Abstract
This archival cross-sectional investigation examined the impact of mood, reproductive status (RS), and age on polysomnographic (PSG) measures in women. PSG was performed on 73 normal controls (NC) and 64 depressed patients (DP), in the course of studies in menstruating, pregnant, postpartum, and peri- and postmenopausal women. A two-factor, between-subjects multivariate analysis of variance (MANOVA) was used to test the main effects of reproductive status (RS: menstrual vs pregnant vs postpartum vs menopausal) and diagnosis (NC vs DP), and their interaction, on PSG measures. To further refine the analyses, a two-factor, between subjects MANOVA was used to test the main effects of age (19 to 27 vs 28 to 36 vs 37 to 45 vs 46+ years) and diagnosis on the PSG data. Analyses revealed that in DP women, rapid eye movement (REM) sleep percentage was significantly elevated relative to NC across both RS and age. Significant differences in sleep efficiency, Stage 1%, and REM density were associated with RS; differences in total sleep time, Stage 2 percentage, and Stage 4 percentage were associated with differences in age. Both RS and age were related to differences in sleep latency, Stage 3 percentage, and Delta percentage. Finally, wake after sleep onset time, REM percentage, and REM latency did not vary with respect to RS or age. Overall, this investigation examined three major variables (mood, RS, and age) that are known to impact sleep in women. Of the variables, age appeared to have the greatest impact on PSG sleep measures, reflecting changes occurring across the lifespan.
Keywords: polysomnography (PSG), reproductive status, depression, aging, sleep quality
Abstract
Esta investigación evaluó el impacto del ánimo, del estado reproductive (ER) y de la edad en las mediciones polisomnográficas (PSG) de registros de corte transversal en mujeres. Las mediciones PSG se realizaron en 73 controles normales (CN) y 66 pacientes con depresión (PD), en estudios durante la etapa menstrual, el embarazo, el postparto y, la peri y postmenopausia. Para evaluar los principales efectos del estado reproductivo (ER: menstrual v/s embarazo v/s postparto v/s menopausia) y del diagnóstico (CN v/s PD) en la interacción con las mediciones PSG se utilizó un análisis de la varianza multivariado (MANOVA) entre sujetos, para dos factores. Para perfeccionar aun más los análisis en la evaluación de los principales efectos de la edad (19 a 27 v/s 28 a 36 v/s 37 a 45 v/s 46 y más años) y del diagnóstico respecto a los datos PSG se empleó MANOVA entre sujetos, para dos factores. Los análisis revelaron que en las mujeres con depresión el porcentaje de sueño de movimientos oculares rápidos (REM) fue significativamente mayor en relación a los CN tanto para ER como para edad. Hubo diferencias significativas para la eficiencia del sueño, el porcentaje de la etapa 1 y la densidad del sueño REM que se asociaron con el ER. Diferencias para el tiempo total de sueño y el porcentaje de las etapas 2 y 4 se asociaron con diferencias en la edad. Tanto el ER como la edad se relacionaron con diferencias en la latencia de sueño, el porcentaje de la etapa 3 y el porcentaje de delta. Por último, el tiempo para despertar después de iniciar el sueño, el porcentaje de sueno REM y la latencia REM no variaron con relación al ER ni a la edad. Esta investigación examinó globalmente tres importantes variables (ánimo, ER y edad) que se sabe que influyen en el sueño en la mujer. De estas variables la edad tuvo el mayor impacto en las mediciones PSG del sueño, reflejando los cambios que ocurren a lo largo de la vida.
Abstract
Cette étude croisée d'archives a analysé l'impact de l'humeur, de l'état reproducteur (ER) et de l'âge sur les mesures polysomnographiques (PSG) des femmes. Des mesures PSG ont été réalisées sur 73 patientes témoins normales (TN) et 64 patientes déprimées (PD), au cours d'études chez des femmes ayant leurs règles, enceintes, pendant le postpartum, en périménopause ou déjà ménopausées. Une analyse de variance multivariée (MANOVA) à deux facteurs intersujets a testé les principaux effets de l'état reproducteur (ER: femmes ayant leurs règles vs enceintes vs pendant le postpartum vs ménopausées), du diagnostic (TN vs PD), et leur interaction sur les mesures PSG. Pour affiner ultérieurement les résultats, une analyse MANOVA à deux facteurs intersujets a été utilisée pour tester les principaux effets de l'âge (19 à 27 vs 28 à 36 vs 37 à 45 vs + de 46 ans) et du diagnostic sur les données PSG. Les analyses ont montré que chez les PD, le pourcentage de sommeil à mouvements oculaires rapides (REM) ou sommeil paradoxal était significativement élevé par rapport aux TN, à ER et âge équivalents. L'ER était associé à des différences significatives de fonctionnement du sommeil, de pourcentage de sommeil de stade 1 et de densité de sommeil REM ; avec l'âge, la durée totale du sommeil et le pourcentage de sommeil des stades 2 et 4 étaient changé. L'ER et l'âge ont influé sur la latence du sommeil, le pourcentage de sommeil de stade 3 et le delta. Finalement, l'ER ou l'âge n'ont rien changé au réveil après l'installation du sommeil, au pourcentage et à la latence de sommeil REM. Cette étude a analysé globalement trois variables principales (humour, ER et âge) connues pour influer sur le sommeil chez les femmes. Parmi les variables, l'âge semble avoir le plus grand impact sur les mesures PSG du sommeil, reflétant les changements intervenant au cours de la vie.
Background
Sleep is vital for normal health and well-being. Without sufficient sleep, adults often experience functional decrements that may lead to accidents,1 increased risks for physical2,4 and mental illness,3,5,6 decreased cognitive performance4,7 (especially with aging8), and increased mortality.9 A recent Centers for Disease Control (CDC) analysis of 2006 data from the Behavioral Risk Factor Surveillance System (BRFSS) also determined that women are at higher risk of sleep disturbance (12.4%) than males (9.9%)10 and therefore, understanding the factors that impact sleep in women is an important focus for clinical research.11 Women report more sleep disturbance than men, but objective measures show less sleep disturbance.12,13
Measured objectively by polysomnography (PSG), sleep shows changes in architecture and distribution of sleep stages across the lifespan. For example, a meta-analysis by Ohayon et al14 showed that important sleep measures such as total sleep time (TST), sleep efficiency (SE), percentage of slow- wave sleep (SWS), percentage of REM sleep, and REM latency significantly decreased with advancing age in adults. Conversely, measures of sleep typically associated with less restful sleep (sleep latency [SL], Stage 1% and Stage 2% sleep, and wake after sleep onset [WASO] times) significantly increased with age. Furthermore, Ohayon et al found differences in quantitative sleep measures related to gender. Generally speaking, both sexes showed similar effects of aging on most sleep variables; however, larger effect sizes were observed in women for TST, SE, Stage 1 percentage, and REM latency, suggesting that aging had a greater impact on these variables in women than men. As well, women appeared to have longer TST and SL, lower Stage 2 percentage sleep, and greater percentage of SWS than agematched men.
In addition to age-related changes, women also experience gender-specific physiological changes that potentially disrupt their sleep. Changes during the menstrual cycle,15 pregnancy,16 in the postpartum period,17 and at menopause18 are associated with alterations in qualitative and quantitative sleep measures. Women are also more predisposed to develop depressed mood,19 especially during these periods of hormonal change20 which may further compromise their nighttime sleep.
Nevertheless, while subjective reports of sleep disturbances in association with disturbed mood, aging, and altered reproductive status (RS) are widely reported, carefully controlled studies of objectively measured sleep alterations associated with alterations in mood and RS are uncommon (see review in ref 21).
Qualitative and quantitative sleep measures during the menstrual cycle
Research on sleep during the menstrual cycle in normal women has failed to show significant variation across the menstrual cycle in objective ratings of TST, SE, SL, REM sleep latency, or SWS.22 However, Shechter, et al found that total REM sleep decreased and total Stage 2 and SWS increased during the luteal compared with the follicular phase.23 In women with premenstrual syndrome (PMS) or premenstrual dysphoric disorder (PMDD), quantitative EEG studies show those with severe PMS experienced more WASO and microarousals during the late luteal phase compared with the follicular phase; however, comparable, effects occurred in normal control (NC) women.24 Compared with NCs, women with PMS/PMDD also showed increased REM latency in both menstrual phases, and increased slow-wave activity during the late luteal phase. Additionally, Parry et al found that women with PMDD had more Stage 2 and less REM sleep (minutes and percentage) than NCs, irrespective, of menstrual phase.25 Stage 3 sleep minutes also decreased, and intermittent awakenings increased during the late luteal phase in both symptomatic and asymptomatic women.
Qualitative and quantitative sleep measures during pregnancy
To date, PSG studies of sleep in pregnant women have yielded equivocal results. For example, in thirdtrimester women Hertz et al26 found that total WASO and Stage 1 sleep increased significantly, while SE and REM percentage sleep decreased compared with nonpregnant NC women. Brunner et al27 reported that WASO increased from the second to the third trimesters while REM sleep decreased from the first to second trimester. Similarly, Schorr et al28 found reduced amounts of Stages 3 and 4 sleep in pregnant women relative to non-pregnant NCs. Lee et al29 found an increase in TST by 11 to 12 weeks' gestation, but with less deep sleep and more awakenings. Conversely, Driver et al30 found that SWS was significantly greater at 27 to 39 weeks than at 8 to 16 weeks, while REM sleep remained unchanged. Other studies examining SWS have generally shown that total Stage 4 sleep decreases before delivery.31-33
With regards to mood disturbance during pregnancy, few prospective studies have been performed. For example, women with a history of mood disorder appear to experience more sleep disturbance and a reduction in REM latency from early to late pregnancy.34 Shorter (or longer) REM sleep during pregnancy has been found to precede postpartum mood disorders.12 Field et al found more sleep disturbance in depressed than nondepressed women during the second and third trimesters.35 In contrast, Skouteris et al found that sleep quality and depressive symptoms remained relatively stable across pregnancy; however, poor sleep quality early in pregnancy predicted higher levels of depression later in pregnancy, while depressive symptoms early in pregnancy were unrelated to sleep quality later.36
Qualitative and quantitative sleep measures during postpartum
As with pregnancy, few comprehensive, objective studies of postpartum sleep have been published. PSG studies in NC typically show a restoration of normal SE and WASO relative to pregnant levels by 3 to 5 months postpartum. Additionally, REM sleep typically decreases after delivery.12 Coble et al,34 in a home-based EEG study of women from 12 weeks' gestation through 8 months postpartum, found that the most significant effects on sleep were observed at 4 weeks postpartum, at which time sleep continuity became disrupted due to wakefulness (approximately 1 hour per night) associated with infant care.34
In women with a history of depression, childbearing has been associated with greater changes in TST and with reduced REM latency. Studies indicate that depression risk increases substantially postpartum,37 especially in women who report depression and sleep disturbances during the month before delivery; they also reported more depressive symptoms 3 months postpartum.38 Frank et al39 found that women with pregnancy-related depression showed longer REM sleep time and more REM activity.
Qualitative and quantitative sleep measures during menopause
Research on objective sleep measures in menopausal women has produced mixed scientific findings. In a study of 82 midlife women classified as poor or good sleepers according to either self-reported sleep quality or sleep efficiency, Shaver et al found that menopausal women showed more wakefulness and Stage 2 sleep and less REM sleep than good sleepers.40 In one large epidemiologic study,41 objective, sleep quality was not found to be worse in peri- or post-menopausal women than in premenopausal women. In fact, postmenopausal woman had more deep sleep and significantly longer TST Kalleinen et al found that while TST was similar in premenopausal and postmenopausal women, TST was significantly longer in younger women and SE was greater in younger women, while pre- and postmenopausal women had less SWS and a higher frequency and duration of WASO than younger women.42
To our knowledge, few researchers have examined the effects of mood disturbance on PSG measures of sleep in menopausal women. One investigation43 determined that depressive and/or anxiety symptoms were not significantly associated with shorter REM latency and/or lower levels of deep sleep as hypothesized from previously published research. In another report, Polo-Kantola et al found that impaired subjective sleep quality was associated with climacteric vasomotor symptoms, but did not manifest as abnormalities in PSG sleep recordings.44
In an effort to clarify findings from the extant literature, we have, in this archival cross-sectional investigation, simultaneously examined the impact of mood, reproductive status (RS), and age on PSG measures of objective sleep in women. We hypothesized that these factors would contribute cumulatively to alter sleep architecture, thereby impacting the quality and quantity of sleep women experience across their reproductive lifespan.
Methods
Subjects
We obtained overnight PSG on 73 normal controls (NC) and 64 unipolar depressed patients (DP), in the course of studies of clinical depression in menstruating, pregnant, postpartum, and women in peri- and post-menopause.
Data for this report were collected between November 1989 and July, 2010. Participant recruitment procedures are described in detail elsewhere.45 Briefly, prospective subjects residing in San Diego, California were screened via telephone. Women were eligible to participate if they did not: i) smoke; ii) use hormonal contraceptives; or iii) use medications, herbs, or over-the-counter preparations (eg, antihistamines, asthma medications, valerian root, black cohosh, melatonin, St John's Wort, and/or decongestants with epinephrine [pseudoephedrine]) that would interfere with neuroendocrine measures. As per Benloucif et al, Tylenol was permitted (but not aspirin).46 Participants agreed to multiple overnight hospital stays in the General Clinical Research Center, where they were permitted to bring their child if necessary. Eligible women underwent the following laboratory tests: a chemistry panel, thyroid indices, complete blood count, urinalysis, and urine toxicology screening. Eligible women could not have significant medical illness, and women who were receiving drug treatment were required to discontinue any medication that would interfere with study measures. We required DP women to discontinue antidepressant treatment >2 weeks (>4 weeks for fluoxetine treatment) before the start of the study. For participants who stopped antidepressant use prior to the study, their baseline mood ratings were obtained only after antidepressant withdrawal. In addition to ratings done during the evaluation phase, we also collected mood ratings on the evening before the PSG data collection. No participants were permitted to have had an alcohol abuse or dependency problem within the past year. Women with bipolar or primary anxiety disorders were excluded from the study, but women with personal or family histories of unipolar depression were included in both the NC and DP groups.
To establish DSM-IV entrance and baseline criteria, trained clinicians administered the following assessments to each participant: the Structured Clinical Interview for DSM-IV (SCID)47 and at least two baseline evaluation ratings, scheduled 1 week apart, using objective ratings with the Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders (SIGH-SAD version),48 which includes the 21-item Hamilton Depression Rating Scale49 and an eight-item atypical depressive symptom inventory,50 plus subjective ratings with the Beck Depression Inventory (BDI).51 We also evaluated mood in pregnant and postpartum women with the Edinburgh Postnatal Depression Scale (EPDS),52 which has been validated for use during pregnancy.53 For study inclusion, DP women were required to have a mean SIGH-SAD score >14 and BDI and EPDS scores >10 for 2 weeks. In postpartum women, the onset of a major depressive episode needed to be within 3 months postpartum. Normal control subjects were required to have a mean SIGH-SAD score <8 and a mean BDI score <5. We based diagnosis of PMDD subjects, in part, on daily mood ratings showing symptoms of major depression in proximity to menstruation for two consecutive menstrual cycles.54 We studied menstruating women twice, once in the follicular and once in the luteal menstrual phase, one full cycle apart, based on the time of the mid-cycle luteinizing hormone (LH) surge using a eoiorimetric urinary immunoassay (Clearblue® colorimetric LH assay, Princeton, New Jersey) to document ovulation. (We report here only the data from the luteal phase, the time period when PMDD symptoms appear.) Finally, three of 21 NC women were perimenopausal, with irregular menses for at least 1 year; the remaining NC women were postmenopausal, being without menses for at least 1 year. One depressed woman was perimenopausal; the remaining 10 depressed women were postmenopausal, without menses for at least 1 year, who met DSM-IV criteria for a major depressive episode.55 To confirm verbal reports, postmenopausal status was also verified by FSH > 40 mlU/mL at the time of testing.
Procedure
Women who met entrance criteria were admitted to the University of California San Diego (UCSD) General Clinical Research Center (GCRC) at 16:00 h. Following one night of adaptation to the sleep room, licensed nurses inserted an intravenous catheter at 17:00 h. and drew blood (3 cc) every 30 min from 18:00 h to 11:00 h. for measurement of hormone levels (reported elsewhere). To minimize sleep disturbances, nurses withdrew blood samples from an adjacent room, through an intravenous catheter connected to a tube threaded through a porthole in the wall. Serum for E2 and P4 was obtained at 18:00 h and again at 6:00 h. Participants remained on bed rest in a single room with double doors, with windows covered with heavy drapery to block extraneous light from entering between 16:00 h and 11:00 h. Nurses or sleep technicians entered the room only when necessary (recorded by infrared camera), using a pen-sized dim red flashlight.
Sleep measures
Sleep studies were conducted during two consecutive nights in the J. Christian Gillin Laboratory of Sleep and Chronobiology. The first night was used to acclimate subjects to the sleep laboratory environment and to rule out intrinsic sleep disorders other than insomnia. We analyzed sleep measures only from the second, postadaptation night. (Women studied during the menstrual cycle had adaptation nights before both the follicular and luteal phase measurements. This report includes only the data from the luteal phase.) Subjects were required to be in bed by 22:00 and were allowed to sleep and awaken at their habitual wake time.
The recording montage consisted of a minimum 10 electrophysiologic signals. The basic montage included 2 electrooculograms (EOGs) referenced to a single mastoid, 6 EEGs referenced to linked mastoids [F3, F4, C3, C4, O1 and O2], a bipolar mentalis electromyogram (EMG), and an electrocardiogram (ECG). Several measures, in addition to our core montage, were obtained. These included 1 channel of nasal/oral airflow and 2 channels of leg-related motor activity (right and left tibial EMGs).The airflow and tibial data were used to detect obstructive sleep apnea (OS A) and periodic limb movements (PLMs), respectively. The second PSG night was used to characterize subjects' sleep. The montage was the same as the first night except that OSA and PLMs were not measured.
All-night PSG recordings (EEG, EOG, submental EMG) were digitized, stored on optical discs and scored visually in 30-second epochs without knowledge of conditions for sleep stages according to Rechtschaffen and Kales56 criteria by trained sleep technicians (inter-rater reliability coefficient 0.85). We measured PSG-derived TST, SL, SE, WASO, and percentages of Stages 1-4, slow wave sleep (SWS: sum of Stage 3 and 4), and REM, as well as REM latency, and REM density.
The UCSD Institutional Review Board approved the study protocol. All subjects gave written informed consent after study procedures were explained fully.
Statistical analyses
Subject RS was incompletely crossed with age; eg, menstruating, pregnant, and postpartum women spanned ages from 19 to 46, but none were over 46 years of age. Therefore, to assess effects of RS and age on PSG, we analyzed the data using two approaches:
Reproductive status x diagnosis
We used a two-factor, between subjects multivariate analysis of variance (MANOVA) to test the main effects of RS (menstrual vs pregnant vs postpartum vs menopausal) and diagnosis (NC vs DP), and their interaction, on PSG measures. To control for the contribution of age to the RS differences, we reanalyzed the results including age as a covariate in the MANCOVA in those cases where its significance was P<.10. When the main effect of RS was significant, we did post-hoc comparisons of paired reproductive epochs, using the Bonferroni adjustment for multiple comparisons.
Age category x diagnosis
To further refine our analyses of age effects on PSG measures, we used a two-factor, between-subjects MANOVA to test the main effects of age category (1927 vs 28-38 vs 39-72 vs 46+ years of age) and diagnosis on our PSG data. When the age category was significant as a main effect we reanalyzed the results applying RS as a covariate in the MANCOVA only in cases where RS reached a significance level of P<.10. As above, we used Bonferroni-adjusted paired comparisons for post-hoc analyses of significant main effects of age category.
Results
Subject characteristics
Complete data were obtained from 73 NC and 62 DP. Table I shows the distribution of women at different reproductive stages along with their ages and SIGHSAD scores at the time of data collection. Detailed descriptions of subject characteristics are provided in Parry et al.57-59 None of the pregnant participants brought children to the GCRC on test nights. Six of 13 postpartum NC women brought their children and nursed them on test nights; two others pumped their breasts but did not nurse children in the GCRC. Statistical analyses of total sleep time, sleep latency, sleep efficiency, and wake after sleep onset showed no significant differences between NC and DP groups as a function of breastfeeding status, child's presence in the room, or their interaction.
Table I. Distribution of age ranges and mean (SD), Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal.
| Menstrual (luteal) | Pregnant | Postpartum | Menopausal | |||||||||
| Diagnosis | (n) | Age range | SIGH-SAD | (n) | Age range | SIGH-SAD | (n) | Age range | SIGH-SAD | (n) | Age range | SIGH-SAD |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||||||||
| NC (n=73) | 15 | 23-44 | 4.0 (4.3) | 24 | 19-38 | 9.2 (46) | 13 | 23-35 | 4.7 (3.4) | 21 | 46-72 | 5.1 (4.6) |
| DP (n=64) | 16 | 22-44 | 15.3 (12.1) | 16 | 19-41 | 23.8 (6.0) | 21 | 19-39 | 24.9 (8.2) | 11 | 48-59 | 26.2 (11.2) |
| Total (n) | 31 | 40 | 34 | 32 |
Effects of reproductive status and diagnosis on polysomnographic measures (with age as a covariate)
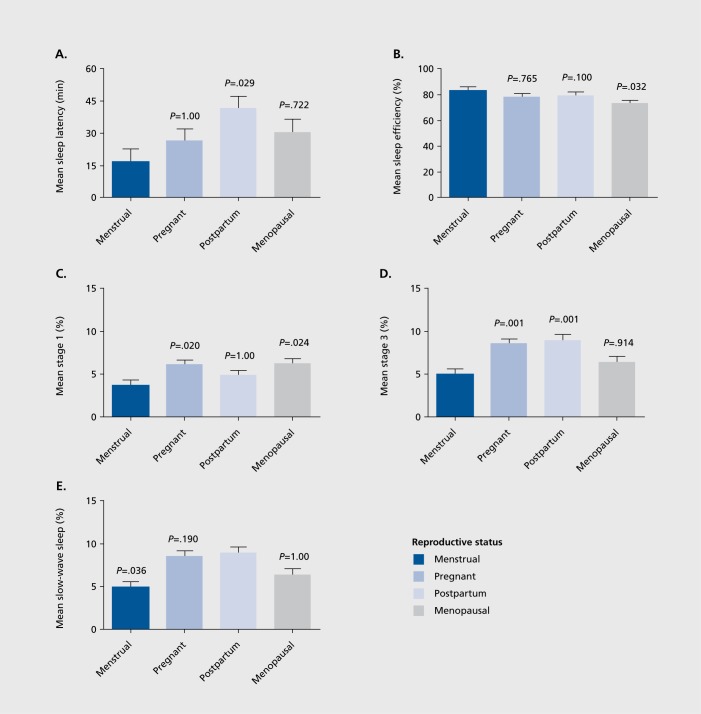
The omnibus MANOVA (without covariate) was highly significant for RS (P<.00001) but was non-significant for diagnosis (P=.364) and the RS x diagnosis interaction (P=.811). Univariate ANOVA showed significant effects of RS in 11 of the 14 PSG variables examined. However, the covariate of age was correlated to a substantial degree (P<.10, at least) in five of the PSG measures, and after including age as a covariate in the analyses, significant main effects of RS were obtained for SE (P=.027), SL (P=.041), S1 % (P=.008), S3% (P=.0001), SWS % (P=.0001), and REM density (P.020) (Table II). Pair-wise comparisons of age-adjusted significant values (with Bonferroni correction) are displayed in Figure 1.
Figure 1. Mean + SEM polysomnography (PSG) measures as a function of reproductive status. P-values denote Bonferroni-adjusted significance of differences from menstrual mean (A - D) or postpartum mean (E).
Table II. F-ratios and P-values for analyses of effects of reproductive status (RS: menstrual vs pregnant vs postpartum vs menopausal) and age category (19-27, 28-36, 37-45, 46+) on polysomnographic (PSG) variables, covariate adjustment was applied when the covariate (age, for reproductive status; reproductive status, for age category) was significant at P<. 10. Significant effects (P<.05) are highlighted in bold. * Results of analyses with covariate, where the P-value of the age covariate was P<.10. + Results of analyses with covariate, where the P-value of the RS covariate was P<.10.
| Effect of → | Reproductive status | Age category | ||
| PSG variable | F(3,130) | P | F(3,130) | P |
| Total sleep time | 0.092* | .985* | 5.511 | .001 |
| Sleep efficiency | 3.146 | .027 | 2.401 | .071 |
| Sleep latency | 2.832 | .041 | 2.905+ | .037+ |
| Wake after sleep onset | 2.338* | .077* | 1.258+ | .292 |
| Stage 1 % | 4.074 | .008 | 2.482 | .064 |
| Stage 2 % | 0.885* | .451* | 3.450+ | .019+ |
| Stage 3 % | 7.930 | .0001 | 7.203+ | .001+ |
| Stage 4 % | 2.055* | .109* | 8.592+ | .0001+ |
| Slow-wave sleep % | 4.838* | .003* | 9.386+ | .0001+ |
| REM % | 1.364 | .257 | 1.771 | .156 |
| REM latency | .0460 | .711 | 0.257 | .856 |
| REM density | 3.404* | .020* | 1.766 | .157 |
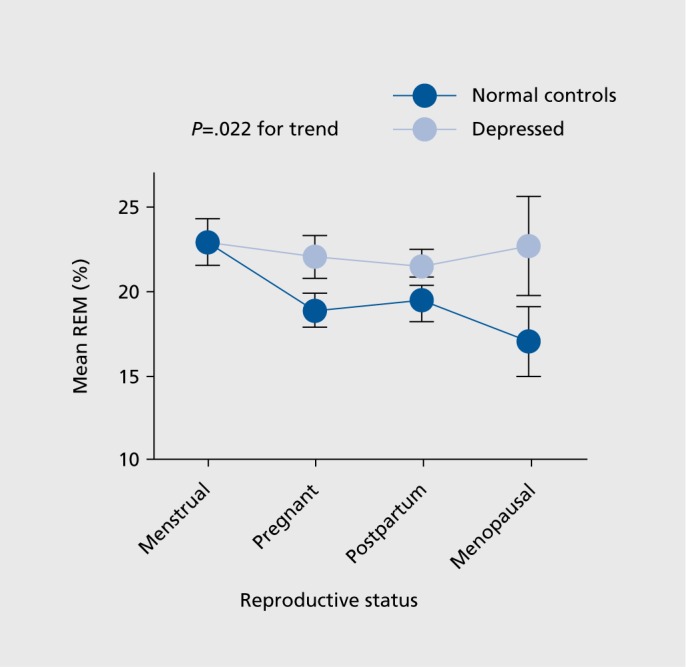
We found only one significant mood-related effect: REM percentage was significantly greater in DP vs NC across RS groups (Group means + SEM = 22.3+0.9 vs 19.6+0.8%, F(1,130) = 5.335, P .022) as illustrated in Figure 2.
Figure 2. Mean + SEM REM percentage in norma! controls and depressed patients as a function of reproductive status.
Effects of age category and diagnosis on polysomnographic measures (with reproductive status as a covariate)
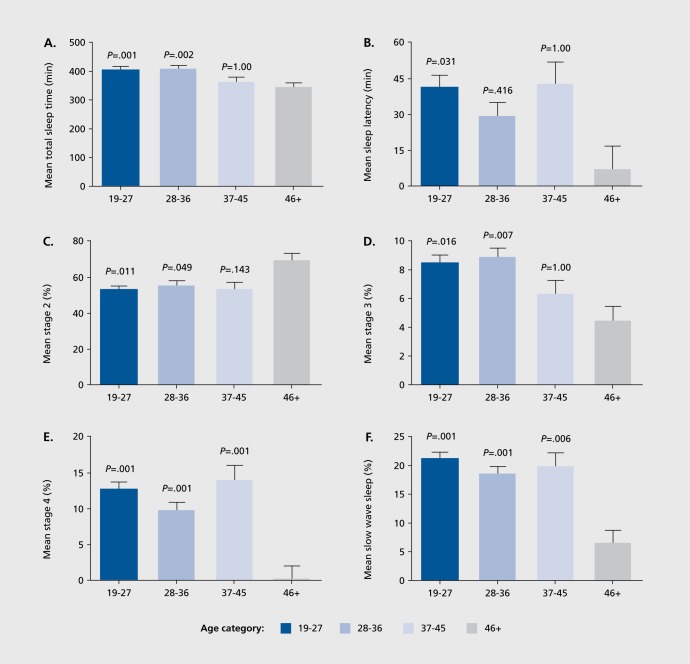
The omnibus MANOVA (without covariate) was significant for age category (P<.00001) but was non-significant for diagnosis (P=.127) and the age category x diagnosis interaction (p = .728). Univariate ANOVA showed significant effects of age category in 8 of the 14 PSG variables examined. However, RS was correlated with four of the PSG measures to a substantial degree (P< .10, at least), and after including RS as a covariate in the analyses, significant main effects of age category were obtained for TST (P=.001), SL (P=.037), S2 % (P=.019), S3 % (P=.001), S4% (P=. 0001), and SWS % (P=.0001) (Table II). Pair-wise comparisons of age-adjusted significant values (with Bonferroni correction) are displayed in Figure 3.
Figure 3. Mean + SEM polysomnography measures as a function of age category. P-values denote Bonferroni-adjusted significance of differences from menopausal mean.
In confirmation of the RS analyses, we found only one significant effect of diagnosis in relation to the age category: REM percentage was significantly greater in DP vs NC across age groups (Group means + SEM =22.3+0.8% vs 19.6+0.8%, F(1,130) = 7.190, P=.008). Post-hoc analyses showed that DP who were 37 years of age or older had significantly greater REM percentage than NC (F(1,134) =9.285, P=.003); DP younger than 37 years old did not differ significantly from NC (P> .05).
Discussion
We evaluated objectively measured PSG sleep cross-sectionally in women, examining the combined effects of mood, reproductive status, and age on sleep parameters.
Given the unique challenges to sleep that women may face during periods of reproductive change, we sought to elucidate the contribution of these changes to sleep alteration, beyond those accounted for by the aging process alone.
In the first level of our analyses we examined the contribution of depressed mood on PSG. Contrary to expectation, we found a reliable effect of depressed mood on only one PSG measure: REM percentage, which was significantly elevated in DP versus NC across both RS and age.
These results are consistent with earlier evidence showing increased REM percentage as a characteristic marker of sleep in depressed individuals (eg, Frank et al,39 Walker60) and a decrease from baseline in REM percentage in depressed patients during recovery.61 With this exception of REM percentage, the absence of reliable changes in objective sleep measures in depressed women across ages and reproductive epochs confirms earlier findings (see review in ref 20), and raises the question: why are subjective complaints of sleep disturbances in depression not uniformly confirmed by PSG analysis? While our data cannot provide a definitive answer, it is conceivable that measurements made under strictly controlled laboratory conditions lack sufficient ecological validity relative to natural sleep, thereby leading to systematic underestimates of the sleep alterations depressed women experience under natural conditions.29,62,63 Alternatively, subjective reports may actually provide an accurate representation of a patient's sleep, but procedures by which “objective” sleep is measured may fail to identify small EEG differences (ie, microarousals and high-frequency EEG) that may contribute to the perception of poor sleep.64,65
Consistent with other reports14 we also observed a trend toward decreased REM percentage across the lifespan which was observed only in the NC but not DP women. Conversely, the lack of difference in other objective PSG sleep measures between the groups appears to be at odds with studies that have found that DP typically exhibit reduced REM latencies, as well as increased SL, and WASO.60 While our investigation failed to find compelling evidence for sleep alterations in DP versus NC, some evidence did emerge supporting the observation that mood disturbance in DP women may lead to changes in REM sleep over the reproductive lifespan. In the second level of our analyses we examined the contribution of RS and age on sleep. As Table II shows, our analyses revealed that PSG measures differed in response to changes in RS, age, or both, while some differed in response to neither. For example, significant differences in SE, Stage 1 percentage, and REM density were associated primarily with differences in RS rather than age; differences in TST, Stage 2 percentage, and Stage 4 percentage were associated primarily with differences in participant's ages, rather than their RS. On the other hand, both RS and age were related significantly to differences in SL, Stage 3 percentage, and SWS percentage. Finally, WASO, REM percentage, and REM latency did not vary significantly with changes in either RS or age.
To examine these results more completely we ran a separate multivariate analysis on RS, including age as a covariale in those instances where age contributed substantially to the dependent variable (s) of interest. Results of these analyses showed that menstruating women had significantly shorter SL than postpartum women and greater SE than menopausal women. Menstruating women also had significantly less light (Stage 1 percentage) sleep relative to pregnant and menopausal women and less Stage 3 percentage sleep than pregnant and postpartum women. Postpartum women, however, appeared to have the most deep sleep (highest SWS percentage) of the groups, especially when compared with menstruating women. Since this is a novel investigation examining PSG sleep across RS, it is unclear how these findings relate to previously published literature, on specific RS groups. The observation that postpartum women had the most SWS activity could be interpreted in the light of likely sleep pressure brought on by infant care needs. Since the PSG was performed in a clinic environment, it is possible that postpartum women may have been able to use this setting to “catch up” on needed rest while experiencing a break from their normal nocturnal responsibilities at home.
When age was examined separately in the multivariate analyses using RS as a covariate age groups differed on several PSG variables. More specifically, the paired comparison data (Figure 3) showed that older women (46 years old+) had significantly less TST than younger women (19 to 36 years old), more Stage 2 percentage than women 19 to 36 years old, less Stage 3 percentage sleep than women 19 to 36 years old, and considerably less Stage 4 percentage and SWS percentage than women 19 to 45 years old. Only SL appeared to be problematic for younger women as 19 to 26 years old had significantly longer SL than older (46+ years old) women. In keeping with other published reports on the aging effects on sleep (eg, ref 13), it appeared from our analyses that advancing age had profound impact on sleep quality and quantity, beginning at middle age. While such changes in sleep may be an inevitable consequence of aging, it is not clear that such changes necessarily lead to decrements in general health, functioning or mood. As such, further examination of these findings may reveal how these age-related changes impact individual well-being.
In summary, this investigation simultaneously examined three major variables (mood, RS, and age) that are known to impact sleep in women. We found that age appeared to have the greatest impact on PSG sleep measures, though RS showed considerable overlap with age and was independently related to significant changes in several PSG measures, most notably SE. Conversely, mood effects on PSG measures were minimal, being restricted to REM percentage. As expected, younger and menstruating women experienced better sleep versus older and menopausal women, although postpartum women obtained the most SWS of any group. Taken together, the results of this study support the hypothesis that significant differences in PSG result from changes that women experience across the reproductive lifespan. Therefore, researchers and clinicians need to be cognizant of these factors when designing studies and/or dealing with clinical issues related to women's health.
Limitations
The primary limitation to the study is the cross-sectional nature, of the sample. Some reproductive status and age effects are unavoidably confounded, with individuals experiencing simultaneous changes in both (eg, menopause and age), making it impossible to completely separate the two factors for analysis. Second, in an effort to examine sleep more broadly in our sample, we did not control for various factors within each reproductive epoch that might modulate qualitative and quantitative PSG measures (eg, weeks pregnant or postpartum, luteal versus follicular phase in postpartum women who had resumed menstruation, peri- versus post-menopausal status). Lastly, the data collected for this investigation were obtained over an extended period of time, which may have lead to cohort effects and/or other subtle variations in data acquisition, and this could have affected the results.
Conclusions
Overall, this investigation examined three major variables (mood, RS, and age) that are known to impact sleep in women. Age appeared to have the greatest impact on PSG sleep measures, although RS showed considerable overlap with age. Taken together, the results of this study support the hypothesis that significant differences in PSG result from changes that women experience across the reproductive lifespan.
Abbreviations
- DP
depressed patients
- NC
normal controls
- PMDD
premenstrual dysphoric disorder
- PMS
premenstrual syndrome
- PSG
polysomnographic
- RS
reproductive status
- SE
sleep efficiency
- SIGH-SAD
Structured Interview Guide for the Hamilton Rating Scale - Seasonal Affective Disorders
- SL
sleep latency
- SWS
short-wave sleep
- TST
total sleep time
- WASO
wake after sleep onset
Contributor Information
Henry J. Orff, Department of Psychiatry, University of California San Diego, California, USA.
Charles J. Meliska, Department of Psychiatry, University of California San Diego, California, USA.
Ana Lopez, Department of Psychiatry, University of California San Diego, California, USA.
Fernando Martinez, Department of Psychiatry, University of California San Diego, California, USA.
Diane Sorenson, Department of Psychiatry, University of California San Diego, California, USA.
Barbara L. Parry, Department of Psychiatry, University of California San Diego, California, USA.
REFERENCES
- 1.Balter MB., Uhlenhuth EH. New epidemiologic findings about insomnia and its treatment. J Clin Psychiatry. 1992;53 (suppl):34–39; discussion 40-32. [PubMed] [Google Scholar]
- 2.Akerstedt T., Nilsson PM. Sleep as restitution: an introduction. J Intern Med. 2003;254:6–12. doi: 10.1046/j.1365-2796.2003.01195.x. [DOI] [PubMed] [Google Scholar]
- 3.Strine TW., Chapman DP. Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Med. 2005;6:23–27. doi: 10.1016/j.sleep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Banks S., Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 5.Ford DE., Cooper-Patrick L. Sleep disturbances and mood disorders: an epidemiologic perspective. Depress Anxiety. 2001;14:3–6. doi: 10.1002/da.1041. [DOI] [PubMed] [Google Scholar]
- 6.Kamphuis J., Meerlo P., Koolhaas JM., Lancel M. Poor sleep as a potential causal factor in aggression and violence. Sleep Med. 2012;13:327–334. doi: 10.1016/j.sleep.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Van Dongen HP., Maislin G., Mullington JM., Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-lsrael S. Sleep and aging: prevalence of disturbed sleep and treatment considerations in older adults. J Clin Psychiatry. 2005;66 (suppl 9):24–30 quiz 42-23. [PubMed] [Google Scholar]
- 9.Kripke DF., Garfinkel L., Wingard DL., Klauber MR., Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. Perceived insufficient rest of sleep among adults-United States, 2008. MMWR Weekly. 2009;58:1175–1179. [PubMed] [Google Scholar]
- 11.Lee KA., Kryger MH. Women and sleep. J Womens Health (Larchrnt). 2008;17:1189–1190. doi: 10.1089/jwh.2007.0574. [DOI] [PubMed] [Google Scholar]
- 12.Parry BL., Martinez LF., Maurer EL., Lopez AM., Sorenson DL., Meliska CJ. Sleep rhythms and women's mood. Part I: Menstrual cycle, pregnancy and postpartum. Sleep Med Rev. 2006;10:129–144. doi: 10.1016/j.smrv.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Parry BL., Martinez LF., Maurer EL., Lopez AM., Sorenson DL., Meliska CJ. Sleep, rhythms and women's mood. Part II: Menopause. Sleep Med Rev. 2006;10:197–208. doi: 10.1016/j.smrv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon MM., Carskadon MA., Guilleminault C., Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 15.Hachul H., Andersen ML., Bittencourt LR., Santos-Silva R., Conway SG., Tufik S. Does the reproductive cycle influence sleep patterns in women with sleep complaints? Climacteric. 2010;13:594–603. doi: 10.3109/13697130903450147. [DOI] [PubMed] [Google Scholar]
- 16.Wilson DL., Barnes M., Ellett L., Permezel M., Jackson M., Crowe SF. Decreased sleep efficiency, increased wake after sleep onset and increased cortical arousals in late pregnancy. Aust NZJ Obstet Gynaecol. 2011;51:38–46. doi: 10.1111/j.1479-828X.2010.01252.x. [DOI] [PubMed] [Google Scholar]
- 17.Insana SP., Stacom EE., Montgomery-Downs HE. Actual and perceived sleep: associations with daytime functioning among postpartum women. Physiol Behav. 2011;102:234–238. doi: 10.1016/j.physbeh.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pien GW., Sammel MD., Freeman EW., Lin H., DeBlasis TL. Predictors of sleep quality in women in the menopausal transition. Sleep. 2008;31:991–999. [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Mental Health. Available at: http://www.nimh.nih.goV/statistics/1 MDD_ADULT.shtml. xx. xx;xx:xx–xx. [Google Scholar]
- 20.Lasiuk GC., Hegadoren KM. The effects of estradiol on central serotonergic systems and its relationship to mood in women. Biol Res Nurs. 2007;9:147–160. doi: 10.1177/1099800407305600. [DOI] [PubMed] [Google Scholar]
- 21.Meliska CJ., Martinez LF., Parry BL. Sleep-endocrine relationships in depressed women across the reproductive cycle. In: Pandi-Perumal SR, Kramer M, eds. Sleep and Mental Illness. Cambridge, UK: Cambridge University Press; 2010;xx:xx–xx. [Google Scholar]
- 22.Driver HS., Dijk DJ., Werth E., Biedermann K., Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728–735. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- 23.Shechter A., Varin F., Boivin DB. Orcadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33:647–656. doi: 10.1093/sleep/33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker FC., Kahan TL., Trinder J., Colrain IM. Sleep quality and the seep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30:1283–1291. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parry BL., Mendelson WB., Duncan WC., Sack DA., Wehr TA. Longitudinal sleep EEG, temperature, and activity measurements across the menstrual cycle in patients with premenstrual depression and in age-matched controls. Psychiatry Res. 1989;30:285–303. doi: 10.1016/0165-1781(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 26.Hertz G., Fast A., Feinsilver SH., Albertario CL., Schulman H., Fein AM. Sleep in normal late pregnancy. Sleep. 1992;15:246–251. doi: 10.1093/sleep/15.3.246. [DOI] [PubMed] [Google Scholar]
- 27.Brunner DP., Munch M., Biedermann K., Huch R., Huch A., Borbely AA. Changes in sleep and sleep electroencephalogram during pregnancy. Sleep. 1994;17:576–582. doi: 10.1093/sleep/17.7.576. [DOI] [PubMed] [Google Scholar]
- 28.Schorr SJ., Chawla A., Devidas M., Sullivan CA., Naef RW., Morrison JC. 3rd. Sleep patterns in pregnancy: a longitudinal study of polysomnography recordings during pregnancy. J Perinatoi. 1998;18:427–430. [PubMed] [Google Scholar]
- 29.Lee KA., Zaffke ME., McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95:14–18. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 30.Driver HS., Shapiro CM. A longitudinal study of sleep stages in young women during pregnancy and postpartum. Sleep. 1992;15:449–453. doi: 10.1093/sleep/15.5.449. [DOI] [PubMed] [Google Scholar]
- 31.Petre-Quadens O., De Lee C. Sleep-cycle alterations during pregnancy, postpartum, and the menstrual cycle. In: Ferin M, Halberg F, Richart R, Van de Wiele RL, eds. Biorhythms and Human Reproduction. New York, NY: John Wiley and Sons; 1974;xx:335–352. [Google Scholar]
- 32.Roffwarg HP., Frankel BL., Pessah M. The nocturnal sleep pattern in pregnancy. Paper presented at: Annual Meeting of the Association for the Psychophysiological Study of Sleep; March, xx. 1968;xx:xx–xx. [Google Scholar]
- 33.Karacan I., Williams WB., Webb H., Agnew RJ. Abstracts. Association for the Psychophysiology Study of Sleep Meeting. 1967;4:378–xx. [Google Scholar]
- 34.Coble PA., Reynolds CF., Kupfer DJ., Houck PR., Day NL., Giles DE. 3rd. Childbearing in women with and without a history of affective disorder. II. Electroencephalographs sleep. Compr Psychiatry. 1994;35:215–224. doi: 10.1016/0010-440x(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 35.Field T., Diego M., Hernandez-Reif M., Figueiredo B., Schanberg S., Kuhn C. Sleep disturbances in depressed pregnant women and their newborns. Infant Behav Dev. 2007;30:127–133. doi: 10.1016/j.infbeh.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Skouteris H., Germano C., Wertheim EH., Paxton SJ., Milgrom J. Sleep quality and depression during pregnancy: a prospective study. J Sleep Res. 2008;17:217–220. doi: 10.1111/j.1365-2869.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 37.Vesga-Lopez O., Blanco C., Keyes K., Olfson M., Grant BF., Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65:805–815. doi: 10.1001/archpsyc.65.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goyal D., Gay CL., Lee KA. Patterns of sleep disruption and depressive symptoms in new mothers. J Perinat Neonatal Nurs. 2007;21:123–129. doi: 10.1097/01.JPN.0000270629.58746.96. [DOI] [PubMed] [Google Scholar]
- 39.Frank E., Kupfer DJ., Jacob M., Blumenthal SJ., Jarrett DB. Pregnancy-related affective episodes among women with recurrent depression. Am J Psychiatry. 1987;144:288–293. doi: 10.1176/ajp.144.3.288. [DOI] [PubMed] [Google Scholar]
- 40.Shaver JL., Giblin E., Paulsen V. Sleep quality subtypes in midlife women. Sleep. 1991;14:18–23. doi: 10.1093/sleep/14.1.18. [DOI] [PubMed] [Google Scholar]
- 41.Young T., Rabago D., Zgierska A., Austin D., Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 42.Kalleinen N., Polo-Kantola P., Himanen SL., et al. Sleep and the menopause - do postmenopausal women experience worse sleep than premenopausal women? Menopause Int. 2008;14:97–104. doi: 10.1258/mi.2008.008013. [DOI] [PubMed] [Google Scholar]
- 43.Kravitz HM., Avery E., Sowers M., et al. Relationships between menopausal and mood symptoms and EEG sleep measures in a multi-ethnic sample of middle-aged women: the SWAN sleep study. Sleep. 2011;34:1221–1232. doi: 10.5665/SLEEP.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polo-Kantola P., Erkkola R., Irjala K., Helenius H., Pullinen S., Polo O. Climacteric symptoms and sleep quality. Obstet Gynecol. 1999;94:219–224. doi: 10.1016/s0029-7844(99)00284-7. [DOI] [PubMed] [Google Scholar]
- 45.Parry BL., Maurer EL. Light treatment of mood disorders. Dialogues Clin Neurosci. 2003;5:353–365. doi: 10.31887/DCNS.2003.5.4/bparry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benloucif S., Burgess HJ., Klerman EB., et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–69. [PMC free article] [PubMed] [Google Scholar]
- 47.First MB., Gibbon M., Spitzer RL., Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - research version. New York, NY: Biometrics Research Dept, New York State Psychiatric Institute; 1995 [Google Scholar]
- 48.Williams JB., Link MJ., Rosenthal NE., Amira L., Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD). Revised edition. New York, NY: New York Psychiatric Institute; 1994 [Google Scholar]
- 49.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 50.Rosenthal NE., Heffernan MM. Bulimia, carbohydrate craving and depression: a central connection? In: Wurtman RJ, Wurtman JJ, eds. Nutrition and the Brain. Vol 7. New York, NY: Raven Press; 1986 [Google Scholar]
- 51.Beck AT., Ward CH., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 52.Cox JL., Holden JM., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 53.Green J., Murray D. The use of the Edinburgh Postnatal Depression Scale in research to explore the relationship between antenatal and postnatal dysphoria. In: Cox J, ed. Perinatal Psychiatry: Use and Misuse of the Edinburgh Postnatal Depression Scale. London, UK: Gaskell; 1994:180–198. [Google Scholar]
- 54.American Psychiatric Association. DSM-IV Sourcebook. Vol 2. Washington, DC: American Psychiatric Association; 1996 [Google Scholar]
- 55.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 56.Rechtschaffen A., Kales AA. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Wash ington, DC: Superintendent of Documents, US Government Printing Office; 1968 [Google Scholar]
- 57.Parry BL., Meliska CJ., Sorenson DL., et al. Plasma melatonin circadian rhythm disturbances during pregnancy and postpartum in depressed women and women with personal or family histories of depression. NIHMS242677. Am J Psychiatry. 2008;165:1551–1558. doi: 10.1176/appi.ajp.2008.08050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parry BL., Meliska CJ., Sorenson DL., et al. Increased melatonin and delayed offset in menopausal depression: role of years past menopause, follicle-stimulating hormone, sleep end time, and body mass index. PMC2190736. J Clin Endocrinol Metab. 2008;93:54–60. doi: 10.1210/jc.2006-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parry BL., Meliska CJ., Martinez LF., et al. Late, but not early, wake therapy reduces morning plasma melatonin: relationship to mood in Premenstrual Dysphoric Disorder. NIHMSID # 242664. Psychiatry Res. 2008;161:76–86. doi: 10.1016/j.psychres.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker MP., van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buysse DJ., Kupfer DJ., Frank E., Monk TH., Ritenour A. Electroencephalographic sleep studies in depressed outpatients treated with interpersonal psychotherapy: II. Longitudinal studies at baseline and recovery. Psychiatry Res. 1992;42:27–40. doi: 10.1016/0165-1781(92)90036-3. [DOI] [PubMed] [Google Scholar]
- 62.Signal TL., Gander PH., Sangalli MR., Travier N., Firestone RT., Tuohy JF. Sleep duration and quality in healthy nulliparous and multiparous women across pregnancy and postpartum. Aust N Z J Obstet Gynaecol. 2007;47:16–22. doi: 10.1111/j.1479-828X.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 63.Thomas KA., Burr RL. Melatonin level and pattern in postpartum versus nonpregnant nulliparous women. J Obstet Gynecol Neonatal Nurs. 2006;35:608–615. doi: 10.1111/j.1552-6909.2006.00082.x. [DOI] [PubMed] [Google Scholar]
- 64.Perlis ML., Giles DE., Mendelson WB., Bootzin RR., Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 65.Perlis ML., Smith MT., Andrews PJ., Orff H., Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]