Abstract
EMBO J (2013) 32 2, 219–230 doi:; DOI: 10.1038/emboj.2012.308; published online November 27 2012
The identification of distinct bone marrow microenvironments that support haematopoietic stem cell (HSC) survival and function is one of the most significant recent advances in our understanding of HSC biology. While the intricate organization of these structures and their effect on HSC is on its own a fascinating biological question, solving this puzzle remains a key step in the development of more efficient stem cell therapies through improved in vitro HSC culture systems. In this issue, a study conducted by Wang and colleagues describes a novel structure within the bone marrow and provides evidence for an additional layer of complexity and compartmentalisation within the bone marrow microenvironment, suggesting the presence of specialised structures that support clonal HSC expansion.
The bone marrow is a complex tissue, containing stem and progenitor cells for the haematopoietic, endothelial and osteogenic lineages. Surrounded by compact bone, the marrow is perfused by a complex network of vessels. Small arteries reach the marrow through the bone and branch into increasingly smaller arterioles. These in turn feed into intricate slow flowing sinusoidal capillaries, drained by venules, which eventually flow into a relatively large central sinus. The architecture of the bone marrow and the relationship between these structural building blocks and the haematopoietic stem cells (HSC) residing there is currently a topic of great interest. Specifically, an understanding of how elements of the endothelium, stromal cells and haematopoietic tissue interact to regulate steady state haematopoiesis is critical for the development and improvement of stem cell therapies based on HSC manipulation and administration.
The close connection between vascular tissue and bone development and remodelling have been documented and studied for a number of years (Wang et al, 2007). The initial discovery that functional alterations in osteoblastic cells in spongy bones had consequences on HSC function and number attracted the interest of researchers in the HSC field (Calvi et al, 2003; Visnjic et al, 2004; Zhang et al, 2003). A growing number of studies have subsequently implicated endothelial cells and bone marrow vasculature in the support of HSC survival and function. HSC were consistently found residing in the proximity of bone marrow vessels in histological studies (Kiel et al, 2005) and it is now clear that endothelial cells can be used to support HSC culture in vitro (reviewed in Butler et al (2010)). Moreover, manipulation of the vascular bone marrow compartment can affect haematopoiesis (Ding et al, 2012). It has been proposed by multiple groups that while the osteoblastic niche favours HSC quiescence and maintenance, perivascular niches are associated with HSC expansion (Li and Li, 2006).
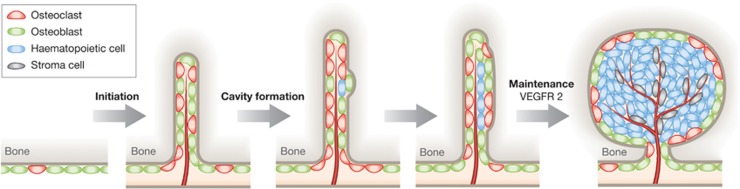
In this issue, a study conducted by Wang et al (2012) presents an in depth histological analysis of distinct bone marrow microenvironments identified using a combination of genetic labelling and traditional immunohistochemistry. Using this approach, they document a functional unit composed of vasculature, bone and HSC, which they name ‘haemosphere’. Structurally, the haemosphere is a cyst-like protrusion of sinusoidal epithelial cells encased by an inner shell of stroma cells and, finally, mineralized bone. The most interesting aspect of haemospheres is that single or multiple, tightly packed CD45+ haematopoietic cells are often found enclosed within this structure. (Figure 1 depicts the proposed stages of development of a haemosphere). Using in situ SLAM marker labelling and cell cycle analysis, the authors identify the cells within the haemosphere as clonal, highly proliferative pockets of haematopoietic cells enriched for primitive HSC, suggesting that this vascular structure has a particularly important role in HSC expansion. Additionally, in lethally irradiated mice, haemospheres were shown to preferentially host the engraftment of transplanted bone marrow cells, adding further weight to the idea that they play a specialised role in the support of stem cell proliferation and expansion. The studies described here provide another step towards dissecting the composition of bone marrow microenvironments and indicate a topological location for expanding HSC clones.
Figure 1.
Haemospheres likely initiate as an invagination of bone, mediated by bone remodelling around a vessel branch and eventually providing space for a growing clone of haematopoietic stem/progenitor cells. Larger cavities, such as the main femur diaphysis marrow space, allow mixing of haematopoietic clones. The maintenance of haemospheres depends on signalling through VEGF receptor 2.
Interestingly, haemospheres were shown to be dependent on vascular endothelial growth factor (VEGF) signalling, suggesting they may be dynamic structures. This is consistent with recent findings implicating a role for angiocrine factors released by endothelial cells in vivo in the replenishment of the HSC pool and the negative effect on reconstitution efficiency when VEGF2 signalling is inhibited (Hooper et al, 2009; Butler et al, 2010). The results presented here suggest an additional layer of complexity as they indicate that VEGFR2 might mediate significant vascular and bone remodelling, that could potentially generate new microenvironments supportive of HSC.
As with most novel findings, the work from Wang et al. opens a handful of new questions whose answer will considerably increase our understanding of the connection between HSC and bone marrow stroma. Most notably, which came first, haemosphere or HSC? And how plastic is this relationship? The studies here demonstrate that active remodelling of bone marrow microenvironments via VEGF signalling is necessary for haemosphere formation. However, it is still unclear how formation of these structures recruits and maintains HSC. Additionally, does the structure of the haemosphere create a unique combination of growth factors and ligands compared with other microenvironments that specifically supports HSC engraftment as well as homoeostatic proliferation? Conversely, could single HSC migrate to these perivascular sites and initiate remodelling of the local environment to their own advantage? And if this is the case, how does an HSC initiate such drastic remodelling and how can we use these factors to address clinical problems in stem cell transplantation therapy? Further studies are needed to obtain temporal information on the generation, maintenance and stability of haemospheres and to functionally compare haematopoiesis in haemospheres and in diaphyseal marrow. It is possible that the two locations may be functionally identical even though anatomically different, with the physical constraint of the encasing bone forcing haematopoietic clones to remain cohesive within haemospheres, while clones outside this environment can readily mix in the central, more fluid marrow.
The findings presented by Wang et al. challenge current paradigms of the plasticity of the niche and its composition. Technological and experimental advances allowing full bone imaging and isolation of live cells from specific bone regions will be critical to obtain a complete picture of the function and dynamics of anatomically distinct bone marrow microenvironment and their relationship with HSC. In the mean time, the haemospheres are a new type of bone marrow structure that deserves further attention and guarantees further insights in our understanding of the HSC niche.
References
- Butler JM, Kobayashi H, Rafii S (2010) Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer 10: 138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, Witte L, May C, Shawber C, Kimura Y, Kitajewski J, Rosenwaks Z, Bernstein ID, Rafii S (2010) Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6: 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846 [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, James D, Witte L, Zhu Z, Wu Y, Pytowski B, Rosenwaks Z, Mittal V, Sato TN, Rafii S (2009) Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4: 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109–1121 [DOI] [PubMed] [Google Scholar]
- Li Z, Li L (2006) Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci 31: 589–595 [DOI] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL (2004) Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103: 3258–3264 [DOI] [PubMed] [Google Scholar]
- Wang L, Benedito R, Bixel MG, Zeuschner D, Stehling M, Sävendahl L, Haigh JJ, Snippert H, Clevers H, Breier G, Kiefer F, Adams RH (2012) Identification of a clonally expanding haematopoietic compartment in bone marrow. EMBO J 32: 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL (2007) The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117: 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836–841 [DOI] [PubMed] [Google Scholar]