Abstract
EMBO J (2013) 32 2, 204–218 doi:; DOI: 10.1038/emboj.2012.302; published online November 30 2012
Eukaryotic cells critically rely on a constant and intensive exchange of macromolecules between the cytoplasm and nucleoplasm across the barrier of the nuclear envelope. Nuclear pore complexes (NPCs) function as gates to mediate this transport, but why they grant the selective passage of transport receptor–cargo complexes and at the same time exclude most other cellular macromolecules is incompletely understood. In this issue of The EMBO Journal, Labokha et al (2012) extend our view on how certain NPC proteins form a sieve-like meshwork within the pore that is crucial for these functions.
Nuclear pore complexes (NPCs), the gatekeepers of the nuclear envelope, perform a dual role. They allow for an active and regulated transport of proteins and nucleic acids across the nuclear envelope and at the same time hamper the passage of inert macromolecules. By that they exclude non-nuclear proteins from the nucleoplasm and restrict the backflow of transported substrates.
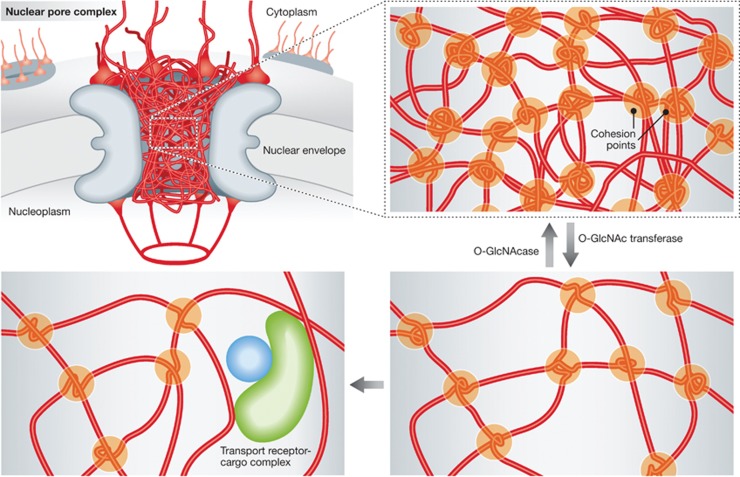
NPCs are formed by multiple copies of about 30 different proteins named nucleoporins. One third of them have been recognized early on as peculiar as they contain up to 50 repetitive sequences of phenylalanine-glycine (FG) (Starr and Hanover, 1991). These FG-motifs are part of large intrinsically disordered domains (Denning et al, 2003) that crowd the central cavity of the pore but also extend to the periphery of the NPC. They function as binding sites for transport receptors, which guide cargos through the NPC. Concurrently, they are important for the exclusion properties of the pore. How they fulfil the second task is still a matter of debate. It has been proposed that the FG-repeat domains behave like brushes limiting space within the pore or repel other macromolecules. According to one model, the binding of transport receptors to FG-repeats funnels the transport receptor–cargo complexes through this bottleneck (Rout et al, 2003). Alternatively, it was proposed to push away (Macara, 2001) or collapse (Lim et al, 2006) the FG brushes freeing the passage through the NPC. All these models predict that FG-repeat regions of nucleoporins repel each other and such a behaviour has been indeed observed for half of FG-repeat containing nucleoporins in S. cerevisiae. However, in the other half, FG-repeat domains rather glue together. This sticky behaviour is a key feature of an alternative hypothesis, the selective phase model (Ribbeck and Gorlich, 2001). It predicts that by multivalent intra- and intermolecular interaction cohesive FG-repeat regions form a sieve-like hydrogel. Binding of transport receptors to FG-motifs replaces FG–FG contacts and locally melts the gel enabling passage of the transport receptor–cargo complex through the pore (Figure 1).
Figure 1.
FG-nucleoporins (red) establish a sieve-like meshwork in the central channel of the pore by multivalent intra- and intermolecular FG-motif-mediated interactions. O-GlcNAc transferase catalysed addition N-acetyl-glycosylation of certain FG-repeat regions weakens these interactions and might be dynamically counteracted by O-GlcNAcase-mediated removal of this modification. Transport receptor binding to FG-motifs competes with FG–FG interactions and locally melts the meshwork allowing the passage of transport receptor–cargo complexes.
Indeed, FG-repeat domains of certain yeast nucleoporins jell in high concentrations in vitro (Frey et al, 2006). These hydrogels exclude inert proteins but strongly enhance their passage if bound to transport receptors (Frey and Gorlich, 2007). Therefore, they could function as a selective barrier in the NPC. However, it remains open how non-cohesive FG-nucleoporins contribute to the transport and exclusion properties of the pore and whether a combination of both brush-like and gel-like features of FG-nucleoporins are crucial for NPC function.
By systematically analysing FG-nucleoporins in Xenopus, Labokha et al (2012) find now that all of them are cohesive and can form hydrogels in vitro. Notably, arginine–glutamine-rich spacer regions between FG-repeats, which contribute to the interaction by forming interchain β-sheets in cohesive yeast FG-nucleoporins (Ader et al, 2010), are replaced by significantly more hydrophobic sequence elements in Xenopus. This indicates that FG-hydrogels can assemble through diverse structural principles, but nevertheless acquire comparable exclusion and transport properties.
Thus, also vertebrate FG-nucleoporins are cohesive and conceivably form a sieve-like meshwork within the NPC preventing uncontrolled passage. However, there might be a trade-off between the strength of interactions that define the tightness of the meshwork and its capability to be locally resolved by transport receptors. Indeed, hydrogels formed in vitro by the nucleoporin Nup98 show efficient exclusion of inert substances but are so tight that they allow only a very slow passage of transport receptor–cargo complexes. Interestingly, a post-translational sugar modification, the addition of N-acetylated glucose to serine and threonine residues of a subset of FG-nucleoporins (Hanover et al, 1987), might be important to fine tune the cohesiveness of these proteins. N-acetyl-glycosylation reduces the cohesiveness of the FG-repeat domains and thereby increases the transport rate of transport receptor–cargo complexes (Figure 1). As most post-translational modifications, N-acetyl-glycosylation is reversible and might therefore offer a possibility for the cell to dynamically regulate the permeability of the pore. Although it is purely speculative at the moment, it will be interesting to see whether this is important as a general cellular response to physiological demands or could even adjust transport capabilities of individual NPCs if large cargos such as ribosomal subunits need to pass.
Whereas in vitro the biophysical properties have mostly been studied on FG-repeat domains from single nucleoporins, the transport and exclusion properties of NPCs are defined by the combination of FG-repeats derived from more than 10 different nucleoporins. Indeed, composite hydrogels of FG-repeat domains of three nucleoporins interacting in the central cavity of the NPC show a better selectivity than hydrogels formed from the individual nucleoporins and within the pore composite hydrogels are presumably prevalent. As different FG-nucleoporins are anchored at distinct loci within the NPC, the meshwork properties may not be uniform within the pore and it will be interesting to see whether local differences in the hydrogel properties define specific pathways for certain substrate classes or could fulfil specialized functions.
Notably, such a specialization is found on the cytoplasmic side of NPCs. Hydrogels formed by the nucleoporins localized there, Nup358 and Nup214, show a surprisingly low diffusion rate for nuclear export complexes formed by the export factor crm1, GTP bound ran and the export cargo. At this site of the NPC, cofactors of ran stimulate GTP hydrolysis, which dissociates the export complex and renders the transport event irreversible. Stalling here might prevent backflow of the export complex until GTP hydrolysis has occurred. It is currently unclear what specifies the FG-repeat domain of Nup358 and Nup214 for this function, but it will be an interesting direction for future research.
Footnotes
The author declares that he has no conflict of interest.
References
- Ader C, Frey S, Maas W, Schmidt HB, Gorlich D, Baldus M (2010) Amyloid-like interactions within nucleoporin FG hydrogels. Proc Natl Acad Sci USA 107: 6281–6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning DP, Patel SS, Uversky V, Fink AL, Rexach M (2003) Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc Natl Acad Sci USA 100: 2450–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Gorlich D (2007) A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 130: 512–523 [DOI] [PubMed] [Google Scholar]
- Frey S, Richter RP, Gorlich D (2006) FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314: 815–817 [DOI] [PubMed] [Google Scholar]
- Hanover JA, Cohen CK, Willingham MC, Park MK (1987) O-linked N-acetylglucosamine is attached to proteins of the nuclear pore. Evidence for cytoplasmic and nucleoplasmic glycoproteins. J Biol Chem 262: 9887–9894 [PubMed] [Google Scholar]
- Labokha A A, Gradmann S, Frey S, Hülsmann BB, Urlaub H, Baldus M, Görlich D (2012) Systematic analysis of barrier-forming FG hydrogels from Xenopus nuclear pore complexes. EMBO J 32: 204–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RY, Huang NP, Koser J, Deng J, Lau KH, Schwarz-Herion K, Fahrenkrog B, Aebi U (2006) Flexible phenylalanine-glycine nucleoporins as entropic barriers to nucleocytoplasmic transport. Proc Natl Acad Sci USA 103: 9512–9517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara IG (2001) Transport into and out of the nucleus. Microbiol Mol Biol Rev 65: 570–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck K, Gorlich D (2001) Kinetic analysis of translocation through nuclear pore complexes. EMBO J 20: 1320–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout MP, Aitchison JD, Magnasco MO, Chait BT (2003) Virtual gating and nuclear transport: the hole picture. Trends Cell Biol 13: 622–628 [DOI] [PubMed] [Google Scholar]
- Starr CM, Hanover JA (1991) A common structural motif in nuclear pore proteins (nucleoporins). Bioessays 13: 145–146 [DOI] [PubMed] [Google Scholar]