millar R, Wang Y (2006) The Populus homeobox gene ARBORKNOX1 reveals overlapping mechanisms regulating the shoot apical meristem and the vascular cambium. Plant Mol Biol 61: 917–932 [DOI] [PubMed] [Google Scholar]
- Guo M, Thomas J, Collins G, Timmermans MCP (2008) Direct repression of KNOX Loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell 20: 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Qin G, Gu H, Qu L-J (2009) Dof5.6/HCA2, a Dof transcription factor gene, regulates interfascicular cambium formation and vascular tissue development in Arabidopsis. Plant Cell 21: 3518–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejatko J, Ryu H, Kim G-T, Dobesova R, Choi S, Choi SM, Soucek P, Horak J, Pekarova B, Palme K, Brzobohaty B, Hwang I (2009) The Histidine Kinases CYTOKININ-INDEPENDENT1 and ARABIDOPSIS HISTIDINE KINASE2 and 3 regulate vascular tissue development in Arabidopsis shoots. Plant Cell 21: 2008–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Kondo Y, Fukuda H (2010a) Regulation of vascular development by CLE peptide-receptor systems. J Integr Plant Biol 52: 8–16 [DOI] [PubMed] [Google Scholar]
- Hirakawa Y, Kondo Y, Fukuda H (2010b) TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 22: 2618–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y, Shinohara H, Kondo Y, Inoue A, Nakanomyo I, Ogawa M, Sawa S, Ohashi-Ito K, Matsubayashi Y, Fukuda H (2008) Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc Natl Acad Sci USA 105: 15208–15213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-Y, Adams JP, Kim H, No K, Ma C, Strauss SH, Drnevich J, Vandervelde L, Ellis JD, Rice BM, Wickett N, Gunter LE, Tuskan GA, Brunner AM, Page GP, Barakat A, Carlson JE, dePamphilis CW, Luthe DS, Yuceer C (2011) FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc Natl Acad Sci USA 108: 10756–10761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, Maxwell BB, Perdue TD, Schaller GE, Alonso JM, Ecker JR, Kieber JJ (2006) The Arabidopsis Histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18: 3073–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañes M, Fàbregas N, Chory J, Caño-Delgado AI (2009) Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc Natl Acad Sci USA 106: 13630–13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilegems M, Vr Douet, Meylan-Bettex M, Uyttewaal M, Brand L, Bowman JL, Stieger PA (2010) Interplay of auxin, KANADI and Class III HD-ZIP transcription factors in vascular tissue formation. Development 137: 975–984 [DOI] [PubMed] [Google Scholar]
- Ishida K, Yamashino T, Yokoyama A, Mizuno T (2008) Three type-B response regulators, ARR1, ARR10 and ARR12, play essential but redundant roles in cytokinin signal transduction throughout the life cycle of Arabidopsis thaliana. Plant Cell Physiol 49: 47–57 [DOI] [PubMed] [Google Scholar]
- Israelsson M, Sundberg B, Moritz T (2005) Tissue-specific localization of gibberellins and expression of gibberellin-biosynthetic and signaling genes in wood-forming tissues in aspen. Plant J 44: 494–504 [DOI] [PubMed] [Google Scholar]
- Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H (2006) Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313: 842–845 [DOI] [PubMed] [Google Scholar]
- Iwakawa H, Ueno Y, Semiarti E, Onouchi H, Kojima S, Tsukaya H, Hasebe M, Soma T, Ikezaki M, Machida C, Machida Y (2002) The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana, required for formation of a symmetric flat leaf lamina, encodes a member of a novel family of proteins characterized by cysteine repeats and a Leucine Zipper. Plant Cell Physiol 43: 467–478 [DOI] [PubMed] [Google Scholar]
- Jenik PD, Gillmor CS, Lukowitz W (2007) Embryonic patterning in Arabidopsis thaliana. Annu Rev Cell Dev Biol 23: 207–236 [DOI] [PubMed] [Google Scholar]
- Jeong S, Trotochaud AE, Clark SE (1999) The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. Plant Cell 11: 1925–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Strable J, Shimizu R, Koenig D, Sinha N, Scanlon MJ (2010) WOX4 promotes procambial development. Plant Physiol 152: 1346–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K, Naramoto S, Sawa S, Yahara N, Ueda T, Nakano A, Sugiyama M, Fukuda H (2005) VAN3 ARF-GAP-mediated vesicle transport is involved in leaf vascular network formation. Development 132: 1699–1711 [DOI] [PubMed] [Google Scholar]
- Kondo Y, Hirakawa Y, Kieber JJ, Fukuda H (2011) CLE peptides can negatively regulate protoxylem vessel formation via cytokinin signaling. Plant Cell Physiol 52: 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM, Lewandowski M, Beri S, Bernard J, Borkowski M, Borkowski MH, Burchfield LA, Mathisen B, Normanly J (2008) Auxin gradients are associated with polarity changes in trees. Science 320: 1610. [DOI] [PubMed] [Google Scholar]
- Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 19: 1855–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S, Ehrismann JS, Schlereth A, Takada S, Mayer U, Jurgens G (2010) Cell-cell communication in Arabidopsis early embryogenesis. Eur J Cell Biol 89: 225–230 [DOI] [PubMed] [Google Scholar]
- Lau S, Slane D, Herud O, Kong J, Jürgens G (2012) Early embryogenesis in flowering plants: setting up the basic body pattern. Annu Rev Plant Biol 63: 483–506 [DOI] [PubMed] [Google Scholar]
- Laux T, Mayer KFX, Berger J, Jurgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122: 87–96 [DOI] [PubMed] [Google Scholar]
- Li X, Wu HX, Southerton SG (2010) Seasonal reorganization of the xylem transcriptome at different tree ages reveals novel insights into wood formation in Pinus radiata. New Phytol 187: 764–776 [DOI] [PubMed] [Google Scholar]
- Li Z, Li L (2006) Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci 31: 589–595 [DOI] [PubMed] [Google Scholar]
- Love J, Björklund S, Vahala J, Hertzberg M, Kangasjärvi J, Sundberg B (2009) Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc Natl Acad Sci USA 106: 5984–5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahonen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Tormakangas K, Ikeda Y, Oka A, Kakimoto T, Helariutta Y (2006a) Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 311: 94–98 [DOI] [PubMed] [Google Scholar]
- Mahonen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y (2000) A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev 14: 2938–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahonen AP, Higuchi M, Tormakangas K, Miyawaki K, Pischke MS, Sussman MR, Helariutta Y, Kakimoto T (2006b) Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Curr Biol 16: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Mansfield SG, Briarty LG (1991) Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can J Bot 69: 461–476 [Google Scholar]
- Mallory A, Reinhart B, Jones-Rhoades M, Tang G, Zamore P, Barton M, Bartel D (2004) MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5' region. EMBO J 23: 3356–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Václavíková K, Miyawaki K, Kakimoto T (2008) Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA 105: 20027–20031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriat M, Moritz T (2009) Analyses of GA20ox- and GID1-over-expressing aspen suggest that gibberellins play two distinct roles in wood formation. Plant J 58: 989–1003 [DOI] [PubMed] [Google Scholar]
- Mayer KFX, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815 [DOI] [PubMed] [Google Scholar]
- McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK (2001) Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411: 709–713 [DOI] [PubMed] [Google Scholar]
- Miyashima S, Koi S, Hashimoto T, Nakajima K (2011) Non-cell-autonomous microRNA165 acts in a dose-dependent manner to regulate multiple differentiation status in the Arabidopsis root. Development 138: 2303–2313 [DOI] [PubMed] [Google Scholar]
- Motose H, Sugiyama M, Fukuda H (2004) A proteoglycan mediates inductive interaction during plant vascular development. Nature 429: 873–878 [DOI] [PubMed] [Google Scholar]
- Moyle R, Schrader J, Stenberg A, Olsson O, Saxena S, Sandberg G, Bhalerao RP (2002) Environmental and auxin regulation of wood formation involves members of the Aux/IAA gene family in hybrid aspen. Plant J 31: 675–685 [DOI] [PubMed] [Google Scholar]
- Muller R, Bleckmann A, Simon R (2008) The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 20: 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N, Asami T, Yoshida S (2001) Brassinazole, an inhibitor of brassinosteroid biosynthesis, inhibits development of secondary xylem in cress plants (Lepidium sativum). Plant Cell Physiol 42: 1006–1011 [DOI] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN (2001) Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413: 307–311 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Fujioka S, Sunohara H, Kamiya N, Hong Z, Inukai Y, Miura K, Takatsuto S, Yoshida S, Ueguchi-Tanaka M, Hasegawa Y, Kitano H, Matsuoka M (2006) The role of OsBRI1 and Its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol 140: 580–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen K, Immanen J, Laxell M, Kauppinen L, Tarkowski P, Dolezal K, Tähtiharju S, Elo A, Decourteix M, Ljung K, Bhalerao R, Keinonen K, Albert VA, Helariutta Y (2008) Cytokinin signaling regulates cambial development in poplar. Proc Natl Acad Sci USA 105: 20032–20037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Karlberg A, Antti H, Lopez-Vernaza M, Mellerowicz E, Perrot-Rechenmann C, Sandberg G, Bhalerao RP (2008) Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 20: 843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodine MD, Yadegari R, Tax FE (2007) RPK1 and TOAD2 Are two receptor-like kinases redundantly required for Arabidopsis embryonic pattern formation. Dev Cell 12: 943–956 [DOI] [PubMed] [Google Scholar]
- Ogawa M, Shinohara H, Sakagami Y, Matsubayashi Y (2008) Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science 319: 294. [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Demura T, Fukuda H (2002) Promotion of transcript accumulation of novel zinnia immature xylem-specific HD-Zip III homeobox genes by brassinosteroids. Plant Cell Physiol 43: 1146–1153 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Fukuda H (2003) HD-Zip III homeobox genes that include a novel member, ZeHB-13 (Zinnia)/ATHB-15 (Arabidopsis), are involved in procambium and xylem cell differentiation. Plant Cell Physiol 44: 1350–1358 [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Fukuda H (2010) Transcriptional regulation of vascular cell fates. Curr Opin Plant Biol 13: 670–676 [DOI] [PubMed] [Google Scholar]
- Prigge MJ, Clark SE (2006) Evolution of the class III HD-Zip gene family in land plants. Evol Dev 8: 350–361 [DOI] [PubMed] [Google Scholar]
- Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE (2005) Class III Homeodomain-Leucine Zipper Gene Family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17: 61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni L, Nieminen K, Pacheco-Villalobos D, Sibout R, Schwechheimer C, Hardtke CS (2011) Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. Plant Cell 23: 1322–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D (2003) Vascular patterning: more than just auxin? Curr Biol 13: R485–R487 [DOI] [PubMed] [Google Scholar]
- Robischon M, Du J, Miura E, Groover A (2011) The Populus Class III HD ZIP, popREVOLUTA, influences cambium initiation and patterning of woody stems. Plant Physiol 155: 1214–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland-Lagan A-G, Prusinkiewicz P (2005) Reviewing models of auxin canalization in the context of leaf vein pattern formation in Arabidopsis. Plant J 44: 854–865 [DOI] [PubMed] [Google Scholar]
- Sablowski R (2011) Plant stem cell niches: from signalling to execution. Curr Opin Plant Biol 14: 4–9 [DOI] [PubMed] [Google Scholar]
- Sachs T (1981) The control of the patterned differentiation of vascular tissues. In Advances in Botanical Research Woolhouse HW (ed) Vol. 9: pp 151–262London: Academic Press, [Google Scholar]
- Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T (2007) Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446: 811–814 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Barkoulas M, Tsiantis M (2010) Control of Leaf and Vein Development by Auxin. Cold Spring Harb Perspect Biol 2: a001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml JÃ, Berleth T (2006) Control of leaf vascular patterning by polar auxin transport. Genes Dev 20: 1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B (2007) Stem-cell niches: nursery rhymes across kingdoms. Nat Rev Mol Cell Biol 8: 345–354 [DOI] [PubMed] [Google Scholar]
- Scheres B, Di Laurenzio L, Willemsen V, Hauser MT, Janmaat K, Weisbeek P, Benfey PN (1995) Mutations affecting the radial organisation of the Arabidopsis root display specific defects throughout the embryonic axis. Development 121: 53–62 [Google Scholar]
- Scheres B, Wolkenfelt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P (1994) Embryonic origin of the Arabidopsis primary root and root meristem initials. Development 120: 2475–2487 [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KFX, Jurgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644 [DOI] [PubMed] [Google Scholar]
- Schrader J, Baba K, May ST, Palme K, Bennett M, Bhalerao RP, Sandberg G (2003) Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc Natl Acad Sci USA 100: 10096–10101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J, Moyle R, Bhalerao R, Hertzberg M, Lundeberg J, Nilsson P, Bhalerao RP (2004a) Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. Plant J 40: 173–187 [DOI] [PubMed] [Google Scholar]
- Schrader J, Nilsson J, Mellerowicz E, Berglund A, Nilsson P, Hertzberg M, Sandberg G (2004b) A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell 16: 2278–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehr EM, Agusti J, Lehner R, Farmer EE, Schwarz M, Greb T (2010) Analysis of secondary growth in the Arabidopsis shoot reveals a positive role of jasmonate signalling in cambium formation. Plant J 63: 811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE, Muday GK, King EJ, Benton G, Kim S, Metcalf KE, Meyers L, Seamen E, Van Norman JM (2006) SCARFACE encodes an ARF-GAP that is required for normal auxin efflux and vein patterning in Arabidopsis. Plant Cell 18: 1396–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414: 98–104 [DOI] [PubMed] [Google Scholar]
- Stahl Y, Wink RH, Ingram GC, Simon R (2009) A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr Biol 19: 909–914 [DOI] [PubMed] [Google Scholar]
- Suer S, Agusti J, Sanchez P, Schwarz M, Greb T (2011) WOX4 imparts auxin responsiveness to cambium cells in Arabidopsis. Plant Cell 23: 3247–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Adler HT, Parks DW, Comai L (1995) The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development 121: 2723–2735 [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Truernit E, Bauby H, Belcram K, Barthelemy J, Palauqui JC (2012) OCTOPUS, a polarly localised membrane-associated protein, regulates phloem differentiation entry in Arabidopsis thaliana. Development 139: 1306–1315 [DOI] [PubMed] [Google Scholar]
- Tucker MR, Laux T (2007) Connecting the paths in plant stem cell regulation. Trends Cell Biol 17: 403–410 [DOI] [PubMed] [Google Scholar]
- Tuominen H, Puech L, Fink S, Sundberg B (1997) A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiol 115: 577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B (1996) Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci USA 93: 9282–9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbel AJE (1990) Xylem-phloem exchange via the rays - the undervalued route of transport. J Exp Bot 41: 631–644 [Google Scholar]
- Van den berg C, Willemsen V, Hage W, Weisbeek P, Scheres B (1995) Cell fate in the Arabidopsis root-meristem determined by directional signaling. Nature 378: 62–65 [DOI] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wiśniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml JÃ (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531 [DOI] [PubMed] [Google Scholar]
- Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel CL, Schuetz M, Yu Q, Mattsson J (2007) Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J 49: 387–398 [DOI] [PubMed] [Google Scholar]
- Whitford R, Fernandez A, De Groodt R, Ortega E, Hilson P (2008) Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proc Natl Acad Sci USA 105: 18625–18630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Perales M, Gruel J, Girke T, Jönsson H, Reddy GV (2011) WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes Dev 25: 2025–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Demura T, Fukuda H (1997) Brassinosteroids induce entry into the final stage of tracheary element differentiation in cultured Zinnia cells. Plant Cell Physiol 38: 980–983 [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Fujioka S, Demura T, Takatsuto S, Yoshida S, Fukuda H (2001) Brassinosteroid levels increase drastically prior to morphogenesis of tracheary elements. Plant Physiol 125: 556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Fujioka S, Iwamoto K, Demura T, Takatsuto S, Yoshida S, Fukuda H (2007) Co-regulation of brassinosteroid biosynthesis-related genes during xylem cell differentiation. Plant Cell Physiol 48: 74–83 [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Yamashino T, Amano Y-I, Tajima Y, Imamura A, Sakakibara H, Mizuno T (2007) Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol 48: 84–96 [DOI] [PubMed] [Google Scholar]
- Yordanov YS, Regan S, Busov V (2010) Members of the LATERAL ORGAN BOUNDARIES DOMAIN transcription factor family are involved in the regulation of secondary growth in Populus. Plant Cell 22: 3662–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Ye Z-H (1999) IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell 11: 2139–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]