Abstract
Background
The presumed benefits of centralization and minimum case numbers often guide health-policy decisions, but these benefits remain inadequately documented, particularly in oncology. In this study, we aim to measure the effect of the type of treatment center and/or the number of patients treated in it on the outcome of patients with Hodgkin’s lymphoma.
Methods
From 1988 to 2002, 8121 patients with newly diagnosed Hodgkin’s lymphoma were treated in Germany in multicenter randomized and controlled trials (RCTs) of the German Hodgkin Study Group (GHSG). Center-related effects on progression-free survival (PFS) were assessed univariately with Kaplan-Meier plots and log-rank tests, as well as with a multivariate Cox regression model.
Results
The 500 participating centers in Germany included 52 university hospitals, 304 non-university hospitals, and 144 medical practices specializing in hematology and oncology. No significant differences in PFS were found between patients from centers with high or low case numbers (5-year-PFS: 78.7% and 78.6% for centers with fewer than 50 and more than 50 patients, respectively) or from different types of centers [5-year-PFS: university hospital, 77.7%; non-university hospital, 79.4%; practice, 79.8%]. Even after statistical controls for the effect of other known and unknown prognostic factors and validation in further datasets, no center effects were found.
Conclusions
The type of center and the minimum number of patients treated in a center have no impact on the treatment outcome of patients with Hodgkin’s lymphoma in Germany. In all GHSG centers, regardless of type, the quality standards for successful treatment are apparently met on all levels of patient care.
The past few decades have seen considerable improvement in the treatment of Hogdkin’s lymphoma, with a corresponding improvement in outcomes: this disease now ranks among the more curable types of human cancer. Most patients now achieve long-term tumor-free survival (1, 2). In view of the low incidence of the disease (ca. 2–3 cases per 100 000 patients per year), this success is due in large part to the effective planning and performance of multicenter randomized and controlled trials (RCTs) to compare different chemo- and radiotherapeutic strategies against one another for safety and efficacy depending on the stage of disease. The German Hodgkin Study Group (GHSG; www.ghsg.org) has devoted itself to the improvement of diagnosis, treatment, and aftercare for patients with Hodgkin’s disease for more than 25 years. It has treated more than 15 000 patients in randomized trials and has received national and international recognition for its work in furthering the development of primary treatment (3– 11). Patients are treated in more than 500 centers of varying size, including university hospitals, non-university hospitals, and medical practices specializing in hematology and oncology.
Now that organized medicine is increasingly subject to economic controls and regulation, so-called center effects have become a topic of interest for research. The variables studied for possible effects on outcome include characteristicss of the treating physician, such as personal experience and qualifications, and characteristics of the treatment center itself, such as size, equipment, and caseload. A major question is whether the type and size of the treatment center or the number of patients treated there with a particular disease has any correlation with the quality of care. Center effects and minimum caseloads have long been an object of study in surgical disciplines and in the care of premature neonates, but data of this type are scarce in oncology.
The aim of this study is to identify any effect of the type of treating oncological center, or of the number of patients treated, on treatment outcomes in Hodgkin’s disease. This research project, including a predefined plan for statistical analysis, was financially supported by German Cancer Aid (Deutsche Krebshilfe e.V.), reg. no. 109273.
Methods
9150 patients with Hodgkin’s lymphoma were treated in three successive generations of clinical trials (G2, G3, and G4) under the auspices of the GHSG from 1988 to 2002. The final evaluations of these trials, after a median follow-up interval of 6.7 years, provided the database for the current study (Table 1). The evaluations of later generations of studies have not yet been completed, because not enough time has yet elapsed for follow-up and because the event numbers are not yet high enough. Depending on the stage of disease and the known risk factors at initial diagnosis, the patients were classified as having early, intermediate, or advanced disease and were given treatment in trials HD4 through HD12. Upon inclusion in the trials, they were randomized and treated accordingly. For a patient to qualify for inclusion in a trial, certain criteria had to be fulfilled regardless of the type of center, including written informed consent, histological confirmation of the diagnosis by biopsy, complete staging, adequate organ function, and the absence of exclusion criteria that would hinder treatment according to the protocol. Treatment usually consisted of combination chemotherapy followed by radiotherapy. The treatment strategies of trials HD4 to HD12 and the results of their final evaluations were described in detail in the relevant publications for each (3– 11).
Table 1. Database for this study.
| Recruitment years | Generation | Study | Stage | Reference |
| 1988–1994 | G2 | HD4 | early | (3) Dühmke E; JCO 2001 |
| HD5 | intermediate | (4) Sieber M; JCO 2002 | ||
| HD6 | advanced | (5) Sieber M; Ann Oncol 2004 | ||
| 1994–1998 | G3 | HD7 | early | (6) Engert A; JCO 2007 |
| HD8 | intermediate | (7) Engert A; JCO 2003 | ||
| HD9 | advanced | (8) Diehl V; NEJM 2003 | ||
| 1998–2002 | G4 | HD10 | early | (9) Engert A, NEJM 2010 |
| HD11 | intermediate | (10) Eich HT; JCO 2010 | ||
| HD12 | advanced | (11) Borchmann P; JCO 2011 |
Database of 9150 patients with a new diagnosis of Hodgkin’s lymphoma who were treated in three generations of prospective, randiomized clinical trials of theGerman Hodgkin Study Group. GHSG, German Hodgkin Study Group; HD, Hodgkin’s disease; G2, 2 nd trial generation; G3, 3 rd trial generation; G4, 4 th trial generation; Ann Oncol, Annals of Oncology; JCO, Journal of Clinical Oncology; NEJM, New England Journal of Medicine
The 9150 patients treated in RCTs included 8121 who were treated in German centers and 1029 who were treated in participating centers in other countries. Information on the type, size, site, and caseload of each center were available from the GHSG database, as were data on patient characteristics, treatments, response rates, recurrence rates, causes of death, and aftercare.
Three collectives were created, in order to:
develop an analytical model for the study and testing of center effects,
validate the findings in the same population, and
check whether the results could be confirmed in a subsequent generation of clinical trials that involved different treatments and that were carried out under different societal conditions.
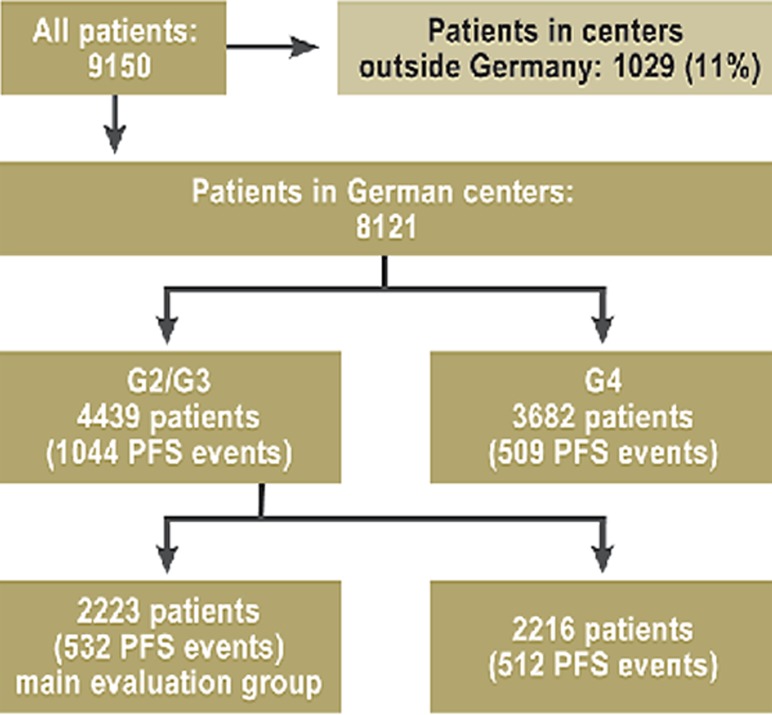
Moreover, the patients treated in the second and third trial generations (G2 and G3) were randomly divided into two groups and served as the main collective:
for the initial testing of center effects (2223 patients, 532 with progression-free survival [PFS events] and the collective);
for validation (2216 patients, 512 PFS events).
For a second validation under altered therapeutic conditions, patients from trial generation G4 were analyzed (3682 patients, 509 PFS events). The events in this collective were used to test the generalizability and stability of the findings under altered treatment conditions (Figure 1).
Figure 1.
The data sets that were analyzed.
G2, 2nd trial
generation;
G3, 3rd trial
generation;
G4, 4th trial
generation;
PFS, progression-free survival.
The large number of PFS events in the collectives (more than 500 in each) made it possible to include a large number of predictive factors in the Cox regression model used for the main analysis.
The primary endpoint, progression-free survival (PFS), is the most commonly applied measure of therapeutic success in Hodgkin’s disease. PFS is calculated from the day of randomization and is defined as the elapsed time to the first occurrence of any critical event (progression, recurrence, or death from any cause). Patients who sustained no critical events at all were censored after the date of the last available information about their tumor status. The following variables for centers were analyzed:
caseload (the number of patients recruited),
type of center (university hospital, non-university hospital, or hematology-oncology practice), and
a center effect for unobserved heterogeneity in a shared-frailty model.
The center variables were tested in Cox regression models with adjustment for relevant covariates at the 5% significance level.
Results
The 500 participating German centers included 52 university hospitals, 304 non-university hospitals, and 144 hematology-oncology practices, corresponding to 10%, 61%, and 29% of centers, respectively. These German centers recruited 8121 patients for the GHSG trials; of this total, the three types of centers recruited 3412 (42%), 3842 (47%), and 867 (11%) patients, respectively.
A secular trend was found across trial generations, in which a diminishing percentage of patients were treated in university hospitals (G2: 58%, G3: 41%, G4: 36%), while rising percentages were treated in non-university hospitals (G2: 32%, G3: 52%, G4: 50%) and hematology-oncology practices (G2: 6%, G3: 8%, G4: 15%). The median and range of the number of patients treated per center was 50 (1–247) for the overall collective, 103 (1–247) for university hospitals, 33 (1–117) for non-university hospitals, and 12 (1–47) for practices.
The characteristics of patients in the main analyzed group (2223 patients) are shown in Table 2, broken down both by center caseload (< or ≥ 50 patients from 1988 to 2002) and by type of center (university hospital, non-university hospital, practice). The chosen cutoff value of 50 patients was the median for center size. Not all demographic variables were evenly distributed. In centers with a lower caseload (fewer than 50 patients from 1988 to 2002), the patients tended to be older; such centers treated more patients in earlier stages of disease, and they also treated relatively fewer patients in the second generation of trials and relatively more in the third. Moreover, hematology-oncology practices treated more patients in early stages of disease than did university or non-university hospitals.
Table 2. Patient characteristics by caseload and type of center.
| Patient characteristics | Caseload < 50 patients (870 pts. total) | Caseload ≥ 50 patients (1353 pts. total) | University hospitals (1060 patients) | Other hospitals (1000 patients) | Practices (163 patients) | |
| Sex (percent female) | 43% | 44% | 41% | 47% | 39% | |
| Age (median) | 38 years | 35 years | 35 years | 37 years | 36 years | |
| Stage | ||||||
| early | 22% | 17% | 18% | 20% | 26% | |
| intermediate | 38% | 43% | 43% | 39% | 40% | |
| advanced | 39% | 39% | 38% | 41% | 34% | |
| B symptoms | 39% | 41% | 40% | 41% | 36% | |
| Generation G2 | 26% | 47% | 48% | 31% | 34% | |
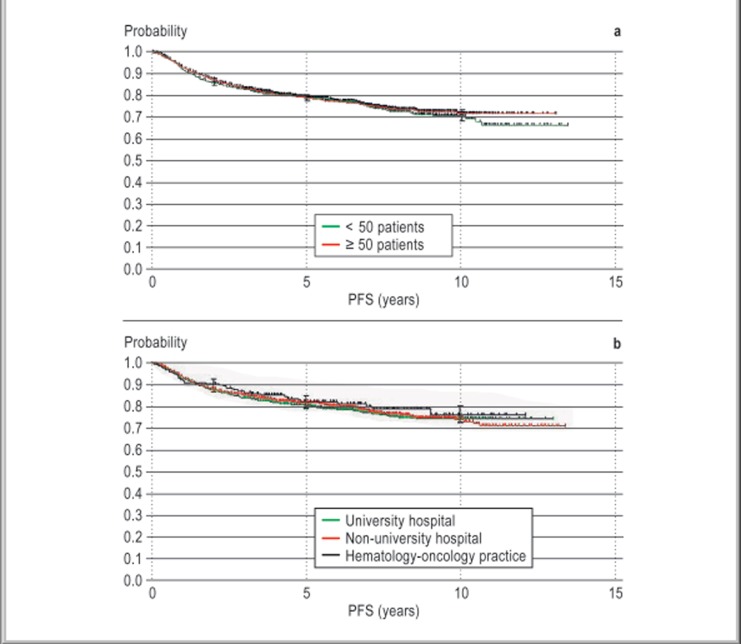
Kaplan-Meier plots of progression-free survival (PFS) are shown in Figure 2. After a median follow-up interval of 6.7 years, there was no statistically significant difference in PFS between patients treated in centers with a lower or higher caseload (5-year PFS 78.7% and 78.6% for centers treating <50 and ≥50 patients, respectively; p = 0.614). Nor was there any statistically significant difference in PFS from one type of center to another (5-year PFS for university hospitals, 77.7%; for other hospitals, 79.4%; for practices, 79.8%; p = 0.655).
Figure 2.
Kaplan-Meier plots of progression-free survival (PFS) by caseload and type of center. Median follow-up, 6.7 years.
a) PFS by caseload (at 5 years). Centers with < 50 pts.: 78.7%, 95% confidence interval (CI) 76%-81% (870 patients). Centers with ≥ 50 pts.: 78.6%, 95% CI 76%-81% (1353 patients).
b) PFS by type of center (at 5 years). University hospital: 77.7%, 95% CI 75%-80% (1060 patients). Non-university hospital: 79.4%, 95% CI 77%–82% (1000 patients). Hematology-oncology practice: 79.8%, 95% CI 72%-85% (163 patients).
The putative effects of caseload and center type on PFS were also tested in multivariate analyses with relevant confounders. In this way, the influence of unevenly distributed demographic variables was optimally accounted for. In a Cox regression model, the following factors were found to be associated with a significantly worse PFS, as expected: male sex, higher age, more advanced stage of disease, presence of B symptoms, and treatment in an earlier generation of trials (G2) or with a less up-to-date protocol (without BEACOPP chemotherapy [bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone] or only with radiotherapy). Even after adjustment for these factors, no independent center effect on outcome could be detected: neither the center caseload nor the type of center had any significant association with PFS. To validate the findings, the same analyses were carried out on further, independent data sets—the second patient collective from G2 and G3 (2216 patients) and patients from G4 (3682 patients, Figure 1). The multivariate analysis did not reveal any significant association of center type or caseload with PFS in either of these two collectives, or in the group of all G2 and G3 patients combined (4439 patients), or in the overall patient group (G2 + G3 + G4, 8121 patients).
The hazard ratios and 95% confidence intervals for all tested variables of the patients in G2 and G3 (4439 patients) are shown in Table 3. Because the centers could theoretically have differed from one another in further relevant ways, a further variable for unobserved heterogeneity was estimated in a shared-frailty model. 5-year PFS was found not to be significantly associated with this variable either (p = 0.38), just as it was not associated with either center type or center caseload.
Table 3. Multivariate analysis.
| A) Effect of caseload on progression-free survival (PFS) | ||||||
| Factor | p | Hazard ratio (95% CI) | ||||
| Caseload | continuous (per 100 pts.)* | 0.176 | 0.93 (0.84–1.04) | |||
| Sex | male | < .0001 | 1.29 (1.14–1.46) | |||
| Age at diagnosis | continuous (per year older) | < .0001 | 1.03 (1.02–1.03) | |||
| Stage (German Hodgkin Study Group [GHSG]) | intermediate + advanced | 0.0003 | 2.11 (1.40–3.17) | |||
| only advanced | < .0001 | 1.95 (1.64–2.31) | ||||
| Treatment | without BEACOPP chemotherapy protocol | < .0001 | 1.98 (1.62–2.41) | |||
| radiotherapy only | < .0001 | 3.12 (2.05–4.75) | ||||
| B symptoms | present | 0.0003 | 1.32 (1.13–1.53) | |||
| Generation | G2 | 0.200 | 1.10 (0.95–1.27) | |||
| Unobserved heterogeneity of centers (frailty) | 0.380 | |||||
| B) Effect of type of center on progression-free survival (PFS) | ||||||
| Factor | p | Hazard ratio (95% CI) | ||||
| Type of center | non-university hospital | 0.194 | 0.92 (0.81–1.05) | |||
| hematology-oncology practice | 0.283 | 1.16 (0.89–1.51) | ||||
| Sex | male | < .0001 | 1.29 (1.14–1.47) | |||
| Age at diagnosis | continuous (per year older) | < .0001 | 1.03 (1.02–1.03] | |||
| Stage (German Hodgkin Study Group [GHSG]) | intermediate + advanced | 0.0004 | 2.09 (1.39–3.14) | |||
| only advanced | < .0001 | 1.95 (1.64–2.31) | ||||
| Treatment | without BEACOPP chemotherapy protocol | < .0001 | 1.98 (1.62–2.41) | |||
| radiotherapy only | < .0001 | 3.01 (2.03–4.73) | ||||
| B symptoms | present | 0.0003 | 1.32 (1.14–1.53) | |||
| Generation | G2 | 0.236 | 1.09 (0.94–1.26) | |||
| Unobserved heterogeneity of centers (frailty) | 0.380 | |||||
*The hazard ratio here denotes the ratio of risk per 100 additionally treated patients.Multivariate analysis of G2 and G3 overall, with estimation of unobserved heterogeneity of centers; testing of the effects of caseload (A) and type of center (B) on progression-free survival (PFS) with relevant confounders. The factors expected to be associated with significantly worse PFS were male sex, older age, advanced stage of disease, presence of B symptoms, treatment in an early trial generation (G2), and less up-to-date type of treatment (without BEACOPP chemotherapy protocol, or with radiotherapy only). There was no independent prognostic effect of centers after adjustment for these factors; nor did unobserved heterogeneity of the centers play any significant role in PFS.
Discussion
We analyzed the effect of the type and caseload of oncological treatment centers on the outcome of treatment for Hodgkin’s lymphoma in clinical trials. Even after statistical controls for certain prognostically unfavorable factors, no significant association could be detected between center type (university hospital, non-university hospital, or hematology-oncology practice) or center caseload and 5-year progression-free survival. Because of the large sample size and the large number of PFS events, these negative findings can be used to draw valid inferences. In general, the data from the patient collectives analyzed here, which were drawn from prospective, randomized trials of the GHSG, are of very high quality. Moreover, bias due to the influence of other interests (e.g., those of pharmaceutical companies) on data analysis and on the publication of trial findings can be ruled out (12, 13).
A large fraction of patients with Hodgkin’s lymphoma in Germany are treated in an RCT of the GHSG. These patients are presumably representative of the entire collective of all treatable patients. Nonetheless, patients who do not meet the inclusion criteria for trial participation or for standard treatment, e.g., because of age, poor organ function, or severe accompanying disease, may have significantly lower survival rates; thus, the use of trial patients implies some extent of positive selection (14). An earlier analysis of patients with Hodgkin’s disease revealed that those treated in GHSG trials had a longer PFS than those treated outside GHSG trials, even after known prognostic factors were controlled for (15). There can also be differences within a patient collective in a given clinical trial: trial patients who were not treated exactly according to protocol may have a significantly shorter PFS (3).
In the present analysis, no center effects were found. This implies that patients were, in general, treated properly and according to protocol, even in centers with a lower caseload, and regardless of center type (type of hospital or practice). This encouraging finding about the treatment of a rare disease is mainly attributable, in the authors’ opinion as experienced clinicians, to the following quality-assurance measures undertaken by the GHSG:
Detailed trial protocols are available, and physicians participating in the trials always have the opportunity to obtain advice from experts at trial headquarters with regard to treatment strategies, side effects, or other problems.
A quality-assurance program for diagnostic testing and for radiotherapy was established, with reference assessment of imaging findings and development and communication of radiotherapy plans.
Moreover, the highly qualified physicians at the participating institutions remain in close contact with the physicians at trial headquarters. Annual trial meetings and courses, as well as international scientific meetings organized by the GHSG, contribute to the training of the participating oncologists and radiation oncologists and enable them to exchange information. The large number of treatment centers is good for patients, who can often be treated near their homes in accordance with established standards, or else receive outpatient follow-up near their homes after a period of in-hospital care. Expert qualifications aside, patient satisfaction has been found to be very high in specialized hematology-oncology practices in Germany, as long as the treating physicians can easily gain access to, and collaborate with, the nearest hospital in case of an emergency (16).
Center effects and minimum caseloads have long been a major topic of health-care policy debates in other branches of medicine, e.g., surgery, interventional cardiology, and the care of premature neonates; in oncology, however, data of this type are scarce and mostly retrospective. Most of the existing data concern mainly the period just after an oncologic-surgical procedure; it has been shown in this context that the intensity and detectability of center effects rise with the procedure-associated risk (17, 18). Center-dependent differences of up to 15% in 100-day mortality have been documented for complex hematological treatments such as allogeneic stem-cell transplantation for leukemia, while differences of up to 4% have been found for autologous stem-cell transplantation, a less dangerous procedure (19). In addition to the caseload treated by a physician or center, qualitative factors such as around-the-clock availability of a physician for urgent problems have been shown to affect patient survival significantly (19). There is also evidence that the effects of known prognostic factors, e.g., age, depend on individual centers’ experience (20). Center effects have not been well investigated to date for hematologic diseases that have a first-line treatment with curative intent (17). Only a single large-scale study has shown that, among patients with Hodgkin’s lymphoma treated in the USA from 1977 to 1982, those treated in comprehensive cancer centers (2278 patients) lived significantly longer than those who received so-called “community care” (3607 patients). This remained the case after controls for other factors including disease stage, histology, and age (21).
The findings reported here for Hodgkin’s lymphoma are in contrast with the documented significant effects of center size, caseload, and other factors on the outcome of other diseases: for example, the surgeon’s type of specialty training and level of expertise have been found to affect survival in ovarian carcinoma (22, 23). The predictive value of “volume data” is, however, subject to bias from multiple sources, e.g., preselection of patient collectives (24). Many of the studies that have been performed to detect center effects are problematic in that the data were retrospectively drawn from registries, with the result that there can be little or no statistical control for potential confounders (25). Even in retrospective data sets concerning large numbers of patients, any center effects that might be detected are hard to interpret unless further characteristics of the centers and patients are taken into account (26). A further problem in the evaluation of minimum caseloads is that a classification into arbitrarily defined high- and low-caseload groups can easily be misleading, as the result may well depend on the particular cutoff values that are chosen (27, 28).
In summary, the findings reported here imply that objectifiable center effects—in particular, effects of caseload or center type—play no role in the outcome of treatment for Hodgkin’s lymphoma in appropriately qualified GHSG centers in Germany.
Key Messages.
This evaluation of 500 German centers that participated in the GHSG trials did not reveal any correlation of the type of center (university hospital, non-university hospital, or hematology-oncology practice) with progression-free survival of patients with Hodgkin’s lymphoma.
Nor was there any correlation between a center’s caseload of patients with Hodgkin’s lymphoma and their progression-free survival.
These findings were obtained in a large (8121 patients) and homogeneous group of patients with optimal consideration of other known and unknown prognostic factors.
It follows that there are no objectively justifiable criteria for minimum caseload or center type for the treatment of Hodgin’s lymphoma in Germany. The necessary quality standards for successful chemotherapy and radiotherapy are evidently being met to the same extent at all levels of care in Germany, so that patients with this diagnosis need not be allocated on the basis of minimum case numbers.
In view of the prominence of minimum caseload requirements in current debates and decisions on health-care policy, scientifically well-grounded health-care policy research remains highly important not only in hematology and oncology, but in other disciplines as well
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Dr. Brillant is employed by Aptiv Solutions GmbH. Dr. Klimm has received reimbursement of travel costs from HEXAL. The other authors state that they have no conflicts of interest.
This project was supported by German Cancer Aid (Deutsche Krebshilfe), reg. no. 109273.
References
- 1.Diehl V. Therapie des Morbus Hodgkin: Erfahrungen der Deutschen Hodgkin-Studiengruppe über vier Studiengenerationen. Dtsch Arztebl. 2002;99(25) [Google Scholar]
- 2.Klimm B, Diehl V, Pfistner B, Engert A. Current treatment strategies of the German Hodgkin Study Group (GHSG) Eur J Haematol Suppl. 2005;66:125–134. doi: 10.1111/j.1600-0609.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 3.Dühmke E, Franklin J, Pfreundschuh M, et al. Low-dose radiation is sufficient for the noninvolved extended-field treatment in favorable early-stage Hodgkin’s disease: long-term results of a randomized trial of radiotherapy alone. J Clin Oncol. 2001;19:2905–2914. doi: 10.1200/JCO.2001.19.11.2905. [DOI] [PubMed] [Google Scholar]
- 4.Sieber M, Tesch H, Pfistner B, et al. Rapidly alternating COPP/ABV/IMEP is not superior to conventional alternating COPP/ABVD in combination with extended-field radiotherapy in intermediate-stage Hodgkin’s lymphoma: final results of the German Hodgkin’s Lymphoma Study Group Trial HD5. J Clin Oncol. 2002;20:476–484. doi: 10.1200/JCO.2002.20.2.476. [DOI] [PubMed] [Google Scholar]
- 5.Sieber M, Tesch H, Pfistner B, et al. Treatment of advanced Hodgkin’s disease with COPP/ABV/IMEP versus COPP/ABVD and consolidating radiotherapy: final results of the German Hodgkin’s Lymphoma Study Group HD6 trial. Ann Oncol. 2004;15:276–282. doi: 10.1093/annonc/mdh046. [DOI] [PubMed] [Google Scholar]
- 6.Engert A, Franklin J, Eich HT, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended-field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin’s lymphoma: final results of the GHSG HD7 trial. J Clin Oncol. 2007;25:3495–3502. doi: 10.1200/JCO.2006.07.0482. [DOI] [PubMed] [Google Scholar]
- 7.Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s Lymphoma: Results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601–3608. doi: 10.1200/JCO.2003.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348:2386–2395. doi: 10.1056/NEJMoa022473. [DOI] [PubMed] [Google Scholar]
- 9.Engert A, Pluetschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s Lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 10.Eich HT, Diehl V, Görgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199–4206. doi: 10.1200/JCO.2010.29.8018. [DOI] [PubMed] [Google Scholar]
- 11.Borchmann P, Haverkamp H, Diehl V, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol. 2011;29:4234–4242. doi: 10.1200/JCO.2010.33.9549. [DOI] [PubMed] [Google Scholar]
- 12.Schott G, Pachl H, Limbach U, Gundert-Remy U, Ludwig WD, Lieb K. The financing of drug trials by pharmaceutical companies and its consequences: Part 1 A Qualitative, systematic review of the literature on possible influences on the findings, Protocols, and quality of drug trials. Dtsch Arztebl Int. 2010;107(16):279–285. doi: 10.3238/arztebl.2010.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schott G, Pachl H, Limbach U, Gundert-Remy U, Lieb K, Ludwig WD. The Financing of Drug Trials by Pharmaceutical Companies and Its Consequences: Part 2 A Qualitative, Systematic Review of the Literature on Possible Influences on Authorship, Access to Trial Data, and Trial Registration and Publication. Dtsch Arztebl Int. 2010;107(17):295–301. doi: 10.3238/arztebl.2010.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terschüren C, Gierer S, Brillant C, Paulus U, Löffler M, Hoffmann W. Are patients with Hodgkin lymphoma and high-grade non-Hodgkin lymphoma in clinical therapy optimization protocols representative of these groups of patients in Germany? Ann Oncol. 2010;21:2045–2051. doi: 10.1093/annonc/mdq214. [DOI] [PubMed] [Google Scholar]
- 15.Brillant C, Terschueren C, Gierer S, et al. Differences in survival rates for patients with Hodgkin Lymphoma, who were treated inside vs. outside therapy optimisation protocols in Germany. ASH Annual Meeting Abstracts. Blood. 2007;110 [Google Scholar]
- 16.Baumann W, Nonnenmacher A, Weiß B, Schmitz S. Patient satisfaction with care in office-based oncology practices. Dtsch Arztebl Int. 2008;105(50):871–877. doi: 10.3238/arztebl.2008.0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: Importance in quality of cancer care. J Clin Oncol. 2000;18:2327–2340. doi: 10.1200/JCO.2000.18.11.2327. [DOI] [PubMed] [Google Scholar]
- 18.Hollenbeck BK, Dunn RL, Miller DC, Daignault S, Taub DA, Wei JT. Volume-based referral for cancer surgery: informing the debate. J Clin Oncol. 2007;25:91–96. doi: 10.1200/JCO.2006.07.2454. [DOI] [PubMed] [Google Scholar]
- 19.Loberiza FR, Jr, Zhang MJ, Lee SJ, et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood. 2005;105:2979–2987. doi: 10.1182/blood-2004-10-3863. [DOI] [PubMed] [Google Scholar]
- 20.Frassoni F, Labopin M, Powles R, et al. Effect of centre on outcome of bone-marrow transplantation for acute myeloid leukaemia. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 2000;355:1393–1398. doi: 10.1016/s0140-6736(00)02137-1. [DOI] [PubMed] [Google Scholar]
- 21.Davis S, Dahlberg S, Myers MH, Chen A, Steinhorn SC. Hodgkin’s disease in the United States: a comparison of patient characteristics and survival in the Centralized Cancer Patient Data System and the Surveillance, Epidemiology, and End Results Program. J Natl Cancer Inst. 1987;78:471–478. [PubMed] [Google Scholar]
- 22.Schrag D, Earle C, Xu F, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst. 2006;98:163–171. doi: 10.1093/jnci/djj018. [DOI] [PubMed] [Google Scholar]
- 23.Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98:172–180. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 24.Peterson ED, Coombs LP, DeLong ER, Haan CK, Ferguson TB. Procedural volume as a marker of quality for CABG surgery. JAMA. 2004;291:195–201. doi: 10.1001/jama.291.2.195. [DOI] [PubMed] [Google Scholar]
- 25.Lipscomb J. Transcending the volume-outcome relationship in cancer care. J Natl Cancer Inst. 2006;98:151–154. doi: 10.1093/jnci/djj055. [DOI] [PubMed] [Google Scholar]
- 26.Geraedts M, de Cruppe W. Controversial study results in relation to minimum volume standards. Z Arztl Fortbild Qualitatssich. 2006;100:87–91. [PubMed] [Google Scholar]
- 27.Bender R, Grouven U. Possibilities and limitations of statistical regression models for the calculation of threshold values for minimum provider volumes. Z Arztl Fortbild Qualitatssich. 2006;100:93–98. [PubMed] [Google Scholar]
- 28.Grouven U, Kuchenhoff H, Schrader P, Bender R. Flexible regression models are useful tools. Epidemiol. 2008;61:1125–1131. doi: 10.1016/j.jclinepi.2007.11.020. [DOI] [PubMed] [Google Scholar]