Abstract
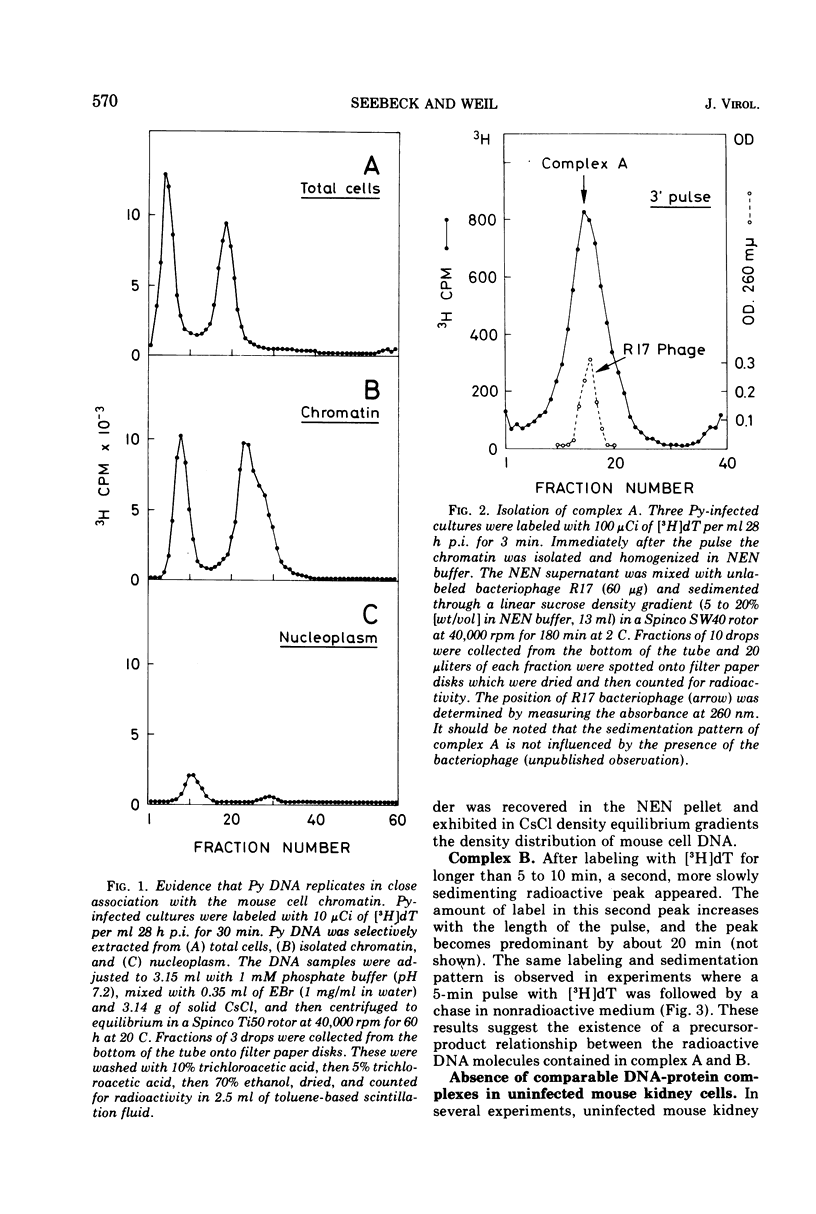
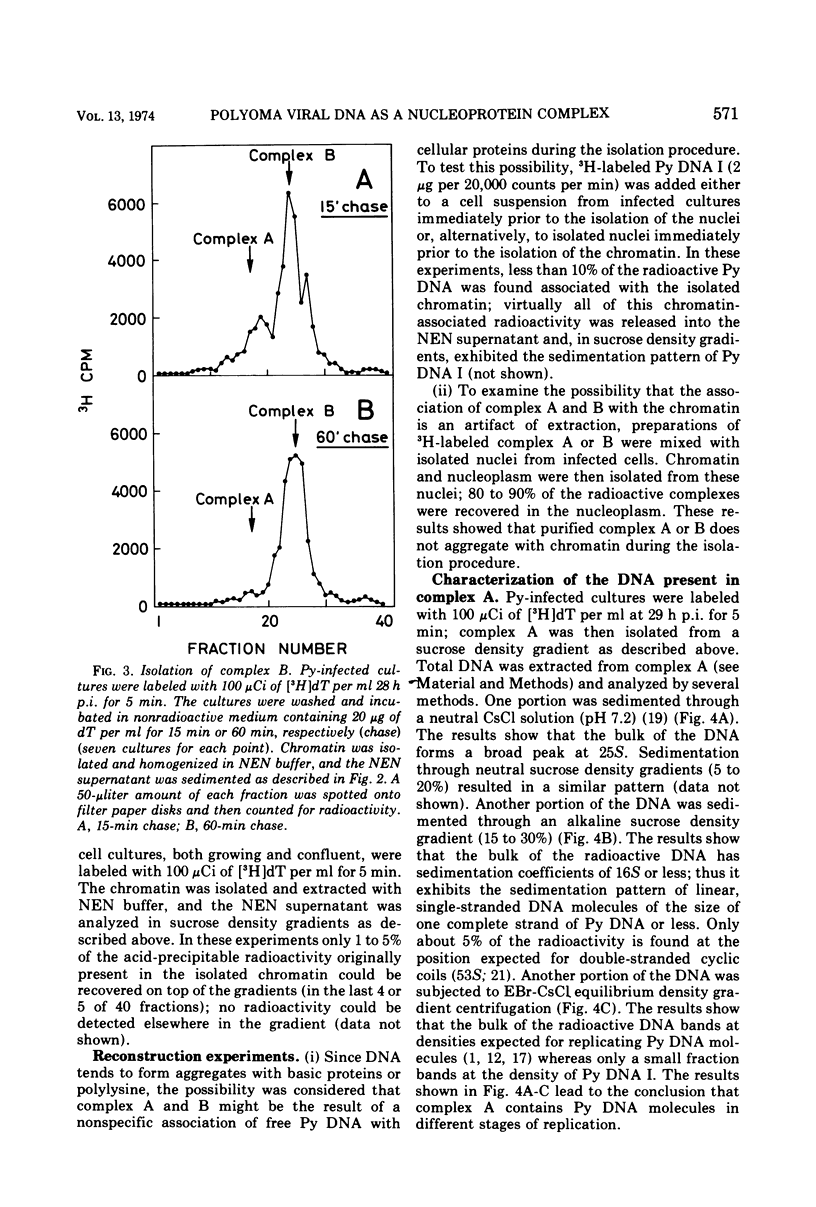
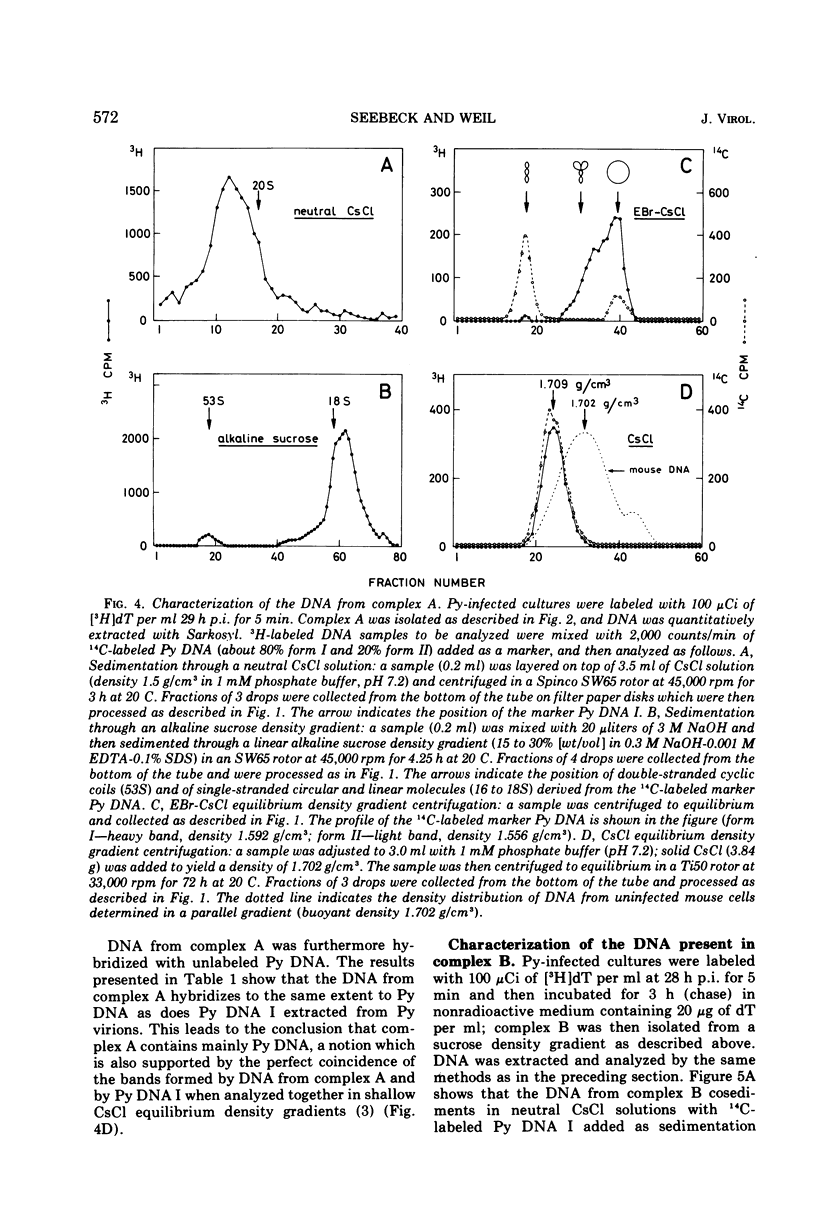
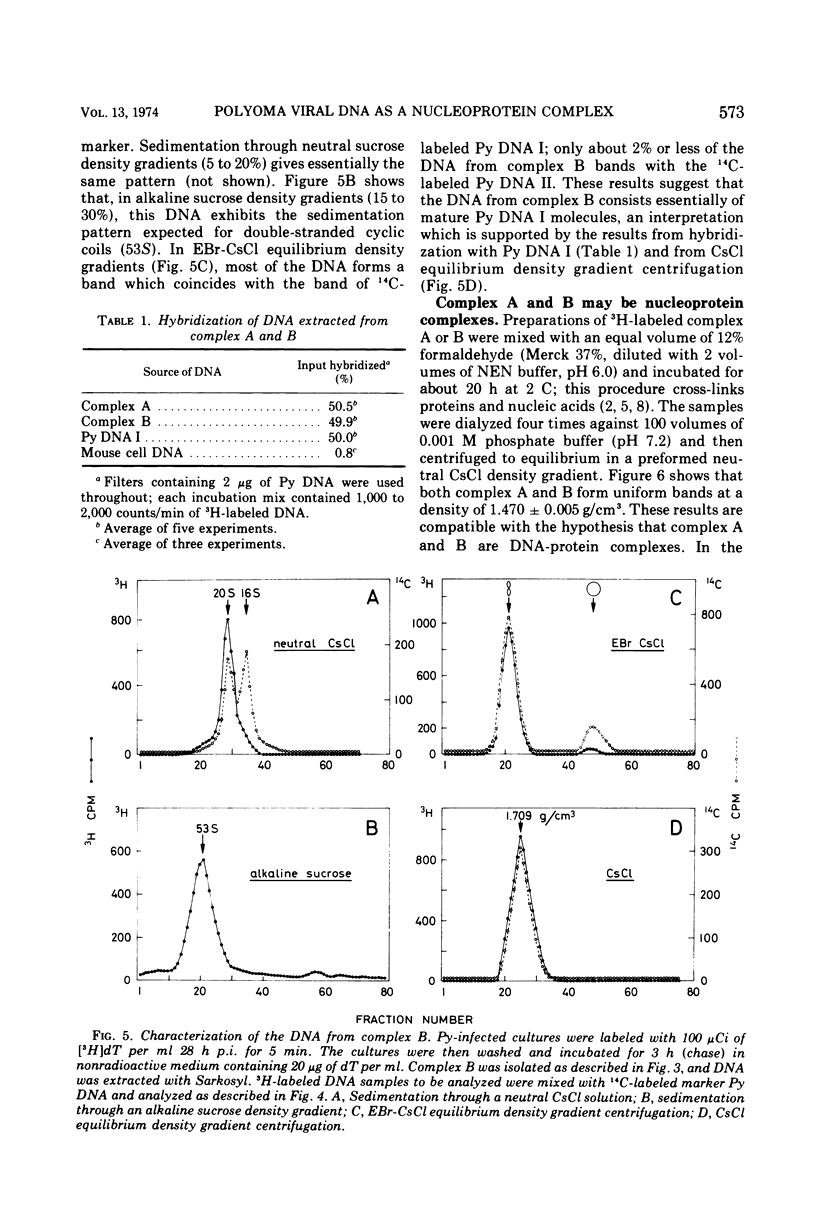
Polyoma viral DNA is shown to be replicated in close association with the mouse cell chromatin. Two virus-specific nucleoprotein complexes, designated complex A and B, can be dissociated from the isolated chromatin by gentle homogenization in 0.5 M NaCl. Complex A contains only replicating polyoma (Py) DNA whereas complex B contains only mature Py DNA I. The results show, furthermore, that complex A, containing viral DNA in different stages of replication, and complex B are both nucleoproteins with the same buoyant density. The data presently available suggest that newly synthesized stretches of Py DNA are immediately complexed with mouse cell histones and that complex B becomes the “core” of progeny Py virions. These results suggested that Py-induced replication of the mouse cell chromatin may be necessary to provide replicating Py DNA with histones.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgaux P., Bourgaux-Ramoisy D. Unwinding of replicating polyoma virus DNA. J Mol Biol. 1972 Oct 14;70(3):399–413. doi: 10.1016/0022-2836(72)90548-7. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Schlehuber C., Bonner J. Properties of formaldehyde-treated nucleohistone. Biochemistry. 1969 Aug;8(8):3214–3218. doi: 10.1021/bi00836a013. [DOI] [PubMed] [Google Scholar]
- Friedmann T., David D. Structural roles of polyoma virus proteins. J Virol. 1972 Oct;10(4):776–782. doi: 10.1128/jvi.10.4.776-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- Green M. H., Miller H. I., Hendler S. Isolation of a polyoma-nucleoprotein complex from infected mouse-cell cultures. Proc Natl Acad Sci U S A. 1971 May;68(5):1032–1036. doi: 10.1073/pnas.68.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. Conservation of histones in chromatin during growth and mitosis in vitro. J Mol Biol. 1969 Mar 28;40(3):457–466. doi: 10.1016/0022-2836(69)90165-x. [DOI] [PubMed] [Google Scholar]
- Hancock R., Weil R. Biochemical evidence for induction by polyoma virus of replication of the chromosomes of mouse kidney cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1144–1150. doi: 10.1073/pnas.63.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ilyin Y. V., Georgiev G. P. Heterogeneity of deoxynucleoprotein particles as evidencec by ultracentrifugation of cesium chloride density gradient. J Mol Biol. 1969 Apr;41(2):299–303. doi: 10.1016/0022-2836(69)90395-7. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Mayer A., Levine A. Replicating SV40 molecules containing closed circular template DNA strands. Nat New Biol. 1971 Sep 15;233(37):72–75. doi: 10.1038/newbio233072a0. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Leidner J., Tremblay G. Y. DNA-DNA hybridization on filters at low temperature in the presence of formamide or urea. Biochimie. 1971;53(10):1111–1114. doi: 10.1016/s0300-9084(71)80201-8. [DOI] [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancock R. Separation by equilibrium centrifugation in CsC1 gradients of density--labelled and normal deoxyribonucleoprotein from chromatin. J Mol Biol. 1970 Mar 14;48(2):357–360. doi: 10.1016/0022-2836(70)90167-1. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin R., Härle E., Dulbecco R. Polyoma virus proteins. 1. Multiple virion components. Virology. 1971 Sep;45(3):555–566. doi: 10.1016/0042-6822(71)90171-1. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Replication of phage lambda DNA. Cold Spring Harb Symp Quant Biol. 1968;33:533–551. doi: 10.1101/sqb.1968.033.01.061. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Weil R., Kára J. Polyoma "tumor antigen": an activator of chromosome replication? Proc Natl Acad Sci U S A. 1970 Oct;67(2):1011–1017. doi: 10.1073/pnas.67.2.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M., Eason R. Nucleoprotein complexes in simian virus 40-infected cells. J Virol. 1971 Oct;8(4):363–371. doi: 10.1128/jvi.8.4.363-371.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Robbins E. Histone synthesis in polyoma- and SV40-infected cells. Virology. 1970 Feb;40(2):307–315. doi: 10.1016/0042-6822(70)90406-x. [DOI] [PubMed] [Google Scholar]



