Abstract
A robust selectivity enhancement of an In(III)-porphyrin ionophore-based chloride-selective electrode under pulsed chronopotentiometric measurement mode that enables the detection of chloride ions in the presence of a normally interfering concentration of salicylate ions is described. This enhancement is achieved by the rapid depletion of the surface concentration of the more dilute lipophilic anion during an initial anodic current pulse period due to extraction of this preferred anion into the membrane phase. Measurement of chloride with a detection limit of 8 mM and near Nernstian response slope in the presence of 1 mM salicylate is possible using the pulstrode method.
Keywords: Ion-selective electrodes, Chloride, Selectivity enhancement, Clinical analysis, Pulsed chronopotentiometry, Solvent polymeric membrane
1. Introduction
The determination of chloride ions is necessary in clinical diagnosis, physiological analysis, environmental monitoring, food analysis and industrial applications. Direct potentiometry with ion-selective electrodes (ISEs) is a simple, inexpensive and robust technique and is very attractive for the detection of chloride ions in solutions. Silver-silver chloride (Ag/AgCl)-based ion-selective electrodes have been explored for the detection of chloride ions in a variety of solutions [1, 2], but these sensors suffer from poor selectivity against bromide and adsorption of proteins on the electrode surface when used in biological samples [3]. More biocompatible liquid membrane ion-selective electrodes based on dissociated ion-exchangers such as quaternary ammonium salts were also widely explored for the detection of chloride ions [4–6]. However, these sensors do not possess recognition elements that exhibit a specific interaction with chloride ions and their selectivity is governed by the relative free energies of hydration/solvation of anions according to the Hofmeister selectivity pattern [7]. Therefore, the detection of chloride ions with these sensors is prone to interferences from more lipophilic anions, such as salicylate.
To overcome these limitations, the development of carrier-based chloride selective electrodes has been of keen interest. A number of compounds, mainly organometallics including organotins [8, 9], organomercury [10], and metalloporphyrins [11–13] have all been investigated as ionophores for chloride. Use of these agents has resulted in potentiometric responses that clearly deviate from the Hofmeister selectivity series, albeit they do not offer adequate selectivity for chloride to reliably avoid the interferences of the thermodynamically preferred more lipophilic anions. For example, when these ion-selective electrodes are utilized under the classical zero current potentiometric measuring mode to measure chloride ions in clinical samples in the presence of dilute lipophilic anions such as salicylate (from patients under aspirin therapy) or thiocyanate (from patients who smoke cigarettes), a large positive error is observed due to the preference of the electrode for these lipophilic anions. Asymmetric cellulose acetate-based membrane electrodes, with hydrophilic outer surfaces have been investigated to eliminate the interference of dilute lipophilic anions on the detection of chloride ions via kinetic/size hindrance of the mass transport of the interfering ions [14, 15]. This approach has shown remarkable reduction of the interferences, but could not eliminate it completely.
Conventional anion exhanger-based polymer membranes possess sites (e.g., quaternary ammonium) that control spontaneous ion extraction processes at the membrane/sample interface. This makes reliable detection of hydrophilic anions in the presence of lipophilic anions with membranes of limited selectivities difficult since the lipophilic anions can be irreversibly extracted into the membrane. Recently, pulsed chronopotentiometric anion-selective electrodes without net ion-exchange properties under non-polarized conditions (and thus without significant spontaneous extraction [16]), were explored for the detection of hydrophilic anions in the presence of lipophilic anions. These membranes possess lipophilic neutral salts of the form R+R−, where R+ and R− are a lipophilic cation and a lipophilic anion, respectively. An anodic current pulse is applied accross the membrane to cause extraction of the anions from the sample phase into the polymeric membrane while membrane potential is measured simultaneously. This is followed by a zero-current measuring pulse and then a stripping potential pulse to expel the ions extracted during the uptake current pulse to regenerate the membrane (see Results and Discussion Section).
In this earlier work [16], as a model system, elimination of the interference of therapeutic levels of salicylate ions on the measurement of physiological level of chloride ions was studied using ionophore-free ion-responsive electrodes (containing the lipophilic salt tetradodecylammonium tetrakis(4-chlorophenyl) borate, ETH 500). Although this technique was shown to be very promising, a 10-fold dilution of a blood mimetic sample for patients under aspirin therapy (0.1 mM salicylate) was required to attain the elimination of the interference from salicylate on the measurement of chloride, since the therapeutic level (1 mM) salicylate was too high to be discriminated by the sensor. It was shown in this previous work [16] that the tolerable interfering ion level at the detection limit of the discriminated primary ions could be increased by increasing the thermodynamic selectivity of the membranes for the primary ion.
In this paper, we demonstrate that a much greater degree of selectivity for chloride over salicylate can be achieved by using a membrane that is composed of a lipophilic salt of In(III)tetraphenylporphyrin (In(III)TPP+) and tetrakis[3,5-bis(trifluoromethyl)phenyl borate (TFPB), using the pulsed chronopotentiometric measurement mode. Indeed, earlier work had shown that membranes doped with In(III) porphyrins exhibit enhanced response to chloride [12], but selectivity over salicylate is still problematic when using classical membrane formulations and zero current potentiometry. Herein, the direct detection of chloride ions in the presence of therapeutic level of salicylate (1 mM) in buffer solutions is possible utilizing pulsed chronopotentiometric ion-selective membranes doped with the salt In(III)TPP+/TFPB.
2. Experimental
2.1 Reagents
High molecular weight poly(vinyl chloride) (PVC), o-nitrophenyl octyl ether (o-NPOE), tetradodecylammonium tetrakis(4-chlorophenyl) borate (ETH 500), tetrahydrofuran (THF), potassium tetrakis[3,5-bis(trifluoromethyl)phenyl borate (KTFPB), 2-morpholinoethanesulfonic acid (MES), sodium hydroxide and all salts were purchased from Fluka (Milwaukee, WI). Meso-tetraphenylporphyrin (TPP) was purchased from Frontier Scientific (Logan, Utah). In(III)TPPCl was prepared by metallation of the free TPP [17]. The lipophilic ionophore salt, In(III)TPP-TFPB, was obtained by mixing equimolar quantities of In(III)TPPCl and KTFPB in the membrane cocktail (see the results and discussion section). Aqueous solutions were prepared by dissolving the appropriate compounds in Nanopure-deionized water (18.2 MOhm cm).
2.2. Membrane Preparation
The chloride-selective membranes (~200 µm thick) were prepared by solvent casting using freshly distilled THF as the solvent. A membrane cocktail containing 5 wt% each of the inert lipophilic salts, ETH 500 and In(III)TPP-TFPB, 30 wt% PVC and 60 wt% o-NPOE was employed for pulsed chronopotentiometry experiments, and 1.5 wt% In(III)TPPCl, 20 mol% (with respect to the ionophore) KTFPB, 32.5 wt% PVC and 66 wt% o-NPOE for zero-current potentiometry experiments.
2.3. Electrodes
The polymer membranes were cut with cork borer (6 mm diameter) from the parent membrane and incorporated onto an electrode body UnilSE MTO50 S7/120 (Oesch Sensor Technology, Sargans, Switzerland). The actual membrane area was 20 mm2. The inner solution (10 mM NaCl) was in contact with an internal Ag/AgCl electrode. The external reference electrode was a double-junction Ag/AgCl electrode with saturated KCl as inner solution and a 1 M LiOAc as the bridge electrolyte (type 13-620-658, Accumet, Fisher scientific, Pittsburgh, PA). A high surface area coiled Pt-wire in contact with the sample solution was used as a counter electrode for the pulstrode experiments. The working electrodes were conditioned in 0.01 M NaCl solution for at least 12 h prior to experiments and kept in the conditioning solution when not in use.
2.4. Experimental set-up
A conventional three-electrode setup was used for the pulsed chronopotentiometric measurements with the In(III)TPP-TFPB-based membrane electrode, with the internal Ag/AgCl electrode of the membrane electrode connected as the working electrode and the external reference and counter electrodes were immersed into the sample solution. The measurements were made with an AFCBI bipotentiostat (Pine Instruments, Grove City, PA) controlled by a PCI-MIO-16E4 interface board and LabVIEW 8.6 data acquisition software (National Instruments, Austin, TX) on a PC computer. The potentials were sampled at 2 ms intervals and recorded and saved in two files as a raw data and as an averaged data during the last 10% time period of the anodic current pulse and at the end of the zero current pulse period. A baseline potential pulse of 0 V versus the Ag/AgCl reference electrode was applied as the stripping potential throughout the experiments. This baseline (equilibrium) potential was determined from an initial measurement in a backgground solution, where a stable and reproducibe potential was observed and the current was decayed to near 0 µA after the baseline potential pulse of 0 V Vs, the reference electrode was applied. This is an indication of the regeneration of the membrane. An uptake (anodic current pulse) time of 1 s, a zero-current pulse of 0.5 s and a stripping (potentiostatic pulse) time of 15 s were used to conduct the pulsed chronopotentiometric experiments. Classical potentiometric measurements were conducted with an 8-channel, high-impedance voltmeter (Lawson Laboratories Inc., Malvern, PA) connected to PC computer. The potentials of the membrane electrode were measured against the same reference electrode used in the pulstrode, but using the conventional two-electrode (galvanic cell) configuration. All experiments were conducted at room temperature (21 – 23 °C).
3. Results and Discussion
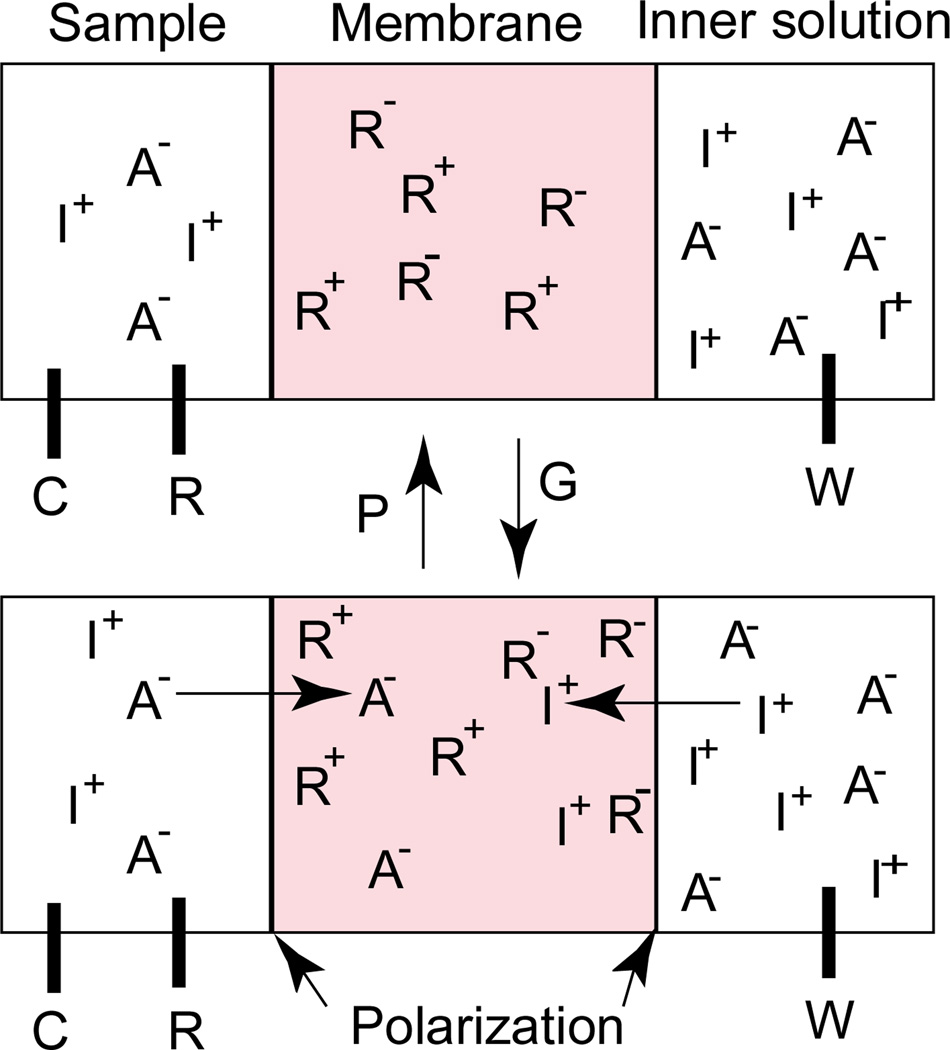
The pulsed chronopotentiometric chloride-selective membrane used here is formulated with a lipophilic neutral salt of the form R+R−, where R+ and R− are the lipophilic ionophore In(III)TPP+ used as a chloride ion recognition element, and the lipophilic anion TFPB−, used as the counteranion of In(III)TPP+ in the membrane phase, respectively (see Figure 1). This formulation was obtained by adding equimolar quantities of the charged lipophilic salts, In(III)TPP+Cl− and K+TFPB−, in the membrane cocktail. It is envisioned that the hydrophilic counterions of the lipophilic salts, i.e., Cl− and K+, diffuse into the contacting aqueous phases upon initial conditioning of the membrane electrode in solution, leaving the neutral salt, In(III)TPP-TFPB, within the membrane (see schematic diagram in Figure 2).
Fig. 1.
Structures of the lipophilic charged chloride carrier, In(III)TPP+ and its counterion, TFPB−, used to prepare membrane electrodes employed in this work.
Fig. 2.
Schematic diagram showing the formulation of pulsed chronopotentiometric polymeric membrane without permanent ion-exchange properties. The hydrophilic counterions of the In(III)TPP+ and TFPB−, i.e., Cl− and K+ diffuse into the aqueous phases upon initial conditioning of the electrodes (see above).
Obviously, this membrane should not possess any ion-exchange properties since it contains equimolar lipophilic cation and lipophilic anion species. This behavior was confirmed by monitoring classical potentiometric response to sodium chloride solution over a wide range of concentration (0.1 to 10 mM), and no significant potentiometric response under zero-current conditions was observed, suggesting that the In(III)TPP+/TFPB− salt prepared was rather pure. However, in pulsed chronopotentiometry, an anodic current pulse of fixed magnitude and duration is applied across the membrane to cause polarization of the membrane surfaces (see schematic in Figure 3). Here G and P show the galvanostatic pulse applied to cause the membrane surface polarization and the potentiostatic pulse applied to regenerate the initial equilibrium ion distribution in the membrane phase, respectively. W, R and C represent the working, reference and counter electrodes, respectively (see the Experimental Section). As shown in Figure 3, In(III)TPP+ moves toward the sample side of membrane and concomitantly induces a flux of anions from the sample side into the polymeric membrane. Note that lipophilic cations are also extracted from the inner filling solution into the membrane to maintain charge neutrality. The phase boundary potential at the membrane/sample interface, which is a function of the anion concentration in the sample phase, is monitored as a function of time during the current pulse. This is followed by a zero-current measurement pulse period, during which the ions extracted under the applied current pulse undergo exchange with the ions in the contacting aqueous phases, akin to classical potentiometry [18]. The equilibrium membrane ion concentrations are then restored by applying a baseline (stripping) potential pulse for a longer time period.
Fig. 3.
Schematic of polarization mechanism of the membrane phase boundary by applying an anodic current pulse of 28 µA. The membrane surfaces are temporarily polarized by applying an appropriate current pulse (G) and ions are extracted into the membrane. Then the membrane is renewed by applying a baseline (stripping) potential pulse (P) before the next measurement pulse.
Pulsed chronopotentiometric ion-selective electrodes are based on similar transduction principles as their classical potentiometric counterparts and provide analogous response curves [16, 19]. Figure 4 shows the calibration curves of chloride ion in a background of 50 mM MES-NaOH buffer, pH 5.5, under classical potentiometric (using conventional membrane with In(III)TPP+ as ionophore with only 20 mol% borate species) and pulsed chronopotentiometric- measuring modes, with the potential recorded as averaged data during the last 10% of the uptake current pulse period. As can be seen, for measurements in the higher concentration range of chloride and when there are no strongly interfering anions present, the two measuring protocols yield essentially the same response curves. However, there are certain situations where pulsed chronopotentiometry offers distinct advantages over classical potentiometry. For example, when the ion-selective membrane exhibits inadequate selectivity over lipophilic anions in the zero current potentiometric measurement mode, if the membranes come in contact with lipophilic anions, these anions would be spontaneously extracted into the membrane according to the zero-current ion-exchange reaction. Then, in subsequent samples that do not contain these lipophilic anions, slow exchange reactions will contaminate the sample phase with the lipophilic ions in the unstirred Nernst layer adjacent to the membrane, yielding erroneous results for the measurement of the hydrophilic anions. Such memory effects due to spontaneous extractions do not occur with pulsed chronopotentiometric sensors since the membranes do not possess any permanent ion-exchange properties. Indeed the membrane is continuously renewed during the potentiostatic pulses [16, 20].
Fig. 4.
Calibration curves of pulsed chronopotentiometric and classical potentiometric chloride-selective electrodes in a background of 50 mM MES-NaOH buffer at pH 5.5. For pulstrode experiments, the potential was recorded as averaged data during the last10% of the uptake current pulse period.
Analytically, the more important advantage of the pulsed chronopotentiometric sensors and indeed the focus of this work is the possibility to greatly reduce the interference from low concentrations of lipophilic anions when trying to measure a higher concentration of hydrophilic anions. For the pulstrode configuration, the applied anodic current pulse causes the extraction of the anions (primary and interfering) from the sample side into the polymeric membrane. As the mass transfer reaction is a diffusion-controlled process, the concentration of the dilute thermodynamically preferred lipophilic ions in the unstirred aqueous diffusion layer on the sample side is depleted more quickly upon application of a high current pulse (see Figure 5). Figure 5 shows the schematic of the mechanism of kinetic discrimination of dilute lipophilic ions (X−, e.g., salicylate in these experiments) on the measurement of abundant hydrophilic ions (A−, chloride in these experiments). Thus, the phase boundary potential is mainly governed by the concentration of the more abundant ions at the sample/membrane phase boundary (chloride), as the lower concentration of the more favorably extracted anion (salicylate) is more quickly depleted.
Fig. 5.
Schematic diagrams showing the depletion phenomena of dilute lipophilic anions at the sample/membrane interface. A− and X− are the abundant primary ions (chloride) and the dilute interfering ions (salicylate), respectively.
It is important to note that this approach can also be utilized in a different measurement mode, termed flash chronopotentiometry, where dilute primary ions are locally depleted at the membrane surface to yield a dramatic break point in the resulting chronopotentiograms [21–24]. However, in the present example, the goal is to use the current pulse to deplete the lower concentration of a strongly interferent anion, not the target analyte. Indeed, Figure 6 shows the calibration curves of the classical potentiometric measurements and the pulsed chronopotentiometric measurements (with the potential recorded as averaged data during the last 10% of the applied 28 µA current pulse period) for chloride-selective membrane electrodes formulated with In(III)TPP+ in 50 mM MES-NaOH buffer, pH 5.5, containing 1 mM salicylate. Measurement under applied current pulse is more preferred than that of zero current pulse in the pulstrode measurement mode, in this case, due to the kinetic nature of the depletion process (see above). If the measurement is conducted under the zero current pulse, the zero-current ion-exchange process may allow more interference from salicylate on the measurement of chloride ion. With classical potentiometry, very low EMF response is observed, even at higher concentration (0.1 M) of chloride. This is because of the interference from the lipophilic salicylate ions on the measurement of chloride. This behavior is predicted based on the Nicolsky-Eisenman equation considering that the potentiometric selectivity coefficient for In(III)TPP-based membranes for chloride relative to salicylate, i.e., [12, 13]. However, near Nernstian response (slope ca −58 mV/decade) with a detection limit of ca 8 mM is observed with pulstrode arrangement showing that the depletion of the salicylate at the membrane/sample interface occurs during the initial 1 s anodic current pulse. It should be noted that earlier work with In(III)octaethylporphyrins in polymer membranes with classical potentiometric detection yielded responses that were super-Nernstian [12], likely due to a porphyrin dimer-monomer equilibrium reaction. In this work, the use of In(TPP)+ which is a bulkier porphyrin and also the presence of TFPB− in the membrane that may ion-pair with the In(III) porphyrin, likely prevents the dimer-monomer chemistry from occurring, yielding the expected near Nernstian behavior.
Fig. 6.
Calibration curves of pulsed chronopotentiometric and classical potentiometric chloride-selective electrodes in a background of 50 mM MES-NaOH buffer containing 1 mM sodium salicylate at pH 5.5. For pulstrode experiments, the potential was recorded as averaged data during the last10% of the applied current pulse period.
4. Conclusions
It has been shown that pulsed chronopotentiometry with an In(III)TPP+ ionophore-based ion-selective electrodes is a promising approach for the discrimination of dilute lipophilic interfering ions such as salicylate, for the measurement of abundant hydrophilic ions, such as chloride. The increased thermodynamic selectivity for the primary ions by the In(III)TPP+ ionophore is proven to offer additional advantages over the application of this concept with simple lipophilic neutral salts such as ETH 500, which do not offer distinct selectivity for chloride in the membrane phase.[16] Thus, it is envisioned that this technique may be useful for the selectivity enhancement of other ionophore based ion-selective electrodes such as nitrite- and fluoride-selective electrodes, where interference from dilute hydroxide ions has been problematic for detections of these ions in samples at their natural pH.
Acknowledgements
The authors are grateful to the National Institutes of Health through grant EB-000784 for supporting this research. In addition, KG thanks Instrumentation Laboratory, Inc. for partial financial support.
References
- 1.Dahms H. Clin. Chem. 1967;13:437. [PubMed] [Google Scholar]
- 2.Warwick WJ, Hansen L. Pediatrics. 1965;36:261. [PubMed] [Google Scholar]
- 3.Buhlmann P, Pretsch E, Bakker E. Chem. Rev. 1998;98:1593. doi: 10.1021/cr970113+. [DOI] [PubMed] [Google Scholar]
- 4.Oka S, Sibazaki Y, Tahara S. Anal. Chem. 1981;53:588. doi: 10.1021/ac00227a007. [DOI] [PubMed] [Google Scholar]
- 5.Ozawa S, Miyagi H, Shibata Y, Oki A, Kunitake T, Keller WE. Anal. Chem. 1996;68:4149. doi: 10.1021/ac960526v. [DOI] [PubMed] [Google Scholar]
- 6.Wegmann D, Weiss H, Ammann D, Morf WE, Pretsch E, Sugahara K, Simon W. Mikrochim. Acta. 1984;3:1. [Google Scholar]
- 7.Hofmeister F. Arch. Exp. Pathol. Pharmacol. 1888;24:247. [Google Scholar]
- 8.Hauser PC. Anal. Chim. Acta. 1993;278:227. [Google Scholar]
- 9.Wuthier U, Pham HV, Zund R, Welti D, Funck RJJ, Bezegh A, Ammann D, Pretsch E, Simon W. Anal. Chem. 1984;56:535. [Google Scholar]
- 10.Rothmaier M, Simon W. Anal. Chim. Acta. 1993;271:135. [Google Scholar]
- 11.Bakker E, Malinowska E, Schiller RD, Meyerhoff ME. Talanta. 1994;41:881. doi: 10.1016/0039-9140(94)e0041-o. [DOI] [PubMed] [Google Scholar]
- 12.Park SB, Matuszewski W, Meyerhoff ME, Liu YH, Kadish KM. Electroanal. 1991;3:909. [Google Scholar]
- 13.Steinle ED, Schaller U, Meyerhoff ME. Anal. Sci. 1998;14:79. [Google Scholar]
- 14.Cha MJ, Shin JH, Oh BK, Kim CY, Cha GS, Shin DS, Kim B. Anal. Chim. Acta. 1995;315:311. [Google Scholar]
- 15.Maj-Zurawska M, Sokalski T, Mulik E, Kotlinska A, Lewandowski R, Hulanicki A. Anal. Lett. 2001;34:1413. [Google Scholar]
- 16.Gemene KL, Shvarev A, Bakker E. Anal. Chim. Acta. 2007;583:190. doi: 10.1016/j.aca.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan NH, Agrawal S, Kureshy RI, Abdi SHR, Mayani VJ, Jasra RV. Eur. Org. Chem. 2006;3175 [Google Scholar]
- 18.Makarychev-Mikhailov S, Shvarev A, Bakker E. J. Am. Chem. Soc. 2004;126:10548. doi: 10.1021/ja047728q. [DOI] [PubMed] [Google Scholar]
- 19.Shvarev A, Bakker E. Anal. Chem. 2003;75:4541. doi: 10.1021/ac034409t. [DOI] [PubMed] [Google Scholar]
- 20.Perera H, Shvarev A. Anal. Chem. 2008;80:7870. doi: 10.1021/ac801210u. [DOI] [PubMed] [Google Scholar]
- 21.Gemene KL, Bakker E. Anal. Chem. 2008;80:3743. doi: 10.1021/ac701983x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gemene KL, Bakker E. Anal. Chim. Acta. 2009;648:240. doi: 10.1016/j.aca.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Gemene KL, Bakker E. Anal. Biochem. 2009;386:276. doi: 10.1016/j.ab.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 24.Gemene KL, Meyerhoff M. Anal. Biochem. 2011;416:67. doi: 10.1016/j.ab.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]