Abstract
Background
Recently, many studies have emphasized the importance of the comprehension of detailed functional anatomy and biomechanics of the elbow and its significant contribution in facilitating good functional outcomes of conservative and surgical treatment in the field of elbow disorders.
Methods
The most common disease of elbow disorders and their treatment was reviewed.
Results
Lateral epicondylitis of the elbow, is defined as a microscopic tear of extensor carpi radialis brevis tendon, and microscopic findings show immature reparative tissue (angiofibroblastic hyperplasia). The patient needs coordinated rehabilitation, range-of motion-exercise, stretching, and bracing in the second phase. Ninety-five percent of patients with lateral epicondylitis heal spontaneously or conservatively. The medial collateral ligament injury of the elbow is most common in the overhead-throwing athlete. Jobe’s procedure, the original reconstruction technique, and its modifications in bone-tunnel creation, allow a tendon graft to be wound in a figure-eight configuration through the tunnels. Further modification of Jobe’s procedure in bone-tunnel configuration reduced the total number of tunnels and facilitates easier graft tensioning. Outcomes with these reconstruction techniques have proven effective in returning high-level throwing athletes back to their sport. Arthroscopic surgery for the elbow in the throwing athlete has evolved and has proven successful results. Arthroscopic treatment includes debridement of posteromedial synovitis, loose-body removal, and excision of the olecranon spur. Posteromedial elbow impingement is also a source of disability in the overhead-throwing athlete. Twenty-five percent of these patients require a medial collateral ligament reconstruction after removal of a posteromedial bony spur. Linked and unlinked total elbow arthroplasty are successful treatment procedures for patients with rheumatoid arthritis, posttraumatic osteoarthritis, and elderly patients with comminuted distal humeral fractures and the salvage of distal humeral nonunion. Proper selection and implantation of prostheses are also important to achieve good functional outcome and longevity.
Conclusion
The success of treatment of elbow disorders depends greatly on surgical design and technique, both of which require comprehensive knowledge of detailed anatomy and biomechanics of the elbow.
Introduction
The field of elbow surgery continues to evolve. Recent emphasis indicates that a surgeon’s comprehensive understanding of the functional anatomy and biomechanics in the elbows yields successful treatment for patients with elbow epicondylitis, ligament injury, rheumatoid arthritis (RA), and posttraumatic osteoarthritis, as well as primary osteoarthritis [1]. Lateral elbow epicondylitis (tennis elbow) is the most common disease in this field. However, for prolonged (>1 year) difficult epicondylitis, the etiologies include secondary intra-articular elbow lesion, and the ideal treatment remains controversial. Techniques for reconstruction of medial collateral ligament injury of the elbow, the most common ligamental injury, have been described since the end of the nineteenth century and include the modern techniques introduced by F.W. Jobe [2]. Arthroscopic debridement, olecranon spur excision, and loose-body removal allow the patient’s return to throwing sports and yield reliable results. Minimally invasive arthroscopic surgery for the elbow in the throwing athlete has been developed and achieves successful results. Patients are able to return to their previous sports level at an early stage of the rehabilitation program [3]. The field of total elbow arthroplasty (TEA) continues to evolve as well. Linked and unlinked implant designs are available for select patients who have RA or posttraumatic contracture, and for distal humerus fractures in the elderly. This review presents the treatment of common elbow disorders, specifically, lateral epicondylitis, ligament reconstruction, arthroscopic debridement for athletes, and TEA.
Functional anatomy and biomechanics of the elbow joint
Knowledge of elbow biomechanics and functional anatomy of the elbow is very important. The anatomical shape of the radial head is not circular but eccentric. The concept of “screw home mechanism of radial head to capitellum [4]” and the physiological movement of the ulna against the humerus through the screw-displacement axes should be clearly recognized. The axis of rotation passes through the center of the arcs formed by the trochlear sulcus and capitellum [1]. Clinical relevance includes elbow-joint design and technique, ligament reconstruction, trauma management, and elbow-joint reconstruction. Motion (kinematics), stability (constraints), and strength (force transmission) are important when designing surgical procedures. The elbow joint has six degrees of freedom: flexion/extension, pronation/supination, and valgus/varus functional motion and anatomy. An electromagnetic tracking device that allows three-dimensional measurement of simulated active elbow motion reveals the amount of physiologic varus–valgus laxity during elbow flexion/extension to an average of 3–4° [1] (Fig. 1).
Fig. 1.
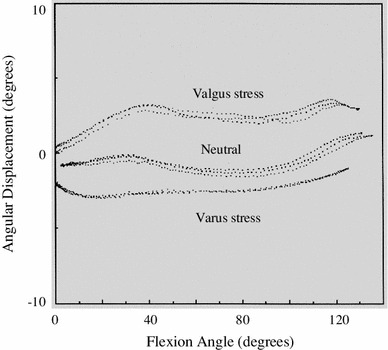
Normal kinematics
The lateral collateral ligament and the anterior bundle of the medial collateral ligament originate from points through which the axis of rotation passes. The medial collateral ligament has two components. The anterior bundle is taught in extension. Owing to the slight difference in anatomical origin between the lateral and medial collateral ligaments, the lateral collateral ligament shows uniform tension during elbow flexion–extension. In contrast, the medial collateral ligament is taught at different positions of elbow flexion [5]. Biomechanics and functional anatomy are excellent bases for diagnostic consideration and options of surgical treatment. In addition to knowledge of elbow-joint function, a comprehensive understanding of shoulder- and wrist-joint motion and dynamic elbow-joint motion is essential. Neurological assessment of the arm and cervical spine is important.
Lateral epicondylitis (tennis elbow)
One of the common diseases of the elbow, lateral epicondylitis, so-called tennis elbow tendinosis, is defined as a microscopic tear of the extensor carpi radialis brevis (ECRB) tendon, which is located on the lateral proximal side, covers the radial head, and runs beneath the supinator and extensor carpi radialis longus (ECRL) tendon. Five- to 10-mm distal to its origin from the lateral epicondyle, the ECRB has a biomechanical weak point where it connects to the joint capsule without covering the supinator muscle. This point is an essential etiological point as it is vulnerable to ECRB microscopic tear.
Lateral epicondylitis is a familiar term used to explain numerous symptoms in the vicinity of the lateral aspect of the elbow; its occurrence is more frequent in nonathletes than in athletes, with a peak incidence in the early fifth decade and a nearly equal gender incidence. Lateral epicondylitis can occur during activities that require repetitive supination and pronation of the forearm with the elbow in near full extension. Although originally described as an inflammatory process, the current consensus is that lateral epicondylitis is initiated as a microtear, most often within the origin of the extensor carpi radialis brevis. Microscopic findings show immature reparative tissue that resembles angiofibroblastic hyperplasia [6]. The pathological process mainly involves the origin of the ECRB (Fig. 2). The diagnosis of tennis elbow is made by confirming symptoms of discomfort localized at the origin of the ECRB. Tenderness is present over the lateral epicondyle approximately 5 mm distal and anterior to the midpoint of the condyle. Pain usually is exacerbated by resisted wrist dorsiflexion and forearm supination, and there is pain when grasping objects. Plain radiographs are usually negative; occasionally, calcific tendinitis may be present. To date, in some cases, magnetic resonance imaging (MRI) may be helpful in demonstrating the pathology at the lateral epicondyle or ECRB (Fig. 3); however, based on our experience, this is rarely indicated to diagnose epicondylitis.
Fig. 2.
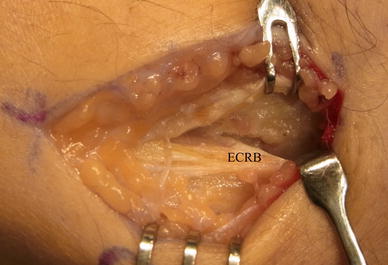
Intraoperative findings of extensor carpi radialis brevis (ECRB) tendon degeneration
Fig. 3.
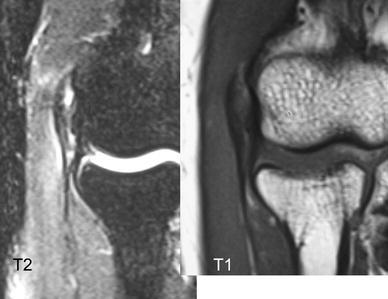
Magnetic resonance imaging (MRI) showing lateral epicondylitis in a 52-year-old man. T1 and T2 images show intraextensor carpi radialis longus (ECRB) tendon tear
There are three stages of this disease: initial acute phase, second subacute phase, and third chronic phase. In the first phase, the patient needs icing, compression, and rest. Coordinated rehabilitation, range-of-motion (ROM) exercise, stretching, and bracing, are needed in the second phase. Corticosteroid injection or platelet-rich plasma injection therapy is also indicated at the second phase [7, 8].
Other entities that can produce pain in this general vicinity are osteochondritis dissecans of the capitellum, lateral-compartment arthrosis, varus instability, and—perhaps most commonly—radial tunnel syndrome. Radial tunnel syndrome is a compressive neuropathy of the posterior interosseous nerve caused by structures of fibrous band and vascular variance that cross the nerve. The pain of radial tunnel syndrome occurs 2 cm distal to the lateral epicondyle and is located more anteriorly. True lateral epicondylitis and radial tunnel syndrome may coexist in 5 % of patients. Ninety-five percent of the patients who have lateral epicondylitis heal spontaneously [5]. Our clinical experience of surgical intervention for elbow epicondylitis suggests, for primary epicondylitis, a suture technique by Nirshl and Mayo groups leads to good results. Arthroscopic release is useful for intra-articular elbow lesion [9, 10].
Ligament injury of the elbow
There are three main mechanisms of injury to the elbow: valgus, posterior translation, and posterolateral rotatory mechanisms. The valgus stress mechanism is the most common and high-incident injury. Injury to the elbow medial collateral ligament (MCL) from valgus repetitive forces was first described in 1946 by Waris in a javelin thrower [11]. The injury has since become well recognized in baseball pitchers and other overhead-throwing athletes, in whom it is now most common. Jobe [12] developed the original MCL reconstruction and described the technique with initial results in 1986. In this technique, the flexor–pronator mass is transected and reflected, the ulnar nerve is transposed to a submuscular position, and humeral tunnels are created that penetrate the posterior humeral cortex. Modifications in bone-tunnel creation have also been made that direct the tunnels anteriorly on the humeral epicondyle to avoid risk of ulnar nerve injury, while maintaining figure-eight graft passage and configuration [13]. Further changes in bone-tunnel configuration reduce the total number of tunnels and facilitate easier graft tensioning [14]. These modified techniques have proven effective in returning high-level athletes back to throwing.
In the reconstruction of MCL, the surgeon should understand elbow biomechanics and natural healing of the grafted tendon. The MCL tendon originates from the bone (medial epicondyle) and inserts into the ulnar tuberosity. Considering the clinical reports of rerupture after reconstruction of the MCL tendon with strenuous sports activity, a comprehensive understanding of interface matrix distribution between bone and grafted tendon is necessary. From the tendon site, soft tissue consisting of fibroblasts changes to cartilage and forms hard, stiff tissue and bone consisting of osteoblasts. The biomechanical transformation of soft tissue, yielding a balanced interface matrix distribution, is important in this new concept. This procedure would minimize stress concentrations and promote fibrocartilage differentiation. The procedure for higher initial strength using a headless screw [15] between the bone and grafted tendon renders a high risk of ligament rerupture after MCL reconstruction.
The anterior bundle of the MCL is the strongest component and the primary restraint and stabilizer against valgus stress [16–19]. The anterior oblique ligament (AOL) is functionally composed of anterior and posterior bands that provide a reciprocal function in resisting valgus stress through the range of flexion–extension motion. Valgus stress is generated at the elbow during throwing maneuvers in baseball, softball, football, tennis serving, and volleyball spiking, as well as other active sports. A cadaver model has demonstrated that the flexor carpi ulnaris is the primary dynamic contributor to valgus stability and the flexor digitorum superficialis is a secondary stabilizer [20]. Thus, the muscular dynamic stability of the medial elbow is essential and must be included in rehabilitation programs.
The postoperative rehabilitation protocol is important as well. The net-throw motion starts from 4 months after surgery, the soft throwing and catching motions begin from 5 months, pitching from 8 months, and full recovery (100 %) of pitching should be expected at 10 months postoperatively. This protocol is relatively gradual, but it is highly sophisticated and safe for reconstructed MCL of the elbow to prevent rerupture. Cain and Andrews stated that MCL reconstruction of the elbow in 1,281 athletes achieved showed excellent results 743 of them [21]; 83 % returned to previous or higher level of competition in <1 year. Thirty-four of 45 major leaguers who underwent operation (75.5 %) returned to the previous or higher level of competition. Jobe’s procedure is thought to be a reliable surgical technique for the overhead-throwing athlete.
Another reliable surgical technique in MCL reconstruction is the docking procedure. Recently, a new docking technique has been developed, and Paletta and Wright [22] reported a review of 25 professional or scholarship collegiate baseball players with a high success rate. They reported the first series using a further modification of the docking technique employing a four-strand palmaris longus graft, and 23 of the 25 (92 %) were able to return to their preinjury levels of competition. These procedures are reliable surgical options as well.
Sports injuries to the elbow in the throwing athlete
Slocum first categorized elbow injuries in baseball athletes into three patterns: medial tension overload injury, lateral compression overload injury, and extension overload injury [23]. In recent years, minimal invasive arthroscopic surgery for the elbow in the throwing athlete has evolved and is proven to represent successful results. Patients return to their previous sports level at the early stage of the rehabilitation program. Arthroscopic treatment includes debridement of the posteromedial synovitis, loose-body removal, and excision of the olecranon spur. Posteromedial elbow impingement is a source of disability in the overhead-throwing athlete. Cohen et al. stated [24] after treating nine throwing athletes on the basis of the Andrews–Carson scale, that the subjective and objective outcome was considered excellent in seven patients and good in two. Their study indicates that MRI identifies a reproducible pattern in throwing athletes with posteromedial elbow impingement. These MRI findings were highly correlated with arthroscopic evaluation. Arthroscopic debridement, olecranon spur excision, and loose-body removal allow return to throwing sports and achieve reliable subjective and objective results in carefully selected patients.
Our experience included 19 throwing athletes who underwent arthroscopic debridement of the posteromedial synovitis, loose-body removal, and excision of the olecranon spur. The mean age was 24.3 (14–47) years. On the basis of the Japanese Orthopaedic Association (JOA) score, subjective and objective outcomes were 82 points preoperatively to 93 points postoperatively. In this series, the first priority regarding the purpose of surgery was pain relief of the elbow by removal of the loose body (Fig. 4). However, a problem following surgery is symptoms due to the remnant spur and also spur fracturing. Complications of arthroscopic excision of the spur create new instability at the medial part the elbow (Fig. 5). Andrews reviewed 72 professional baseball players who underwent arthroscopic or open elbow surgery [3]. The most common diagnoses were posteromedial olecranon osteophyte (65 %) and ulnar collateral ligament injury (25 %). Intra-articular loose bodies were found in 39 % of patients. Eighty percent returned to play for a minimum of one season, and 17 % retired initially because of their elbow injury; one third required two or more surgical procedures, with 25 % of these patients requiring an MCL reconstruction after removal of a posteromedial olecranon osteophyte [3]. The patients with posteromedial olecranon osteophytes had the highest rate of reoperation, and patients who underwent MCL reconstruction had a higher rate of return to play.
Fig. 4.
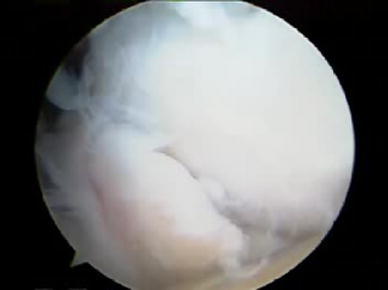
Arthroscopic loose-body removal
Fig. 5.
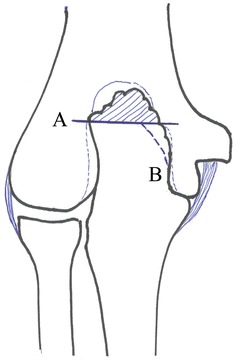
Arthroscopic olecranon resection. Only the A cut is recommended for baseball athletes
Total elbow arthroplasty
The field of TEA continues to evolve [25–28]. Currently, TEA represents a successful treatment alternative for patients with RA, posttraumatic osteoarthritis, and primary osteoarthritis, and for elderly patients with comminuted distal humerus fractures and to salvage distal humerus nonunion [29].
Designs of total elbow implants
In general, there are two broad categories of total elbow prostheses: unlinked and linked. Unlinked implant and surface replacement design are the same category. A common misunderstanding is to equate linked to constraint. The TEA design, of which longevity is <15 years, is more constrained than linked implants. Elbow stability after unlinked TEA is also important to prevent elbow dislocation and eccentric motion [29–33]. In the unlinked TEA, the design is surface replacement and components are not linked. Snap-in implants are basically the surface replacement design, but this implant is classified as a linked TEA. Longevity of the unlinked total elbow implant depends upon the precise positioning of components, ligament integrity, and elbow alignment and instability [33]. The most popular unlinked implant in Japan and Europe is the Kudo type (Fig. 6). The design of the joint surface of the Kudo type is composed of saddle-shaped components that dissipate axial loads from forearm to soft tissue, ligaments, joint capsule, and muscles. The ulnar component has lateral to medial free motion on the joint surface during elbow flexion and extension because there is no radial-head system. However, this unlinked implant is constrained in high rotational torque [34]. This surface replacement total elbow implant is currently the most reliable and ideal design and has been reported to yield long-term satisfactory results [35–39]. The three-component unlinked design of this prosthesis, which includes a radial-head component, is still controversial regarding toleration of its long-term use. Currently, most linked implants are semiconstrained [40–42]. The mechanism of this design is its function as a loose hinge joint, allowing approximately 2–4° rotation and 10° varus–valgus [42]. The early linked implants had a highly constrained joint surface and were associated with a high failure rate secondary to the transmission of marked stress to the stem–cement–bone interface [25–28]. Recent designs of the linked prosthesis are believed to transmit less stress to the bone–cement interface and to result in a more consistent long-term survival rate. The most commonly used linked implant is the Coonrad–Morrey prosthesis developed at the Mayo Clinic (Fig. 7). The humeral component is porous coated distally and has an anterior flange. This design increases rotational stability by bone grafting between the prosthesis and distal humerus at the anterior part. The proximal third of the ulnar component has a plasma spray coating. The components are coupled (linked) with a cobalt–chrome axis pin, employing the unique mechanism of pin within a pin. Both humeral and ulnar components have polyethylene bushing and allow rotational and varus–valgus physiological laxity [40–42].
Fig. 6.

Unlinked Kudo total elbow prosthesis
Fig. 7.

Linked Coonrad–Morrey total elbow prosthesis
Indications for total elbow arthroplasty
The best indication for successful outcome and longevity in TEA is rheumatoid elbow. In the earlier stage of Larsen stage III and in younger RA patients, there is usually sufficient bone stock and ligamentous integrity. In these cases, the use of unlinked implant is the best choice. Posttraumatic lesion and osteoarthritis of the elbow represents the most difficult conditions to treat. Some surgeons may select alternative procedures, such as interposition arthroplasty, but pain relief is not completely reproducible and patients may have postoperative instability. TEA provides more reliable results. The more active patients, such as those of younger age, those at risk for early mechanical failure, and polyethylene wear. In general, TEA should be avoided in patients younger than 60 years [40, 41]. The most common indications are acute comminuted distal humerus fractures and subsequent nonunion in elderly patients. Most patients with distal humerus fractures are successfully treated with open reduction and internal fixation. However, in patients >80 years with severe osteoporotic bone, stable internal fixation is usually difficult. In these circumstances, TEA is successfully used and provides good function from an early postoperative day without rehabilitation.
Implant selection
A reliable implant provides successful outcome in a broad spectrum of elbow disorders. In general, for patients who have unstable elbow, linked implants are generally selected, and for the stiff elbow, unlinked implants are selected. Severe contracture and/or ankylosis at the elbow in extension (20–30°) are contraindications to unlinked TEA, and in particular, implants without a radial head component design are not recommended due to the high risk of dislocation [33]. Osteoarthritis is also a contraindication for unlinked TEA. There is relative indication for linked TEA in elbow osteoarthritis. Acute comminuted distal humerus fractures and subsequent nonunion in elderly patients are the most common indications for a linked TEA, such as Coonrad–Morrey TEA. In the earlier stage of younger RA patients, there is usually sufficient bone stock, and the ligamentous integrity allows the use of an unlinked implant, such as the Kudo TEA.
The success of TEA depends greatly on prosthesis design and surgical technique, both of which require comprehensive knowledge of detailed anatomy of the elbow to facilitate the development of surgical approaches and accurate design of novel prostheses. The proper prosthesis selection and implantation are important for good functional outcome and longevity.
Acknowledgments
The authors acknowledge the contributions of Bernard F. Morrey and Kai-Nan An for help and advice. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1.An KN, Zobitz ME, Morrey BF. Biomechanics of the elbow. In: Morrey BF, editor. The elbow and its disorders, 3rd ed. Philadelphia: Saunders; 2009. p. 39–63.
- 2.Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68:887–891. [PubMed] [Google Scholar]
- 3.Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23:407–413. doi: 10.1177/036354659502300406. [DOI] [PubMed] [Google Scholar]
- 4.Roger P. van Riet, Francis Van Glabbeek, Bernard F. Morrey. Radial head fracture. In: Morrey BF, editor. The elbow and its disorders, 3rd ed. Philadelphia: Saunders; 2009. p. 359–81.
- 5.Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop. 1985;201:84–90. [PubMed] [Google Scholar]
- 6.Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg A. 1979;79:832–839. [PubMed] [Google Scholar]
- 7.Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002;359:657–662. doi: 10.1016/S0140-6736(02)07811-X. [DOI] [PubMed] [Google Scholar]
- 8.Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 9.Yeoh KM, King GJ, Faber KJ, Glazebrook MA, Athwal GS. Evidence-based indications for elbow arthroscopy. Arthroscopy. 2012;28:272–282. doi: 10.1016/j.arthro.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Carofino BC, Bishop AT, Spinner RJ, Shin AY. Nerve injuries resulting from arthroscopic treatment of lateral epicondylitis: report of 2 cases. J Hand Surg Am. 2012;37:1208–1210. doi: 10.1016/j.jhsa.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Waris W. Elbow injuries in javelin throwers. Acta Chir Scand. 1946;93:563–575. [PubMed] [Google Scholar]
- 12.Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68:1158–1163. [PubMed] [Google Scholar]
- 13.Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10:152–157. doi: 10.1067/mse.2001.112881. [DOI] [PubMed] [Google Scholar]
- 14.Rohrbough JT, Altchek DW, Hyman J, Williams RJ, 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30:541–548. doi: 10.1177/03635465020300041401. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31:332–337. doi: 10.1177/03635465030310030201. [DOI] [PubMed] [Google Scholar]
- 16.Callaway GH, Field LD, Deng XH, Torzilli PA, O’Brien SJ, Altchek DW, Warren RF. Biomechanical evaluation of the medial collateral ligament of the elbow. J Bone Joint Surg Am. 1997;79:1223–1231. doi: 10.2106/00004623-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RN, Weiland AJ. Valgus stability of the elbow. J Orthop Res. 1987;5:372–377. doi: 10.1002/jor.1100050309. [DOI] [PubMed] [Google Scholar]
- 18.O’Driscoll SW, Jaloszynski R, Morrey BF, An KN: Origin of the medial ulnar collateral ligament. J. Hand Surg. [Am.] 1992; 17:164-8. [DOI] [PubMed]
- 19.Regan WD, Korinek SL, Morrey BF, An KN. Biomechanical study of ligaments around the elbow joint. Clin Orthop. 1991;271:170–179. [PubMed] [Google Scholar]
- 20.Park MC, Ahmad CS. Dynamic contributions of the flexor-pronator mass to elbow valgus stability. J Bone Joint Surg Am 2004;86-A:2268–74. [DOI] [PubMed]
- 21.EL Cain JR, Andrews JR, Dugas JR, Wilk KE, McMichael CS, Walter JC, 2nd, Riley RS, Arthur ST. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38:2426–2434. doi: 10.1177/0363546510378100. [DOI] [PubMed] [Google Scholar]
- 22.Paletta GA, Wright RW. The modified docking procedure for elbow ulnar collateral ligament reconstruction: 2-year follow-up in elite throwers. Am J Sports Med. 2006;34:1594–1598. doi: 10.1177/0363546506289884. [DOI] [PubMed] [Google Scholar]
- 23.Slocum DB. Classification of elbow injuries from baseball pitching. Tex Med. 1968;64:48–53. [PubMed] [Google Scholar]
- 24.Cohen SB, Valko C, Zoga A, Dodson CC, Ciccotti MG. Posteromedial elbow impingement: magnetic resonance imaging findings in overhead throwing athletes and results of arthroscopic treatment. Arthroscopy. 2011;27:1364–1370. doi: 10.1016/j.arthro.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Lowe LW, Miller AJ, Allum RL, et al. The development of an unconstrained elbow arthroplasty. J Bone Joint Surg. 1984;66B:243–247. doi: 10.1302/0301-620X.66B2.6707061. [DOI] [PubMed] [Google Scholar]
- 26.Dee R. Total replacement arthroplasty of the elbow for rheumatoid arthritis. J Bone Joint Surg. 1972;54B:88–95. [PubMed] [Google Scholar]
- 27.Evans BG, Daniels AU, Serbousek JC. A comparison of the mechanical designs of articulating total elbow prosthesis. Clin Mater. 1988;3:235–248. doi: 10.1016/0267-6605(88)90060-1. [DOI] [Google Scholar]
- 28.Levy RN, Volz RG, Kaufer H, et al. Progress in arthritis surgery. Clin Orthop. 1985;200:299–321. [PubMed] [Google Scholar]
- 29.Ewald FC, Simmonds ED, Sullivan JA, et al. Capitellocondylar total elbow replacement in rheumatoid arthritis. J Bone Joint Surg. 1993;75A:498–507. doi: 10.2106/00004623-199304000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Weiland AJ, Weiss AP, Wills RP, Moore JR. Capitellocondylar total elbow replacement: a long-term follow-up study. J Bone Joint Surg. 1989;71A:217–222. [PubMed] [Google Scholar]
- 31.Souter WA. Arthroplasty of the elbow: with particular reference to metalic hinge arthroplasty in rheumatoid patients. Orthop Clin North Am. 1973;4:395–413. [PubMed] [Google Scholar]
- 32.King GJ, Itoi E, Niebur GL, Morrey BF, An KN. Motion and laxity of the capitellocondylar total elbow prosthesis. J Bone Joint Surg. 1994;76A:1000–1008. doi: 10.2106/00004623-199407000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Inagaki K, O’Driscoll SW, Neal PG, Uchiyama E, Morrey BF, An KN. Importance of a radial head component in Sorbie unlinked total elbow arthroplasty. Clin Orthop. 2002;400:123–131. doi: 10.1097/00003086-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Kamineni S, O’Driscoll SW, Urban M, Garg A, Berglund LJ, Morrey BF, An KN. Intrinsic constraint of unlinked total elbow replacements—the ulnotrochlear joint. J Bone Joint Surg. 2005;87A:2019–2027. doi: 10.2106/JBJS.C.00983. [DOI] [PubMed] [Google Scholar]
- 35.Kudo H, Iwano K, Watanabe S. Total replacement of the rheumatoid elbow with a hingeless prosthesis. J Bone Joint Surg. 1980;62A:277–285. [PubMed] [Google Scholar]
- 36.Kudo H, Iwano K. Total elbow arthroplasty with a non-constrained surface replacement prosthesis in patients who have rheumatoid arthritis. J Bone Joint Surg. 1990;72A:355–362. [PubMed] [Google Scholar]
- 37.Kudo H, Iwano K, Nishino J. Cementless or hybrid total elbow arthroplasty with titanium-alloy implants. J Arthroplast. 1994;9:269–278. doi: 10.1016/0883-5403(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 38.Kudo H, Iwano K, Nishino J. Total elbow arthroplasty with use of a nonconstrained humeral component inserted without cement in patients who have rheumatoid arthritis. J Bone Joint Surg. 1999;81A:1268–1280. doi: 10.2106/00004623-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Qureshi F, Draviaraj KP, Stanley D. The Kudo 5 total elbow replacement in the treatment of the rheumatoid elbow. J Bone Joint Surg 2010;92-B:1416–21. [DOI] [PubMed]
- 40.Morrey BF, Brian RS, Dobyns JH, et al. Total elbow arthroplasty. A five year experience at the Mayo clinic. J Bone Joint Surg. 1981;63A:1050–1063. [PubMed] [Google Scholar]
- 41.Gill DR, Morrey BF. The Coonrad-Morrey total elbow arthroplasty in patients who have rheumatoid arthritis. J Bone Joint Surg. 1998;80A:1327–1335. doi: 10.2106/00004623-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 42.O’Driscoll SW, An KN, Korinek S, Morrey BF. Kinematics of semi-constrained total elbow arthroplasty. J Bone Joint Surg. 1992;74B:297–299. doi: 10.1302/0301-620X.74B2.1544973. [DOI] [PubMed] [Google Scholar]


