Abstract
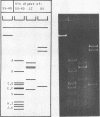
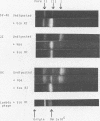
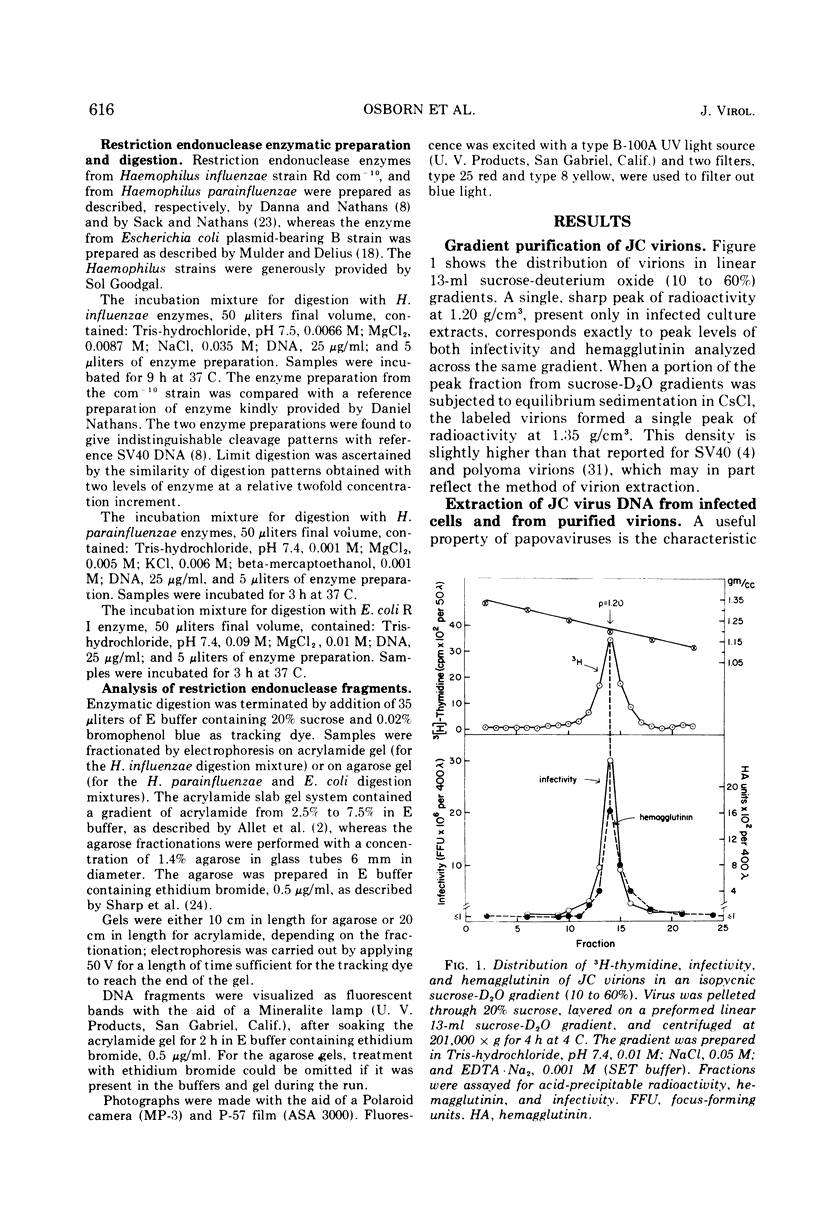
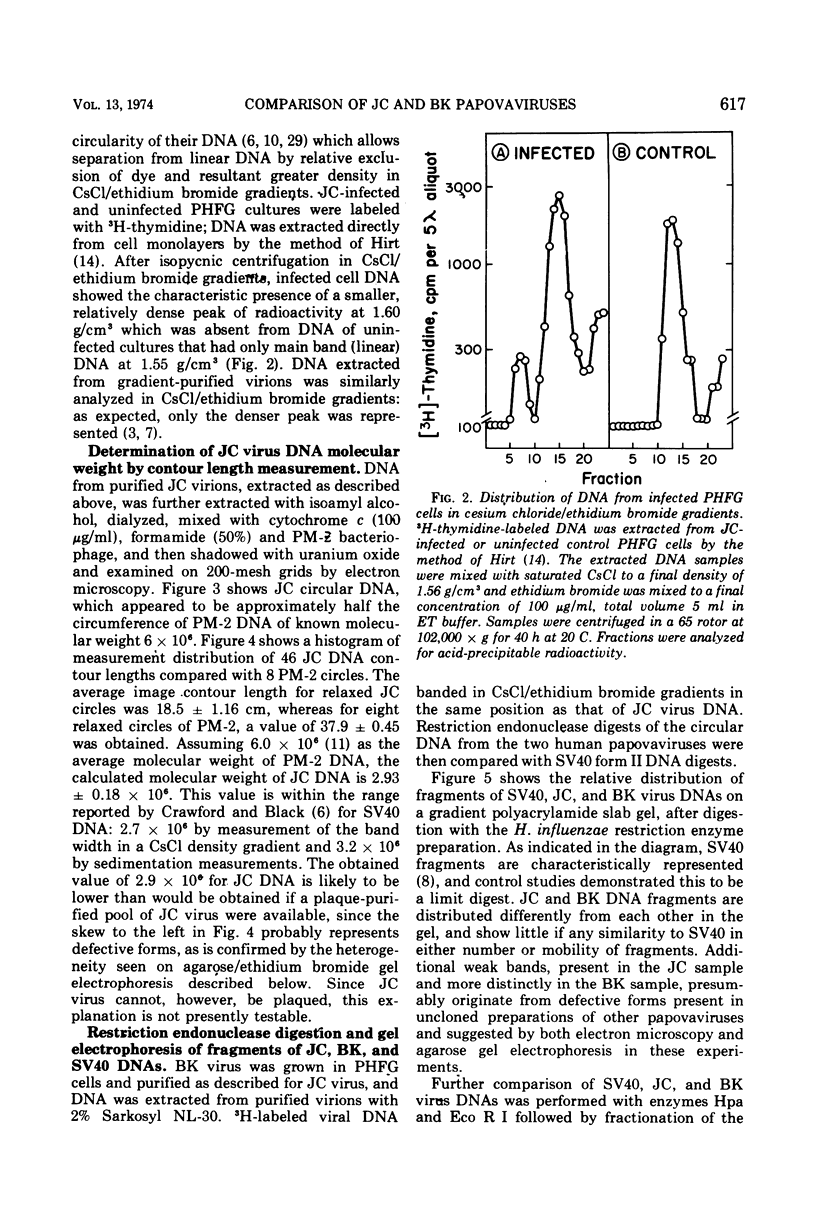
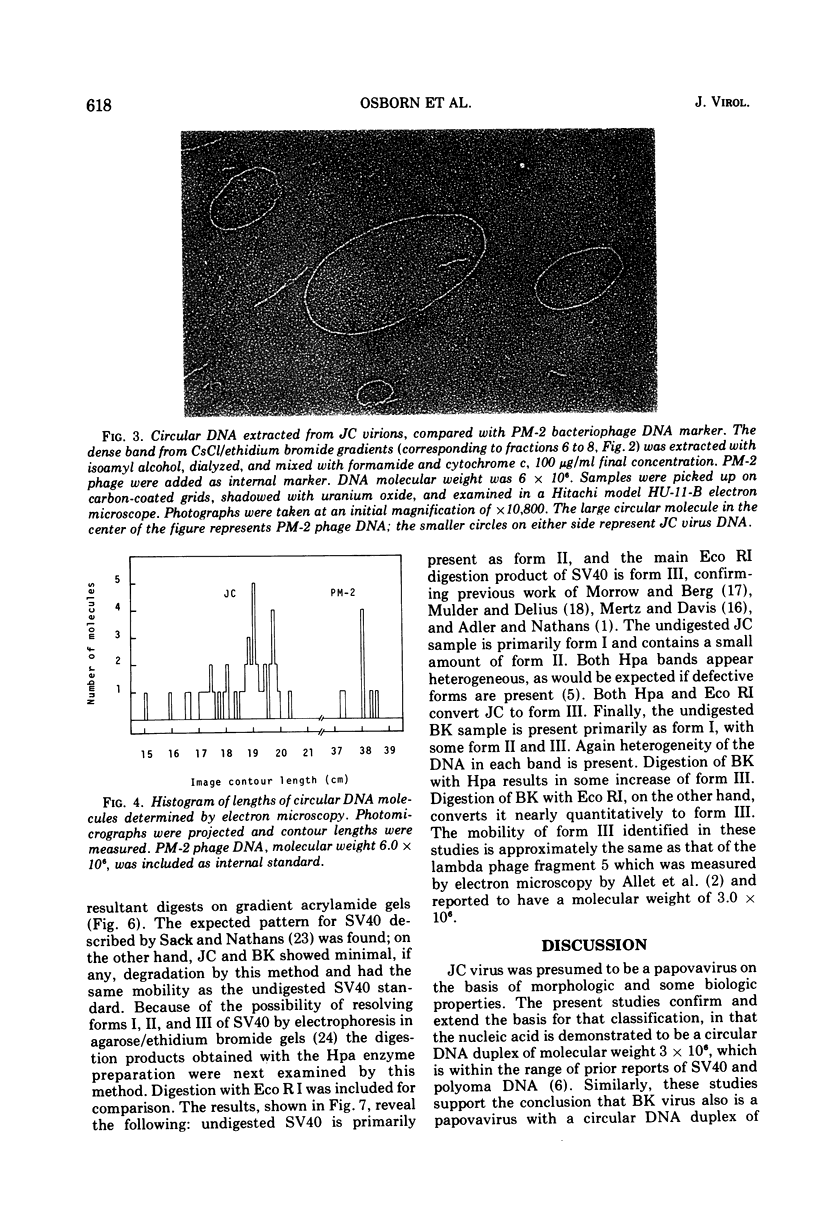
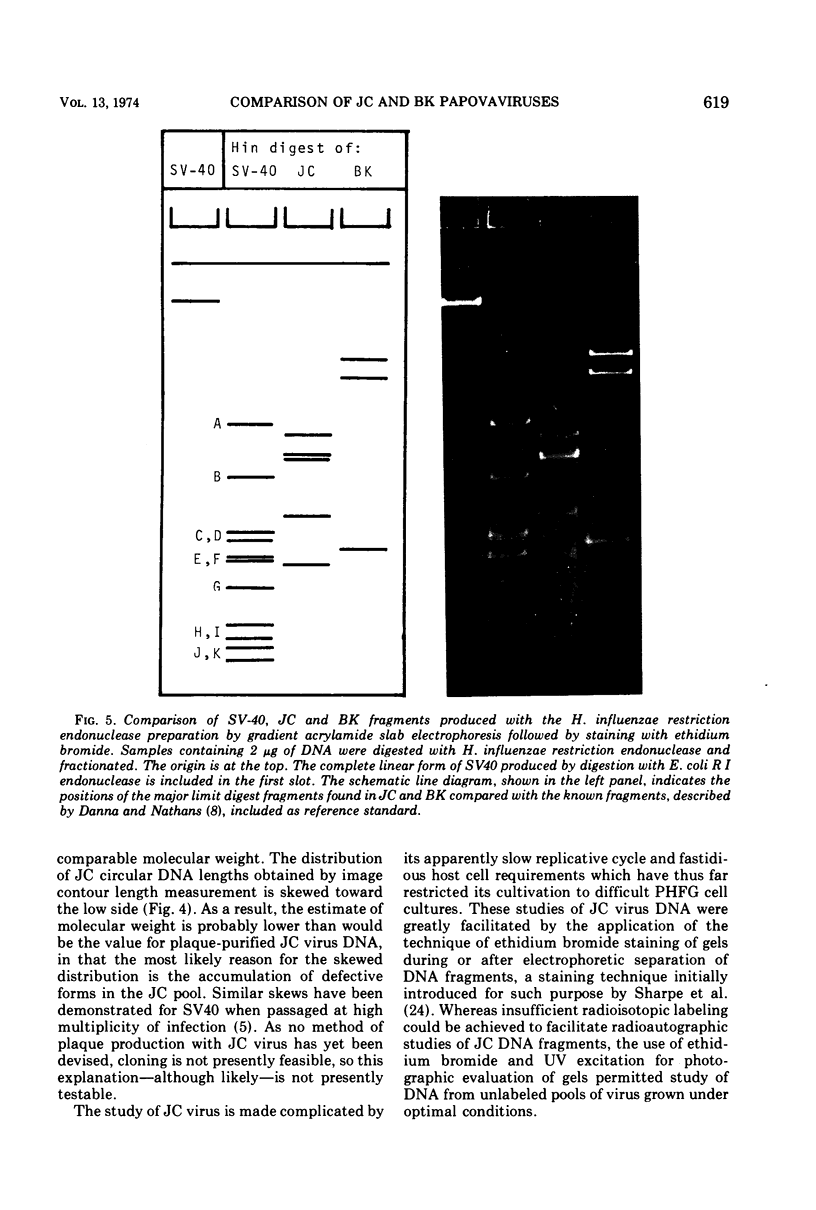
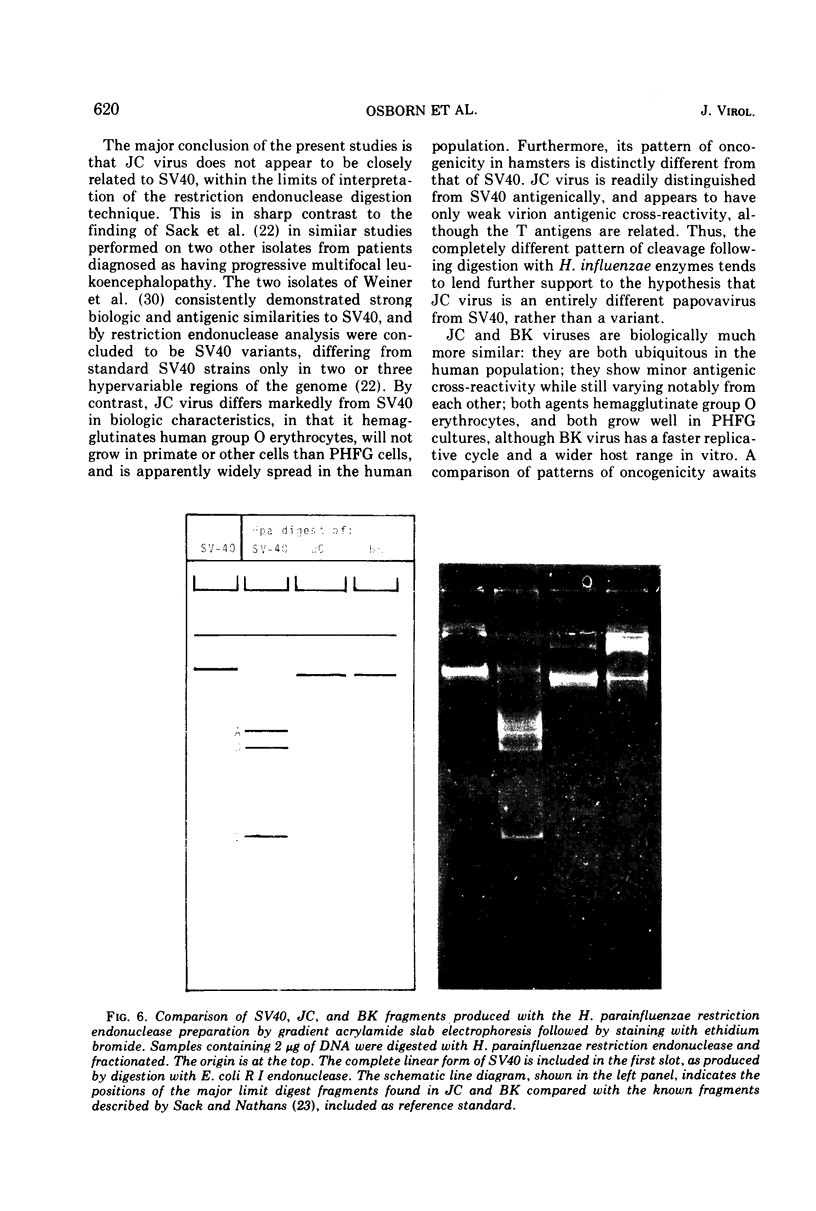
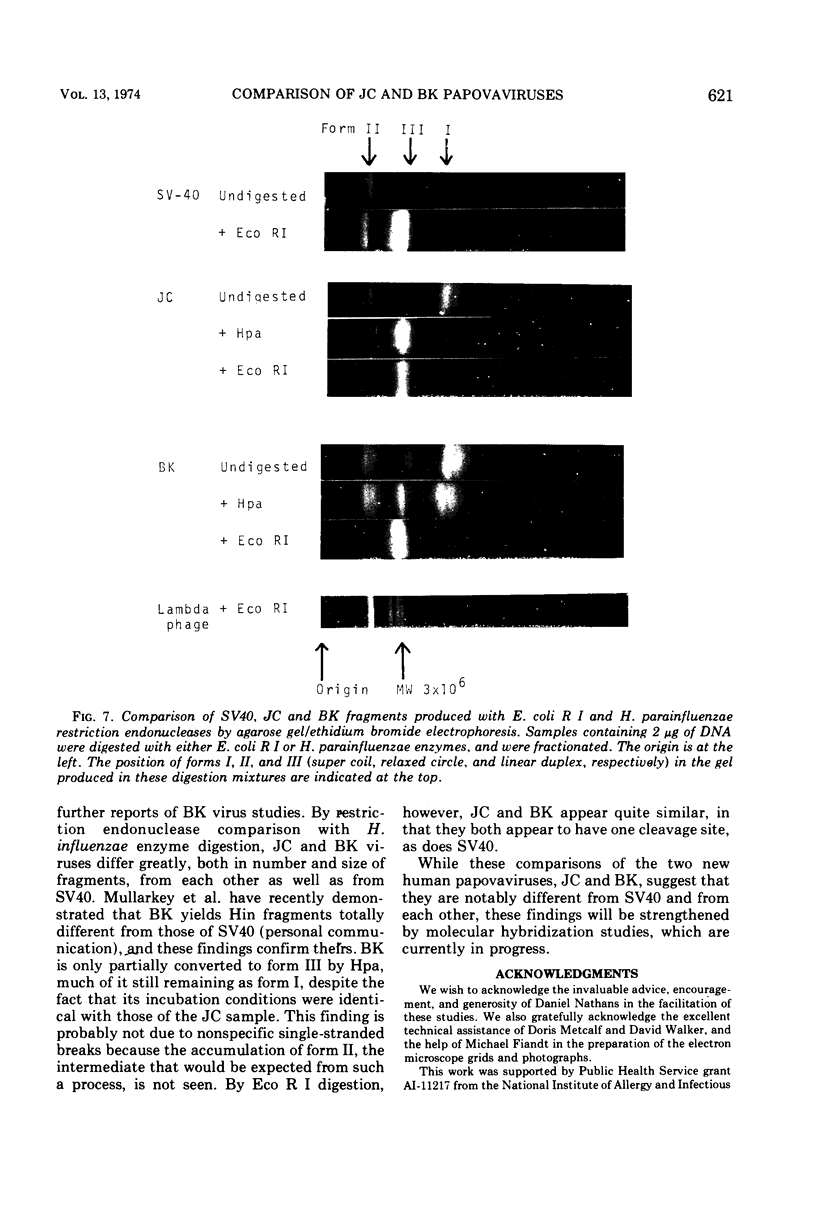
JC virus was found to have a buoyant density of 1.20 g/cm3 in linear sucrose-D2O and 1.35 g/cm3 in cesium chloride isopycnic gradients. DNA extracted either from JC-infected cultures or from gradient-purified virions occupied a dense position relative to linear DNA in cesium chloride/ethidium bromide gradients, and the circular configuration of the extracted DNA was confirmed by electron microscopy, with a measured molecular weight of 2.93 × 106. DNA from BK virus was similarly prepared and compared to JC and to an SV40 DNA standard by digestion with restriction endonuclease preparations from Haemophilus influenzae, Haemophilus parainfluenzae, and Escherichia coli. Digests were electrophoretically analyzed on gradient polyacrylamide slab gels or agarose gels, and the three viruses were found to have distinctly different cleavage patterns by this form of analysis: JC and BK viruses were almost entirely different from SV40 and significantly different from each other. Thus, JC and BK human papovaviruses appear to be discrete new members of the papovavirus group, rather than SV40 variants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P., Nathans D. Studies of SV 40 DNA. V. Conversion of circular to linear SV 40 DNA by restriction endonuclease from Escherichia coli B. Biochim Biophys Acta. 1973 Mar 19;299(2):177–188. doi: 10.1016/0005-2787(73)90340-7. [DOI] [PubMed] [Google Scholar]
- Allet B., Jeppesen P. G., Katagiri K. J., Delius H. Mapping the DNA fragments produced by cleavage by lambda DNA with endonuclease RI. Nature. 1973 Jan 12;241(5385):120–123. doi: 10.1038/241120a0. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., CRAWFORD E. M., CRAWFORD L. V. THE PURIFICATION OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:381–387. doi: 10.1016/0042-6822(64)90175-8. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Brockman W. W., Lee T. N., Nathans D. The evolution of new species of viral DNA during serial passage of simian virus 40 at high multiplicity. Virology. 1973 Aug;54(2):384–397. doi: 10.1016/0042-6822(73)90151-7. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Waring M. J. Supercoiling of polyoma virus DNA measured by its interaction with ethidium bromide. J Mol Biol. 1967 Apr 14;25(1):23–30. doi: 10.1016/0022-2836(67)90276-8. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. EVIDENCE FOR A RING STRUCTURE OF POLYOMA VIRUS DNA. Proc Natl Acad Sci U S A. 1963 Aug;50:236–243. doi: 10.1073/pnas.50.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K., Nathans D. Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2913–2917. doi: 10.1073/pnas.68.12.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gardner S. D. Prevalence in England of antibody to human polyomavirus (B.k.). Br Med J. 1973 Jan 13;1(5845):77–78. doi: 10.1136/bmj.1.5845.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L. Papova virus group. Science. 1962 Mar 30;135(3509):1128–1130. doi: 10.1126/science.135.3509.1128. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Narayan O. Studies of the antigenic relationships of the new human papovaviruses by electron microscopy agglutination. Infect Immun. 1973 Aug;8(2):299–300. doi: 10.1128/iai.8.2.299-300.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack G. H., Jr, Narayan O., Danna K. J., Weiner L. P., Nathans D. The nucleic acid of an SV40-like virus isolated from a patient with progressive multifocal leukoencephalopathy. Virology. 1973 Feb;51(2):345–350. doi: 10.1016/0042-6822(73)90433-9. [DOI] [PubMed] [Google Scholar]
- Sack G. H., Jr, Nathans D. Studies of SV40 DNA. VI. Cleavage of SV40 DNA by restriction endonuclease from Hemophilus parainfluenzae. Virology. 1973 Feb;51(2):517–520. doi: 10.1016/0042-6822(73)90455-8. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shein H. M. Propagation of human fetal spongioblasts and astrocytes in dispersed cell cultures. Exp Cell Res. 1965 Dec;40(3):554–569. doi: 10.1016/0014-4827(65)90234-x. [DOI] [PubMed] [Google Scholar]
- Silverman L., Rubinstein L. J. Electron microscopic observations on a case of progressive multifocal leukoencephalopathy. Acta Neuropathol. 1965 Nov 18;5(2):215–224. doi: 10.1007/BF00686519. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Mullarkey M. F. Human papovavirus, BK strain: biological studies including antigenic relationship to simian virus 40. J Virol. 1973 Sep;12(3):625–631. doi: 10.1128/jvi.12.3.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- ZURHEIN G., CHOU S. M. PARTICLES RESEMBLING PAPOVA VIRUSES IN HUMAN CEREBRAL DEMYELINATING DISEASE. Science. 1965 Jun 11;148(3676):1477–1479. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- Zu Rhein G. M. Association of papova-virions with a human demyelinating disease (progressive multifocal leukoencephalopathy). Prog Med Virol. 1969;11:185–247. [PubMed] [Google Scholar]